Abstract
Purpose
Mesothelin is overexpressed on many pancreatic and ovarian cancers, mesotheliomas, and other tumor types. Clinical trials are ongoing employing immunotoxins to target mesothelin, and patients immunized with allogeneic pancreatic tumor cell lines have demonstrated immune responses to previously defined mesothelin epitopes. The purpose of this study was to define novel mesothelin cytotoxic T lymphocyte (CTL) epitopes, and more importantly, agonist epitopes that would more efficiently activate human T cells to more efficiently lyse human tumors.
Experimental Design and Results
Two novel mesothelin HLA-A2 epitopes were defined. T-cell lines generated from one of these epitopes were shown to lyse pancreatic and ovarian tumor cells. Several agonist epitopes were defined and were shown to (a) have higher affinity and avidity for HLA-A2, (b) activate mesothelin-specific T cells from normal individuals or cancer patients to a greater degree than the native epitope in terms of induction of higher levels of IFN-γ and the chemokine lymphotactin, and (c) lyse several mesothelin-expressing tumor types in a major histocompatibility complex (MHC)–restricted manner more effectively than T cells generated employing the native peptide.
External beam radiation of tumor cells at non-toxic levels was shown to enhance the expression of mesothelin and other accessory molecules, resulting in a modest but statistically significant increase in tumor-cell lysis by mesothelin-specific T cells.
Conclusions
The identification of novel CTL agonist epitopes supports and extends observations that mesothelin is a potential target for immunotherapy of pancreatic and ovarian cancers, as well as mesotheliomas.
Keywords: Agonist peptides, Mesothelin, CTL epitopes, Immunotherapy, HLA-A2 binding peptides
INTRODUCTION
Mesothelin, a glycosylphosphatidylinositol-linked glycoprotein, is a differentiation antigen of mesothelial cells. The cDNA encodes a Mr. 69,000 precursor protein, which is proteolytically processed into two components. One component, corresponding to the COOH-terminal portion of the precursor, is a membrane-bound Mr. 40,000 protein known as mesothelin. The other component is a Mr. 30,000, corresponding to the NH2-terminal secreted protein MPF (megakaryocyte potentiating factor) (1–3). In humans, mesothelin is expressed in normal mesothelium and certain epithelial cells of the tonsil, trachea and fallopian tube (4). Mesothelin is overexpressed in a vast majority of ductal pancreatic adenocarcinomas, mesotheliomas, adenocarcinomas of the ovary, lung and stomach (5–14). Mesothelin has also been reported in squamous cell carcinomas of the esophagus, lung and cervix (15).
The presence of mesothelin in many cancer cells makes it a potential target for cancer therapies. A monoclonal antibody, K1, which recognizes mesothelin, has been developed and used in preclinical studies (4, 16). Later a high affinity anti-mesothelin antibody was isolated by phage display (17) and after affinity improvement (18) was converted to a disulfide linked Fv and fused to a 38 KDa portion of pseudomonas exotoxin A to make immunotoxin SS1(dsFv)-PE38 (3). SS1(dsFv)-PE38 selectively inhibited experimental human lung cancer metastases in nude mice as well as demonstrated anti-tumor activity against tumor cells obtained from patients with ovarian cancer and peritoneal mesotheliomas (19–21). Immunotoxins directed against mesothelin are currently in clinical trials (22,23).
Mesothelin is also a potential target for T-cell direct immunity. Potential cytotoxic T lymphocyte (CTL) epitopes of mesothelin specific for HLA-A2, A3 and A24 have recently been reported. CD8+ T cell responses to these HLA-A2, A3 and A24 epitopes were detected in patients vaccinated with granulocyte macrophage-colony stimulating factor (GM-CSF)–transduced pancreatic cancer cell lines (24). These studies provided evidence of cross-priming post-vaccination, which was directed against mesothelin-specific epitopes (24).
Several preclinical studies have demonstrated that the modification of CTL epitopes to render them more immunogenic will result in enhanced anti-tumor responses. Recent clinical studies in melanoma and colorectal cancer have also shown that vaccination with such agonist epitopes can result in the generation of greater levels of T-cell responses in patients, which have correlated with clinical responses (25, 26). It has also recently been demonstrated that agonist epitopes have the ability to stimulate T cells to produce more lymphotactin. Lymphotactin is a member of the C-chemokine family cloned from activated pro-T cells (27, 28). It is produced by activated CD4+ and CD8+ T cells, natural killer cells, intraepithelial γδ T cells and mast cells (29–31). Lymphotactin is a powerful chemoattractant for CD4+ and CD8+ T cells and a moderate chemoattractant for natural killer cells (28, 30).
The study described here reports the identification and characterization of a novel mesothelin CTL epitope, and the generation of an enhancer agonist of this epitope. T-cell lines, generated from a pancreatic cancer patient with the agonist peptide, showed high levels of lysis of mesothelin-expressing tumor cells and enhanced IFN-γand lymphotactin production when target cells were pulsed with the agonist peptide versus the native peptide. The studies reported here also demonstrate that the use of non-toxic doses of external beam radiation of tumor cells will enhance the expression of mesothelin and other accessory molecules and render tumor cells more susceptible to mesothelin-specific T-cell killing. These studies thus provide the rationale for the potential utility of the novel mesothelin agonist epitope, alone or in concert with previously defined mesothelin epitopes, in peptide- and/or vector-mediated immunotherapy protocols for the treatment of mesothelin-expressing tumors.
MATERIALS AND METHODS
Cell Cultures
The human pancreatic adenocarcinoma cell line CFPAC-1 (32) (HLA-A2 positive and mesothelin positive), a human colon carcinoma SW1463 (HLA-A2 positive, mesothelin negative), a human ovarian adenocarcinoma cell line OVCAR-3 (HLA-A2 positive, mesothelin positive) and a human pancreatic adenocarcinoma cell line AsPC-1 (HLA-A2 negative and mesothelin positive) were purchased from American Type Culture Collection (Manassas, VA). Three HLA-A2 positive and mesothelin positive peritoneal mesothelioma cell lines (YOU, ROB and ORT), a human epidermoid carcinoma cell line A431 (HLA-A2 negative, mesothelin negative), and A431.H9 cell line. A431 cells transfected with pMH107, pcDNA3.1(+) vector containing a full-length mesothelin cDNA are designated as A431.H9. A431.H9 is a stable transfected cell line (33), were obtained from Dr. Raffit Hassan (NCI, NIH). The cultures were free of mycoplasma and were maintained in complete medium [RPMI 1640 (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen Life Technologies)]. The C1R cell line is a human plasma leukemia cell line that does not express endogenous HLA-A or B antigens (34). C1R-A2 cells are C1R cells that express a transfected genomic clone of HLA-A2.1 (35). These cells were obtained from Dr. William E. Biddison (National Institute of Neurological Disorders and Stroke, NIH, Bethesda, MD). The 174CEM-T2 cell line (T2) transport deletion mutant (36) was provided by Dr. Peter Cresswell (Yale University School of Medicine, New Haven, CT). C1R-A2 cells and T2 cells were mycoplasma free and were maintained in RPMI 1640 complete medium and in Iscove’s modified Dulbecco’s complete medium (Invitrogen Life Technologies), respectively.
Peptides
The amino acid sequence of mesothelin was scanned for matches to consensus motifs for HLA-A2 binding peptides. We used the computer algorithm from the BioInformatics and Molecule Analysis Section of NIH (BIMAS), developed by Parker et al. (37), which ranks potential MHC binding peptides according to the predictive one-half-time dissociation of peptide/MHC complexes. The HLA-A2 allele was chosen because it is the most commonly expressed class I allele. Nine-mer and 10-mer peptides were synthesized if they conformed to the respective consensus motif. A panel of 10-mer mesothelin peptides (Table 1) and analogs with one, two or three amino acids substitution of P-547-556 peptide and MUC-1 peptide (38) were made by American Peptide Company (Sunnyvale, CA) with purity >90%.
Table 1.
Binding of human mesothelin peptides and analog peptides to HLA-A2 molecules
| Experiment 1: Analysis of native human mesothelin peptide to HLA-A2 molecules | |||
|---|---|---|---|
| Peptide | Amino acid position in mesothelin | Sequence | T2 bindinga |
| P21 | 21-29 | FLLFSLGWV | 241 (1.5) |
| P547 | 547-556 | KLLGPHVEGL | 407 (2.6) |
| MUC-1 (positive control) | NA | ALWGQDVTSV | 422 (2.7) |
| CAP-7 (negative control) | NA | HLFGYSWYK | 152 |
| Experiment 2: Analysis of analog peptides | |||
| Mesothelin agonist peptides derived from P547 | Designation | Peptide sequence | T2 bindinga |
| P547 (native) | P547 | KLLGPHVEGL | 226 (1.5) |
| 554L | P547-1 | KLLGPHVLGL | 180 (1.2) |
| 554L/556V | P547-2 | KLLGPHVLGV | 289 (2.0) |
| 548M/554L/556V | P547-3 | KMLGPHVLGV | 368 (2.6) |
| 548I/554L/556V | P547-4 | KILGPHVLGV | 342 (2.3) |
| 548M/554L | P547-5 | KMLGPHVLGL | 132 (0.9) |
| 548I/554L | P547-6 | KILGPHVLGL | 185 (1.3) |
| 547Y/554L/556V | P547-7 | YLLGPHVLGV | 234 (1.6) |
| 547Y/554L | P547-8 | YLLGPHVLGL | 161 (1.1) |
| MUC-1 peptide (positive control) | ALWGQDVTSV | 390 (2.7) | |
| CAP-7 (negative control) | HLFGYSWYK | 144 | |
Results are expressed in mean fluorescence intensity (MFI). MUC-1 peptide is an HLA-A2 binding peptide and CAP-7 is an HLA-A3 binding CEA peptide.
NA, not applicable.
Peptides were used at a concentration of 25 μg/ml. Values in parentheses are fold increases as compared with the negative control. Amino acid sequences of the parental P547 peptide (amino acid position 547-556 of mesothelin) and analog peptides. Amino acids are shown by the single-letter code. Substitution amino acids are indicated in bold italic and underlined.
Flow Cytometric Analysis
Dual-color flow cytometric analysis was performed on T-cell lines by using the following antibody combinations: anti-CD56-FITC/anti-CD8-PE, anti-CD8-FITC/anti-CD45RA-PE, anti-CD8-FITC/anti-CD27-PE, and anti-CD8-FITC/anti-CD28 PE. Antibodies were all purchased from BD Biosciences (San Jose, CA). Staining was conducted simultaneously for 1 hour at 4° C; cells were then washed three times with cold Ca2+ and Mg2+ free phosphate-buffered saline (PBS), resuspended in the same buffer, and immediately analyzed using a FACScan and the CELLQuest program (BD Biosciences). Data were gathered from 10,000 live cells, stored and used to generate results.
The procedure for analysis of DCs was similar to the one described above. The following antibody combinations were used: anti-MHC-class II-FITC/anti-CD80-PE, anti-CD58-FITC/anti-CD54-PE, anti-MHC class I-FITC/anti-MHC class II-PE, and anti-IgG1-FITC/anti-IgG2a-PE (isotype controls). Antibodies to MHC-class I and II were purchased from Serotec (Oxford, UK); other antibodies were purchased from BD Biosciences. The method described by Fan et al. (19) using K1 antibody was used for the analysis of mesothelin expression on tumor cell lines. The cells were immediately analyzed using a Becton Dickinson FACScan equipped with a blue laser with an excitation of 15 mW at 488 nm. Data were gathered from 10,000 live cells, stored and used to generate results.
Results were expressed in percent of positive cells and mean fluorescence intensity (MFI). MFI was used to express the levels of fluorescence determined by measuring the average for all the cells in the gated fluorescence dot plot. The MFI value was collected in log scale on the FACScan.
Peptide Binding to HLA-A2
Binding of mesothelin peptides and the mesothelin analogs to HLA-A2 molecules was evaluated by the upregulation of HLA-A2 expression on T2 cells as demonstrated by flow cytometry (39).
Culture of DCs from PBMCs
HLA-A2 normal donor peripheral blood mononuclear cells (PBMCs) were obtained from heparinized blood. PBMCs were separated using lymphocyte separation medium gradient (Organon Teknika, Durham, NC), as described previously (40). Dendritic cells (DCs) were prepared using a modification of the procedure described by Sallusto et al. (41).
Generation of T-cell Lines
Modification of the protocol described by Tsang et al. (42) was used to generate mesothelin-specific CTLs. To generate T-cell line T-1991-P-547, autologous DCs were used as antigen-presenting cells (APCs). Autologous non-adherent cells were then added to APCs at an effector-to-APC ratio of 10:1. Cultures were then incubated for 3 days at 37° C in a humidified atmosphere containing 5% CO2. The cultures were then supplemented with recombinant human IL-2 at a concentration of 20 units/ml for 7 days; the IL-2 containing medium was replenished every 3 days. The 3-day incubation with peptide and 7-day IL-2 supplement constituted one in vitro stimulation (IVS) cycle. Primary cultures were restimulated with autologous DCs as described above on day 11 to begin the next IVS cycle. Autologous DCs were used as APCs for three IVS cycles. Irradiated (23,000 rads) autologous Epstein-Barr virus (EBV)–transformed B cells were used as APCs after the third IVS cycle. For the restimulation with EBV-transformed B cells, peptides at a concentration of 25 μg/ml were used to pulse the autologous EBV-transformed B cells at an effector-to-APC ratio of 1:3 for restimulation. Cultures were then incubated for 3 days at 37° C in a humidified atmosphere containing 5% CO2. After removal of the peptide containing medium, the cultures were then supplemented with recombinant human IL-2 at a concentration of 20unit/ml for 7 days. T-cell lines from patient 55 (T-55-P-547, T-55-P-554L/556V, T-55-P-548M/554L/556V, T-55-P-548L/554L/556V) were generated by stimulation of PBMCs with autologous DCs pulsed with the P-547 or the agonist peptides using the same stimulation protocol as described above. The markers used for the analysis and identification of DCs were CD11c, MHC-class II, CD80, CD54, CD58 and CD83. CD3 was also used as a negative marker.
Cytotoxic Assay
Cytotoxicity assays were used as described previously (42).
Detection of Cytokines
Supernatants of T cells exposed for 24 hours to peptide-pulsed autologous DCs, in IL-2-free medium at various peptide concentrations, were screened for secretion of IFN-γ using an ELISA kit (BioSource International, Camarillo, CA) and lymphotactin using an ELISA assay (43). The results were expressed in pg/ml.
Chemotaxis Assay
Chemotactic responses were examined using the method described previously (44). Briefly, blind Well Chambers (Neuroprobe, Gaithersburg, MD) with polyvinylpyrrolidone-free 5μm-pore size polycarbonate filters previously coated on one side with mouse Collagen IV (Trevigen, Gaithersburg, MD) were used. Supernatants from unstimulated T-55-P547 cells or T-55-P547 cells activated for 24 hours with 25 μg/ml of either P547 or P547-2 peptide were added to the lower chambers, and healthy donor PBMCs (7.5 x 104 cells, 75 μl in complete RPMI medium containing 1% human AB serum) were added to the upper chambers. As negative and positive controls, the lower chambers were loaded with medium alone or recombinant lymphotactin (50 ng/ml in complete RPMI medium containing 1% human AB serum), respectively. After incubation for 4 hours at 37 °C, filters were removed from the chambers, fixed and stained with Diff-Quik stain (Dade Behring Inc., Newark, DE). Blocking assay was performed using anti-lymphotactin antibody (R&D Systems, Inc., Minneapolis, MN). The number of cells associated with the lower side of the membranes was evaluated by direct counting of at least six 40X objective fields for the standard samples or at least nine 40X objective fields for the experimental samples.
Irradiation of Tumor Cells
Human tumor cell lines CFPAC-1, OVCAR-3 and AsPC-1 were irradiated as previously described (45, 46). Tumor cells were harvested in log-growth phase and were placed on ice and irradiated at 10 and 20 Gy by a cesium-137 source (Gammacell-1000; AECL/Nordion, Kanata, Ontario, Canada) at a dose rate of 0.74 Gy/min. Control samples were also placed on ice but not irradiated. Irradiated and nonirradiated cells were then washed in fresh media and seeded in 75-cm2 cell culture flasks. Cells were harvested for surface marker determination after 72 hours in culture by flow cytometry.
Vaccinia Virus Infection of Epidermoid Carcinoma Cells
A recombinant vaccinia vector encoding HLA-A2.1 was used for the infection of A431 and A431.H9 cells. This recombinant virus was constructed by the insertion of the HLA-A2.1 gene into the BamHI J region of the genome of the Wyeth strain of vaccinia virus as described (47). The gene is under control of the vaccinia 40k promoter (48). Target cells at a concentration of 1 x 107/ml in complete RPMI-1640 medium supplemented with 0.1% bovine serum albumin were incubated with equal volume of vaccinia virus (108 pfu/ml) in the same medium at 37°C for 1.0 hours. The cells were then adjusted to a concentration of 5 x 105/ml in complete medium and incubated for 3 hours at 37°C.
Statistical Analysis
Statistical analysis of differences between means was done using a two-tailed paired t test (Stat View statistical software, Abacus Concepts, Berkeley, CA).
RESULTS
The primary amino acid sequence of human mesothelin was analyzed for consensus motifs for novel HLA-A2 binding peptides. One 10-mer peptide and one 9-mer peptide were identified, subsequently synthesized and investigated for binding to the HLA-A2 molecule in a T2 cell binding assay. The amino acid sequences and the positions of these peptides are shown in Table 1. The MUC-1 peptide and a CEA HLA-A3 binding peptide (CAP-7) were used as a positive and negative control, respectively. Two of these peptides (P21-29 and P547-556) were shown to have positive binding in the T2 assay. Experiments were then conducted to compare the ability of the P21-29 and P547-556 peptides to bind HLA-A2 at various peptide concentrations. As seen in Figure 1, the P547-556 peptide bound to HLA-A2 at higher levels than did the P21-29 peptide at concentrations of 50μg/ml, 25μg/ml, 12.5 μg/ml and 6.25 μg/ml. The P547-556 peptide (designated as P547) was thus chosen for further study.
Figure 1.
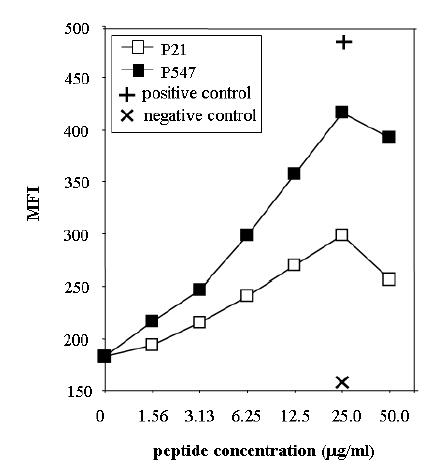
Binding of mesothelin peptides to HLA-A2. Peptides were analyzed for binding to T2 cell line as described in “Materials and Methods.” Peptides were used at concentrations of 0 to 50 μg/ml. P21 peptide (open square), P547 (solid square), positive control (MUC-1 peptide) (+) and negative control (HLA-A3 binding peptide) (X). Results are expressed in mean fluorescence intensity (MFI).
Studies were then initiated to determine whether mesothelin-specific T-cell lines could be established from PBMCs from an apparently healthy donor. Autologous DCs were used as APCs. The specificity of the mesothelin-specific T cells generated (designated T-1991-P547) was analyzed after IVS cycle 3 (see “Materials and Methods”) for the ability to release IFN-γand the chemokine lymphotactin after stimulation with autologous DCs pulsed with the P547 peptide. When T-1991-P547 cells were stimulated with autologous DCs pulsed with P547 peptide, the T cells produced 391 pg/ml IFN-γand 78 pg/ml lymphotactin, whereas the use of autologous DCs pulsed with control CAP1-6D peptide or DCs alone did not result in any IFN-γand lymphotactin production (i.e., less than 16 pg/ml). As determined by flow cytometric analysis, the T-1991-P547 cell line was 98.7% CD8 positive, 62.2% CD45RA positive, 1.1% CD28 positive, and 0.5% CD27 positive. The T-1991-P547 cell line was then analyzed for the ability to lyse mesothelin positive and HLA-A2-positive human tumor cell lines. AsPC-1 (HLA-A2 negative and mesothelin positive pancreatic cancer cell line) was used as a negative control. The expression of HLA-A2 and mesothelin on CFPAC-1, OVCAR-3, AsPC-1, SW1463 and C1R-A2 cell lines was analyzed by flow cytometry. As shown in Table 2, both CFPAC-1 cells and OVCAR-3 cells were lysed by the T-1991-P547 cells. No lysis was observed against AsPC-1 cells. T-1991-P547 cells lysed CFPAC-1 cells to a greater degree as compared with OVCAR-3 cells. This may partly be due to the fact that a higher percentage of CFPAC-1 cells were expressing mesothelin as compared with OVCAR-3 cells.
Table 2.
Ability of T-1991-P547 cells to lyse cancer cells expressing mesothelin*
| Target | HLA-A2 | Mesothelin | % lysis (±SD) |
|---|---|---|---|
| CFPAC-1 (pancreatic cancer cells) | 100 (222) | 93 (21) | 34.9 (3.5)a |
| AsPC-1 (pancreatic cancer cells) | Negative | 92 (19) | 8.8 (1.3) |
| OVCAR-3 (ovarian cancer cells) | 97 (23) | 47.4 (21) | 17.6 (2.0)a |
HLA-A2 and mesothelin expression were tested by flow cytometry (see “Materials and Methods”). Results are expressed in percent of positive cells (MFI). A 16-hour 111In release assay was performed. Results are expressed in percent specific lysis at effector-to-target ratio of 20:1.
Statistical significance (P<0.01, two tailed t test) when comparing lysis of CFPAC-1 cells versus AsPC-1 cells and OVCAR-3 versus AsPC-1 by T-1991-P547 cells.
Analysis of the primary and secondary HLA-A2 binding anchor amino acid residues at positions 1, 2, 8 and 10 of the P547 peptide revealed that modification of amino acids at these positions could potentially enhance the binding ability of the peptide to the HLA-A2 molecule. For this reason, eight different analogs of P547 peptide were synthesized, as shown in Table 1, and were investigated for their binding ability to T2 cells along with the native P547 peptide. The CEA peptide CAP-7, which has previously been shown not to bind to T2 (42), was used as a negative control. As shown in Table 1, three of the eight analogs bound to HLA-A2 at higher levels than the native peptide. They were designated P547-2, P547-3 and P547-4. Experiments were then conducted to compare the ability of these three analogs to bind HLA-A2 at various concentrations. As shown in Figure 2A, all bound to HLA-A2 at higher levels than did the native P547 peptide at all concentrations. These results indicated that these three analogs with modification in the primary anchor position 2 (position 548 of the mesothelin peptide) and position 10 (position 556 of the mesothelin peptide) as well as the secondary position 8 (position 554 of the mesothelin peptide) were potential agonists of peptide P547.
Figure 2.
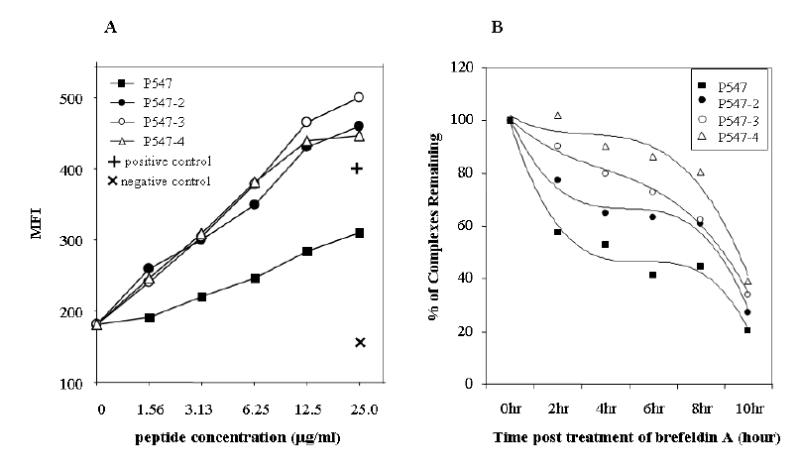
Binding of a native mesothelin peptide and its agonist peptides to HLA-A2. Peptides were analyzed for binding to T2 cell line as described in “Materials and Methods.” (A) Peptides were used at concentrations of 0 to 50 μg/ml. P547 peptide (solid square), P547-2 (solid circle), P547-3 (open circle) and P547-4 (open triangle), positive control (MUC-1 peptide) (+) and negative control (HLA-A3 binding peptide) (X). Results are expressed in mean fluorescence intensity (MFI). (B) Comparison of the stability of HLA-A2 peptide complexes of mesothelin native and agonist peptides. T2 cells were incubated overnight with P547 (solid square), P547-2 (solid circle), P547-3 (open circle), and P547-4 (open triangle) peptides at a concentration of 25 μg/ml and then were washed free of unbound peptide and incubated with brefeldin A to block delivery of new class I molecules to the cell surface. At the indicated times, cells were stained for the presence of surface peptide-HLA-A2 complexes. Results are expressed in relative percentage of binding compared with 100% at time 0.
We then examined the stability of the peptide-MHC complex for P547 (native), and P547-2, P547-3 and P547-4 agonist peptides. The peptides were incubated with T2 cells overnight, and the unbound peptides were washed off and the cells were then incubated with Brefeldin A to block delivery of new class I molecules to the cell surface. Cells were analyzed for the presence of peptide-HLA-A2 complexes at various time points. As shown in Figure 2B, all three HLA-A2 agonist complexes were more stable than the P547-HLA-A2 complex over the 8-hour observation period, with P547-4 slightly more stable than the P547-2 and P547-3 complexes over the same period of time. These data indicated that both the binding to the MHC molecule and the stability of the peptide-MHC complex were greater for the P547-2, P547-3 and P547-4 agonist peptides than the native P547 peptide.
Studies were then undertaken to compare the ability of the P547-2, P547-3 and P547-4 agonist peptides to activate the T-1991-P547 cells, which were generated with the native peptide. As seen in Table 3, pulsing of APCs with P547-2 peptide led to greater levels of both IFN-γand lymphotactin production by T-1991-P547 cells as compared with P547-3, P547-4 or the native P547 peptide. Studies were then conducted to compare the ability of the agonist peptides P-547-2, P547-3, P547-4 and the native P574 peptide at various peptide concentrations to activate T-1991-P547 cells in the production of lymphotactin. As shown in Figure 3, at concentrations of 3.13 μg/ml and higher, the pulsing of APCs with P547-2 led to the highest level of lymphotactin production by the T-1991-P547 cell line.
Table 3.
Production of IFN-γand lymphotactin by T-1991-P547 cells stimulated with autologous DCs pulsed with mesothelin peptide analogs
| Production of |
||
|---|---|---|
| Peptide | IFN-γ (pg/ml) | Lymphotactin (pg/ml) |
| P547 | 94 | 49.7 |
| P547-2 | 449 | 1,835 |
| P547-3 | 103 | 130 |
| P547-4 | 91 | <31.2 |
| None | <15.6 | <31.2 |
T-1991-P547 cells were used as effectors in in vitro stimulation (IVS-5). T cells were stimulated with irradiated autologous DCs pulsed with different mesothelin analogs at a concentration of 25 μg/ml, and an effector-to-APC ratio of 10:1. Twenty-four-hour culture supernatants were collected and screened for the secretion of IFN-γand lymphotactin.
Figure 3.
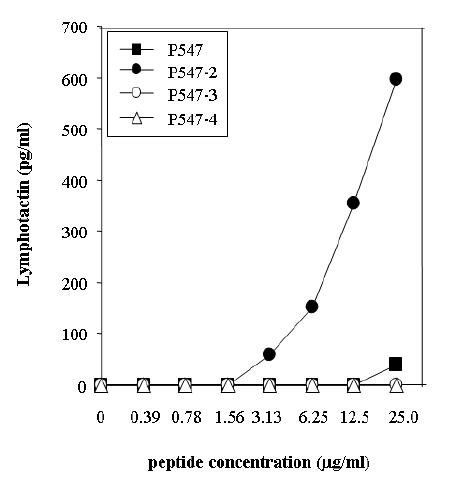
Ability of autologous DCs pulsed with native and agonist mesothelin peptides to induce lymphotactin production by T cells derived from the native peptide (T-1991-P547). Peptides were used at concentrations of 0–25 μg/ml. Results are expressed in pg/ml.
The T-1991-P547 cell line was derived from an apparently healthy individual using the native P547 peptide. Studies were then conducted to determine whether additional T-cell lines could be generated from a patient with pancreatic cancer (patient 55) using the native or the three agonist peptides. Four mesothelin-specific T-cell lines were subsequently established and were designated T-55-P547, T-55-P547-2, T-55-P547-3 and T-55-P547-4. The T-cell lines were generated by stimulation of PBMCs from patient 55 using autologous DCs. The ability of these T-cell lines to lyse mesothelin positive and HLA-A2 positive tumor cells was then investigated. As can be seen in Figure 4, all four T-cell lines were able to lyse the mesothelin positive and HLA-A2 positive CFPAC-1 cells and OVCAR-3 cells but showed no lysis against AsPC-1 (mesothelin positive, HLA-A2 negative) and SW1463 (mesothelin negative, HLA-A2 positive) cells. These results demonstrate the HLA-A2 and mesothelin specificity of the lysis. Furthermore, T-55-P547-2 cells, derived using P547-2 agonist peptide, demonstrated greater lysis of tumor cells than the T cells derived using the native P-547, or the agonist P547-3 and P547-4 peptides. This was seen at three different effector-to-target (E:T) ratios.
Figure 4.
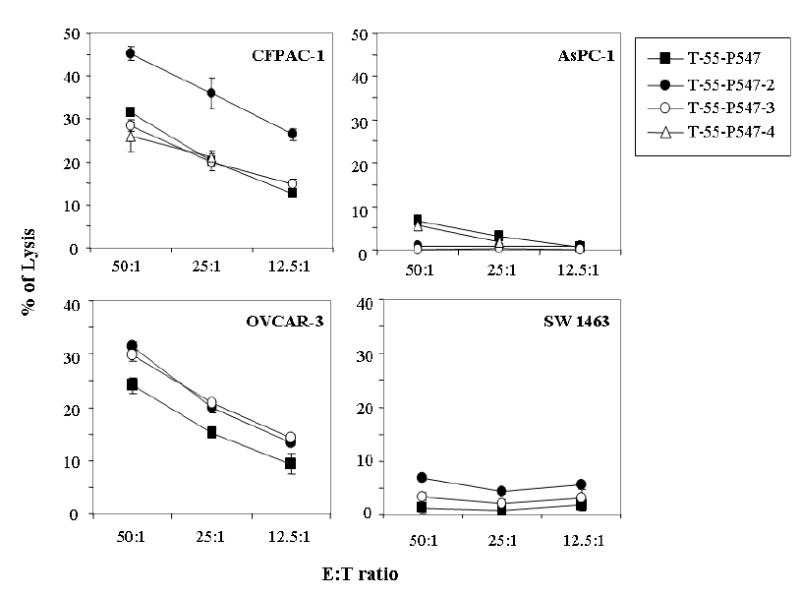
Ability of the mesothelin-specific T-cell lines established for a pancreatic cancer patient (patient 55) to lyse tumor cells expressing native mesothelin. T-55-P547 (derived from the native peptide) (solid square), T-55-P547-2 (derived from the agonist peptide P547-2) (solid circle), T-55-P547-3 (derived from the agonist peptide P547-3) (open circle), and T-55-P547-4 (derived from the agonist peptide P547-4) (open triangle). E:T ratio = 50:1, 25:1 and 12.5:1 in a 16-hour 111In release assay. See “Materials and Methods.” Bars, SD.
Experiments were then conducted to evaluate the ability of T-55-P547-2, T-55-P547-3 and T-55-P547-4 cells to produce lymphotactin when stimulated with the corresponding peptide. As shown in Table 4, a higher level of lymphotactin production was observed when T-55-P547-2 cells were stimulated with the agonist P547-2 peptide as compared with T-55-P547-3, T-55-P547-4 and T-55-P547 when stimulated with the corresponding peptide. Lymphotactin has been reported to enhance chemotaxis responses of various immune cells. A chemotaxis assay was thus performed (see “Materials and Methods”) to confirm the functional activity of the lymphotactin in the supernatants of the mesothelin-specific T-cell line stimulated with APCs pulsed with the P547-2 agonist peptide. T-55-P547 cells were used in this study. As seen in Figure 5A, supernatants from T-55-P547 cells stimulated with P547-2 peptide clearly enhanced migration of PBMCs over the responses to the supernatants from the native P547 peptide and the no peptide control. Purified recombinant human lymphotactin was also used as a positive control in the assay. To ascertain that the observed chemotactin responses were due to the presence of lymphotactin, a chemotaxis assay was performed with and without anti-lymphotactin antibody. As seen in Figure 5B, the addition of anti-lymphotactin antibody inhibited the migration of PBMCs induced by the purified recombinant lymphotactin as well as the migration of PMBCs induced by the supernatants from T-55-P547 cells stimulated with APCs pulsed with the agonist peptide P547-2.
Table 4.
Production of lymphotactin by T-cell lines generated from a pancreatic cancer patient (patient 55) stimulated with P547 and the agonist peptides
| Production of lymphotactin (pg/ml) |
||
|---|---|---|
| T-cell line | Corresponding peptide | CAP1-6D |
| T-55-547 | <31.2 | <31.2 |
| T-55-P547-2 | 2,843 | <31.2 |
| T-55-P547-3 | <31.2 | <31.2 |
| T-55-P547-4 | 73.5 | <31.2 |
Cells from four mesothelin-specific T-cell lines established from a pancreatic cancer patient (patient 55) were used as effector cells at in vitro stimulation (IVS-3). These T-cell lines were established by stimulation with P547-pulsed autologous DCs (T-55-P547), P547-2–pulsed autologous DCs (T-55-P547-2), P547-3–pulsed autologous DCs (T-55-P547-3) or P-547-4–pulsed autologous DCs (T-55-P547-4). For lymphotactin production, T-cell lines were stimulated with the corresponding peptide at a concentration of 25 μg/ml and an effector-to-APC ratio of 10:1. CAP1-6D peptide was used as a negative control. Twenty-four-hour culture supernatants were collected and screened for the secretion of lymphotactin.
Figure 5.
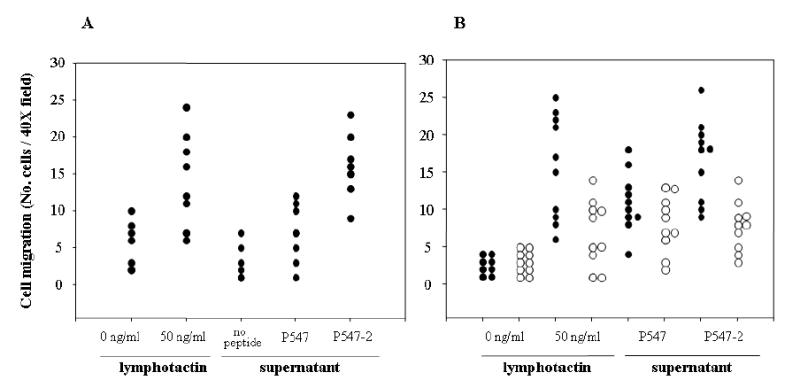
Chemotactic responses of human PBMCs to culture supernatant of a mesothelin-specific T-cell line (T-55-P547). (A) The assay was performed using culture supernatant of T-55-P547 cells stimulated with autologous DCs pulsed with either P547 or P547-2 peptide or without peptide for 24 hours. Recombinant lymphotactin was used as 50 ng/ml as positive control, and medium was used as negative control. Each dot in the scatter plot represents the number of cells in an independently counted field. (B) The assay was performed in the presence (open circle) or absence (closed circle) of anti-lymphotactin antibody at a concentration of 30 μg/ml.
To further characterize the P547-2 peptide, an additional T-cell line was established from a colon carcinoma patient (patient 35) using P547-2 agonist peptide-pulsed autologous DCs. This T-cell line was designated T-35-P547-2 and was 97.4% CD8 positive, <1% CD56 positive, 33.1% CD45RA positive and 29.0% CD27 positive. The T-35-P547-2 cell line was shown to produce IFN-γ(100 pg/ml) and lymphotactin (459 pg/ml) when stimulated with autologous DCs pulsed with P547-2 agonist peptide. As seen in Table 5, the T-35-P547-2 cell line also showed lysis of the mesothelin positive and HLA-A2 positive CFPAC-1 cells and OVCAR-3 cells at various E:T ratios but showed no lysis of the mesothelin positive and HLA-A2 negative AsPC-1 cell line. In addition, the T-35-P547-2 cell line also lysed HLA-A2 positive, mesothelin positive mesothelioma cell lines YOU, ROB and ORT, as shown in Table 5. To confirm our hypothesis that tumor cells endogenously process mesothelin to present mesothelin peptide in the context of MHC for T-cell mediated lysis, CFPAC-1 cells were used as target in a cold target inhibition assay. As shown in Table 6, the addition of peptide-pulsed unlabeled T2 cells decreased the CTL activity of T-35-P547-2 cells against labeled CFPAC-1 cells. The cytotoxic activity of T-35-P547 cells against CFPAC-1 cells was shown to be HLA-A2 restricted, as indicated by the inhibition of lysis with anti-HLA-A2 antibody but not with control antibody (Table 6). To further demonstrate that target cells can endogenously process the entire mesothelin molecule in a manner so as to bind HLA-A2 molecules for presentation at the cell surface, A431 cells were transfected with the entire human mesothelin gene (see Materials and Methods) and used as target cells in a T-cell cytotoxic assay. As seen in Table 7, the mesothelin-transfected A431 cells designated as A431.H9 cells express mesothelin. A431.H9 cells were susceptible to lysis with T-35-P547-2 cells when transfected with rV-HLA-A2 recombinant expressing HLA-A2. These studies further demonstrate the endogenously process of mesothelin in A431.H9 cells and the HLA-A2 restricted nature of the mesothelin-specific lysis of T-35-P547-2 cells.
Table 5.
Ability of the mesothelin-specific T-cell line (T35-P547-2) to lyse human tumor cell lines expressing mesothelin
| % lysis (± SD) |
||||||
|---|---|---|---|---|---|---|
| Target | Type of carcinoma | HLA-A2 | Mesothelin | 50:1 | 25:1 | 12.5:1 |
| CFPAC-1 | Pancreatic | + | + | 29.2 (1.3)a | 26.2 (0.4)a | 22.8 (0.9)a |
| OVCAR-3 | Ovarian | + | + | 25.9 (1.4)a | 20.7 (2.2)a | 14.9 (0.9)a |
| AsPC-1 | Pancreatic | − | + | 2.0 (0.3) | 2.7 (0.3) | 0.4 (0.4) |
| YOU | Mesothelioma | + | + | 26.0 (1.4)a | 26.4 (0.3)a | 17.6 (1.2)a |
| ROB | Mesothelioma | + | + | 51.3 (2.4)a | 46.1 (0.7)a | 31.2 (0.8)a |
| ORT | Mesothelioma | + | + | 49.4 (1.7)a | 42.2 (1.7)a | 31.0 (0.1)a |
A 16-hour 111In release assay was performed. Results are expressed in percent specific lysis at effector-to-target ratios of 50:1, 25:1 and 12.5:1.
Statistical significance (P<0.01, two tailed t test) when comparing lysis of AsPC cells.
Table 6.
Cytotoxicity of a mesothelin-specific T-cell line (T-35-P547-2) against target cells with endogenous mesothelin expression and inhibition of lysis by anti-HLA-A2 antibody
| Target | % lysis (± SD)* | % inhibition |
|---|---|---|
| Experiment 1: cold target inhibitiona | ||
| CFPAC-1 | 14.3 (0.3) | NA |
| CFPAC-1 + T2 | 13.3 (0.6) | NA |
| CFPAC-1 + T2 + P547-2 | 1.5 (0.8)b | 88.7 |
| Experiment 2: anti-HLA-A2 antibody inhibitionc | ||
| CFPAC-1 + anti-HLA-A2 (10 μ g/ml) | 3.5 (0.1)d | 72.6 |
| CFPAC-1 + anti-HLA-A2 (50 μ g/ml) | 3.0 (0.5)d | 76.4 |
| CFPAC-1 + control antibody (10 μ g/ml) | 12.7 (0.4) | NA |
A 16-hour 111In release assay was performed. Results are expressed in percent specific lysis at an effector-to-target ratio of 20:1.
For the cold target inhibition experiment, labeled CFPAC-1 cells and unlabeled T2 cells were used at a ratio of 1:10. T2 cells were incubated with or without P547-2 peptide (25 μg/ml) in serum-free medium for 24 hours at 37°C prior to their addition into the assay.
Statistically significant difference (p<0.01, two-tailed t test) for comparison with CFPAC-1 cells and T2 cells not pulsed with peptide.
For the anti-HLA-A2 antibody inhibition experiment, labeled CFPAC-1 cells were incubated for 1 hour in the presence of medium containing either 10 μg/ml of control antibody (UPC-10), or anti-HLA-A2 antibody (10 μg/ml and 50 μg/ml). Cells were then used as target in 16-hour cytotoxic assays.
Statistically significant difference (p<0.01, two-tailed t test) for comparison with value obtained with control antibody.
Table 7.
Demonstration of HLA-A2 involvement in the ability of a mesothelin- specific T-cell line (T-35-P547-2) to lyse target cells with endogenous mesothelin expression
| HLA-A2* | Mesothelin* | % lysis (+ SD)# | |
|---|---|---|---|
| A431 | |||
| uninfected | Negative | Negative | 8.5 (1.7) |
| rV-HLA-A2 | 95.9 (45) | Negative | 9.0 (0.7) |
| rV-WT | Negative | Negative | 6.0 (0.2) |
| A431.H9 | |||
| uninfected | Negative | 99.7 (288) | 11.5 (1.4) |
| rV-HLA-A2 | 95.9 (55) | 80.0 (46) | 23.3 (0.01)a |
| rV-WT | Negative | 89.1 (96) | 8.0 (0.1) |
HLA-A2 and mesothelin expression were tested by flow cytometry using anti-HLA-A2 and K1 antibodies, respectively. Values represent the percentage of cells reactive to the antibodies. Numbers in parentheses are the mean fluorescence intensity as determined in relative log units.
Results are expressed in percent of specific lysis at an effector-to-target ratio of 40:1.
Statistically significant lysis compared with rV-WT infected and uninfected A431.H9 and uninfected A431 as well as rV-HLA-A2 infected and rV-WT infected A431 cells (p<0.01, paired t test).
It has been previously demonstrated that sublethal irradiation of tumor cells can modulate their phenotype to render them more susceptible to T-cell–mediated killing (45, 46). We investigated whether sublethal doses of radiation would alter the expression of surface markers in CFPAC-1, OVCAR-3 and AsPC-1 cell lines. Tumor cells were subjected to 0 or 10 Gy radiation, and cell surface expression of mesothelin, HLA-A2, and ICAM-1 was analyzed by flow cytometry after 72 hours. No significant increase in cell death was observed with 10 Gy and 20 Gy of radiation. As seen in Figure 6A, slight or no increases in the percentage of cells expressing mesothelin, HLA-A2, and ICAM-1 were detected in the CFPAC-1 cell line at 10 Gy of radiation as compared with no radiation. This is due to the fact that the CFPAC-1 cell line has a high level of expression of these three molecules. The MFI of ICAM-1, mesothelin, and HLA-A2, however, did increase (Fig. 6A). As shown in Figure 6B, increases in the percentage of mesothelin-expressing cells and ICAM-1–expressing cells were observed in the OVCAR-3 cell line after irradiation at 10 Gy. No significant increase in the percentage of cells expressing these three markers was observed on AsPC-1 cells after irradiation at 10 Gy. The MFI of expression of mesothelin, however, did increase after irradiation (Fig. 6C). The functional significance of phenotypic changes of CFPAC-1 and OVCAR-3 cells after irradiation on the susceptibility to be lysed by mesothelin-specific T cells was investigated. The CFPAC-1 and OVCAR-3 cell lines both demonstrated a significant (p<0.01) increase in lysis by T-1991-P547, a mesothelin-specific T-cell line after 10 Gy of irradiation, as compared with the nonirradiated CFPAC-1 and OVCAR-3 cells (Figure 7A and B). The AsPC-1 cell line, a HLA-A2 negative cell line not lysed by T-1991-P547, was used as a negative control (Fig. 7C).
Figure 6.
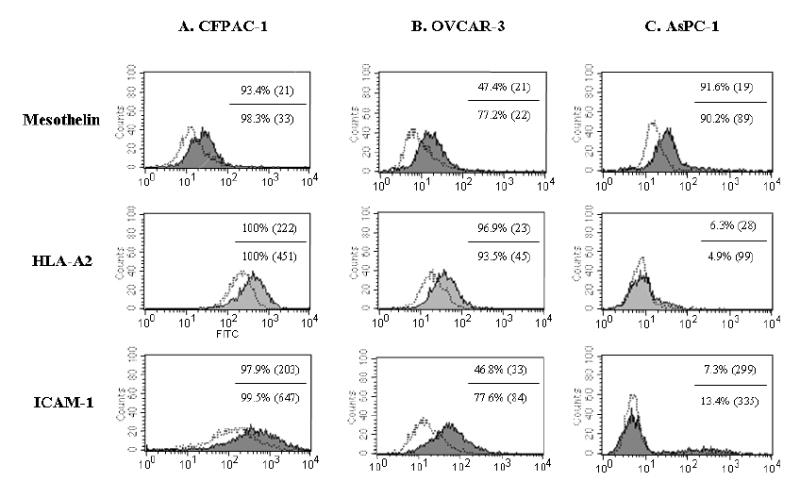
Flow cytometry analysis of surface expression of mesothelin, HLA-A2 and ICAM-1 on human tumor cell lines: (A) CFPAC-1 (pancreatic cancer cells), (B) OVCAR-3 (ovarian cancer cells) and (C) AsPC-1 (pancreatic cancer cells) without (unshaded area) and with irradiation at 10 Gy (shaded area).
Figure 7.
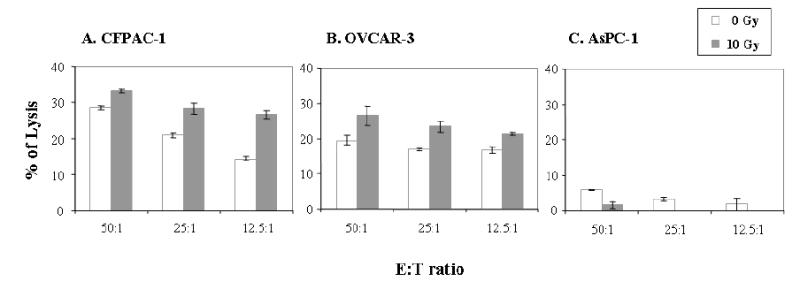
Irradiation increases tumor cell sensitivity to mesothelin-specific T-cell killing. (A) CFPAC-1, a pancreatic cancer cell line, (B) OVCAR-3, an ovarian cancer cell line and (C) AsPC-1, a pancreatic cancer cell line. Tumor cells were mock irradiated (open bar) and irradiated with 10 Gy (solid bar) and cultured for 72 hours. Tumor cells were labeled with 111In and were used in a 16-hour 111In release assay. See “Materials and Methods.” Bars, SD.
Discussion
Mesothelin has previously been shown to be a potential target for both antibody- and vaccine-mediated immunotherapy. Preclinical studies using anti-mesothelin immunotoxin SS1(dsFv)PE38 (SS1P) demonstrated the anti-tumor activity against mesothelin-expressing tumors (20,21). Two Phase 1 clinical trials using SS1P are currently ongoing (22,23). Mesothelin-derived HLA-A2, A3 and A24 restricted CTL 9-mer epitopes have been previously reported (24). These peptides were used for the detection of mesothelin-specific CD8+ T-cell immune responses in pancreatic cancer patients vaccinated with GM-CSF–transduced pancreatic cancer cells. The two HLA-A2 binding peptides used in that study were mesothelin peptides 20–28 (SLLFLLFSL) and 530–538 (VLPLTVAEV) (24). The results from that clinical trial provide evidence that CD8+ T- cell responses can be generated via cross-presentation by an immunotherapy approach designed to recruit APCs to the vaccination site. In the studies reported here, we have modified the primary anchor residues of a novel mesothelin peptide to augment the binding affinity of the peptide to the MHC molecule. Eight analogs were synthesized and analyzed; one of them, designated P547-2, was shown to be superior to the native epitope in terms of affinity of binding to MHC molecules, avidity of the peptide-MHC complex, and the ability to activate CTLs in vitro.
T-cell lines derived from the native or the agonist mesothelin epitope were shown to lyse mesothelin positive and HLA-A2 positive pancreatic cancer, ovarian cancer, and mesothelioma cell lines as well as mesothelin gene-transfected epidermoid carcinoma cells in an MHC-restricted manner. Moreover, such T-cell lines could be derived from a pancreatic cancer patient, a colon cancer patient, and a normal donor.
Preclinical and clinical studies have indicated that lymphotactin may be an important chemokine in attracting effector cells and thus enhancing immune responses. The results reported here demonstrate both enhanced synthesis of lymphotactin and enhanced biologic activity of lymphotactin as a consequence of stimulation of effector T cells with the agonist mesothelin peptide.
Previous studies have demonstrated that when murine and human tumor cells are exposed to sub-lethal irradiation their phenotype can be altered to make them more susceptible to T-cell–mediated killing. This has been shown to be due to upregulation of either tumor antigen, MHC class I, or accessory molecules such as fas or ICAM-1 or combinations of the above. The results reported here demonstrate that non-lethal irradiation of both a pancreatic and ovarian cancer cell line led to the upregulation of both mesothelin and ICAM-1, and MHC class I, which subsequently rendered them more susceptible to lysis by a mesothelin-specific T-cell line.
The studies reported here thus extend previous observations on the suitability of mesothelin as a potential target for immunotherapy of a range of human tumors.
Acknowledgments
We thank the Stehlin Foundation for Cancer Research, Houston, Texas, U.S.A, for providing the tumor cell lines YOU, ROB and ORT, which were established from the ascites of patients with peritoneal mesothelioma. We also thank Debra Weingarten for her editorial assistance in the preparation of this manuscript.
References
- 1.Kojima T, Oh-eda M, Hattori K, et al. Molecular cloning and expression of megakaryocyte potentiating factor cDNA. J Biol Chem. 1995;270:21984–90. doi: 10.1074/jbc.270.37.21984. [DOI] [PubMed] [Google Scholar]
- 2.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen on mesothelium, mesotheliomas and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–40. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–42. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 4.Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992;50:373–81. doi: 10.1002/ijc.2910500308. [DOI] [PubMed] [Google Scholar]
- 5.Chang K, Pai LH, Pass H, Pogrebniak NW, Tsao MS, Pastan I, et al. Monoclonal antibody K1 reacts with epithelial mesothelioma but not with lung adenocarcinoma. Am J Surg Pathol. 1992;16:259–68. doi: 10.1097/00000478-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: Identification of a new pancreatic cancer marker by serial analysis of the gene expression (SAGE) Clin Cancer Res. 2001;7:3862–68. [PubMed] [Google Scholar]
- 7.Hough GD, Sherman-Baust CA, Pizer ES, Montz FJ, Im DD, Rosenshein NB, et al. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000;60:6281–7. [PubMed] [Google Scholar]
- 8.Hippo Y, Yashiro M, Ishii M, Taniguchi H, Tsutsuni S, Hirakawa K, et al. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 2001;61:889–95. [PubMed] [Google Scholar]
- 9.Wang K, Gan L, Jeffrey E, Gayle M, Gown AM, Skelly M, et al. Monitoring gene expression profile changes in ovarian carcinomas using cDNA microarray. Gene. 1999;229:101–8. doi: 10.1016/s0378-1119(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 10.Brinkmann U, Webber K, Di Carol A, Beer R, Chowdhury P, Chang K, et al. Clonong and expression of the recombinant FAb fragment of monoclonal antibody K1 that reacts with mesothelin present on mesotheliomas and ovarian cancers. Int J Cancer. 1997;71:638–44. doi: 10.1002/(sici)1097-0215(19970516)71:4<638::aid-ijc21>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Chang K, Pastan I. Molecular cloning and expression of a cDNA encoding a protein detected by the K1 antibody from an ovarian carcinoma (OVCAR-3) cell line. Int J Cancer. 1994;57:90–7. doi: 10.1002/ijc.2910570117. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, Vasa P, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci USA. 2001;98:13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scholler N, Fu N, Yang Y, Ye Z, Goodman GE, Hellstrom KE, et al. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci USA. 1999;96:11531–6. doi: 10.1073/pnas.96.20.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ordonez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418–28. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Chang K, Pastan I, Willingham MC. Frequent expression of the tumor antigen CAK1 in squamous-cell carcinomas. Int J Cancer. 1992;51:548–54. doi: 10.1002/ijc.2910510408. [DOI] [PubMed] [Google Scholar]
- 16.Hassan R, Viner JL, Wang QC, Margulies I, Kreitman RJ, Pastan I. Anti-tumor activity of K1-LysPE38QQR, an immunotoxin targeting mesothelin, a cell-surface antigen overexpressed in ovarian cancer and malignant mesothelioma. J Immunother. 2000;23:473–9. doi: 10.1097/00002371-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury PS, Viner JL, Beers R, Pastan I. Isolation of high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of recombinant immunotoxin. Proc Natl Acad Sci USA. 1998;95:669–674. doi: 10.1073/pnas.95.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhury PS, Pastan I. Improving antibody affinity by mimicking somatic hypermutation in vitro. Nat Biotechnol. 1999;17:568–572. doi: 10.1038/9872. [DOI] [PubMed] [Google Scholar]
- 19.Fan D, Yano S, Shinohara H, Solorzano C, Van Arsdall M, Bucana CD, et al. Targeted therapy against human lung cancer in nude mice by high-affinity recombinant antimesothelin single-chain Fv immunotoxin. Mol Cancer Ther. 2002;1:595–600. [PubMed] [Google Scholar]
- 20.Hassan R, Lerner MR, Benbrook D, Lightfoot SA, Brackett DJ, Wang QC, et al. Antitumor activity of SS(dsFv)PE38 and SS1(dsFv)PE38, recombinant antimesothelin immunotoxins against human gynecologic cancers grown in organotypic cultures in vitro. Clin Cancer Res. 2002;8:3520–6. [PubMed] [Google Scholar]
- 21.Li Q, Verschraegen CF, Mendoza J, Hassan R. Cytotoxic activity of the recombinant anti-mesothelin immunotoxin, SS1(dsFv)PE38, towards tumor cell lines established from ascites of patients with peritoneal mesotheliomas. Anticancer Res. 2004;24:1327–35. [PubMed] [Google Scholar]
- 22.Hassan R, Kreitman R, Strauss L, et al. SS1(dsFv)PE38 antimesothelin immunotoxin in advanced malignancies: Phase 1 and pharmacokinetic study of alternate-day infusion. Proc Am Soc Clin Oncol. 2002;21:29a. [Google Scholar]
- 23.Kreitman R, Squires D, O’Hagan D, et al. SS1(dsFv)PE38 antimesothelin immunotoxin in advanced malignancies: Phase 1 study of continuous infusion. Proc Am Soc Clin Oncol. 2002;21:22b. [Google Scholar]
- 24.Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ, et al. Mesothelin-specific CD8+ T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valmori D, Fonteneau JF, Lizana CM, Gervois N, Lienard D, Rimoldi D, et al. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J Immunol. 1998;160:1750–8. [PubMed] [Google Scholar]
- 26.Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, et al. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci USA. 2001;98:8809–14. doi: 10.1073/pnas.141226398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelner GS, Kennedy J, Bacon KB, Kleyensteuber S, Largaespada DA, Jenkins NA, et al. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–9. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy J, Kelner GS, Kleyensteuber S, Schall TJ, Weiss MC, Yssel H, et al. Molecular cloning and functional characterization of human lymphotactin. J Immunol. 1995;155:203–9. [PubMed] [Google Scholar]
- 29.Muller S, Dorner B, Korthauer U, Mages HW, D’Apuzzo M, Senger G, et al. Cloning of ATAC, an activation-induced, chemokine-related molecule exclusively expressed in CD8+ T lymphocytes. Eur J Immunol. 1995;25:1744–8. doi: 10.1002/eji.1830250638. [DOI] [PubMed] [Google Scholar]
- 30.Hedrick JA, Saylor V, Figueroa D, Mizoue L, Xu Y, Menon S, et al. Lymphotactin is produced by NK cells and attracts both NK cells and T cells in vivo. J Immunol. 1997;158:1533–40. [PubMed] [Google Scholar]
- 31.Rumsaeng V, Vliagoftis H, Oh CK, Metcalfe DD. Lymphotactin gene expression in mast cells following Fc(ɛ) receptor I aggregation: modulation by TGF-β, IL-4, dexamethasone, and cyclosporine A. J Immunol. 1997;158:1353–60. [PubMed] [Google Scholar]
- 32.Schoumacher RA, Ram J, Iannuzzi MC, Bradbury NA, Wallace RW, Hon CT, et al. A cystic fibrosis pancreatic adenocarcinoma cell line. Proc Natl Acad Sci USA. 1990;87:4012–6. doi: 10.1073/pnas.87.10.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T, Pastan I. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11:3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 34.Anderson KS, Alexander J, Wei M, Cresswell P. Intracellular transport of class I MHC molecules in antigen processing mutant cell lines. J Immunol. 1993;151:3407–19. [PubMed] [Google Scholar]
- 35.Storkus WJ, Howell DN, Salter RD, Dawson JR, Cresswell P. NK susceptibility varies inversely with target cell class I HLA antigen expression. J Immunol. 1987;138:1657–9. [PubMed] [Google Scholar]
- 36.Hogan KT, Shimojo N, Walk SF, Engelhard VH, Maloy WL, Coligan JE, et al. Mutations in the alpha 2 helix of HLA-A2 affect presentation but do not inhibit binding of influenza virus matrix peptide. J Exp Med. 1988;168:725–36. doi: 10.1084/jem.168.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–75. [PubMed] [Google Scholar]
- 38.Tsang KY, Palena C, Gulley J, Arlen P, Schlom J. A human cytotoxic T-lymphocyte epitope and its agonist epitope from the nonvariable number of tandem repeat sequence of MUC-1. Clin Cancer Res. 2004;10:2139–49. doi: 10.1158/1078-0432.ccr-1011-03. [DOI] [PubMed] [Google Scholar]
- 39.Nijman HW, Houbiers JG, Vierboom MP, van der Burg SH, Drijfhout JW, D’Amaro J, et al. Identification of peptide sequences that potentially trigger HLA-A2.1-restricted cytotoxic T lymphocytes. Eur J Immunol. 1993;23:1215–9. doi: 10.1002/eji.1830230603. [DOI] [PubMed] [Google Scholar]
- 40.Boyum A. A one-stage procedure for isolation of granulocytes and lymphocytes from human blood. General sedimentation properties of white blood cells in a 1 g gravity field Scand. J Clin Lab Invest. 1968;97(Suppl):51–76. [PubMed] [Google Scholar]
- 41.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony- stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87:982–90. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 43.Palena C, Schlom J, Tsang KY. Differential gene expression profile in a human T-cell line stimulated with a tumor-associated self peptide versus an enhanced agonist peptide. Clin Cancer Res. 2003;9:1616–27. [PubMed] [Google Scholar]
- 44.Palena C, Arlen P, Zeytin H, Greiner JW, Schlom J, Tsang KY. Enhanced expression of lymphotactin by CD8+ T cells is selectively induced by enhancer agonist peptides of tumor-associated antigens. Cytokine. 2004;24:128–42. doi: 10.1016/j.cyto.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 46.Garnett CT, Palena C, Chakarborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 47.Jenkins S, Gritz L, Fedor CH, O'Neill EM, Cohen LK, Panicali DL. Formation of lentivirus particles by mammalian cells infected with recombinant fowlpox virus. AIDS Res Hum Retroviruses. 1991;7:991–8. doi: 10.1089/aid.1991.7.991. [DOI] [PubMed] [Google Scholar]
- 48.Gritz L, Destree A, Cormier N, Day E, Stallard V, Caiazzo T, Mazzara G, Panicali D. Generation of hybrid genes and proteins by vaccinia virus-mediated recombination: application to human immunodeficiency virus type 1 env. J Virol. 1990;64:5948–57. doi: 10.1128/jvi.64.12.5948-5957.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


