Abstract
Speakers may use laryngeal sensory feedback to adjust vocal fold tension and length before initiating voice. The mechanism for accurately initiating voice at an intended pitch is unknown, given the absence of laryngeal muscle spindles in animals and conflicting findings regarding their existence in humans. Previous reports of rapid changes in voice fundamental frequency following thyroid cartilage displacement suggest that changes in vocal fold length modulate laryngeal muscle contraction in humans. We tested the hypothesis that voice changes resulting from mechanical perturbation are due to rapid responses in the intrinsic laryngeal muscles. Hooked wire electrodes were used to record from the thyroarytenoid, cricothyroid, and sternothyroid muscles along with surface electrodes on the skin overlying the thyroid cartilage in 10 normal adults. Servomotor displacements produced consistent changes in the subjects’ vocal fundamental frequency at 70–80 ms, demonstrating changes in vocal fold length and tension. No simultaneous electromyographic responses occurred in the thyroarytenoid or cricothyroid muscles in any subjects. Instead, short-latency responses at 25–40 ms following stimulus onset occurred in the sternothyroid muscles, simultaneous with responses in the surface recordings. The sternothyroid responses may modulate long-latency changes in voice fundamental frequency (~150 ms). The absence of intrinsic laryngeal muscle responses is consistent with a lack of spindles in these muscles. Our results suggest that other sensory receptors, such as mucosal mechanoreceptors, provide feedback for voice control.
Keywords: somatosensory, sternothyroid, thyroarytenoid, stretch reflex
in speech and singing, humans produce an intended pitch by maintaining active control over laryngeal muscle length and tension, with auditory feedback having a central role in learning and maintaining vocal control (9, 10, 17, 30). Fine adjustments of vocal fold tension and length occur before voice onset and may depend on somatosensory feedback of vocal fold position, length, and tension, as well as previous learning of voice control. Laryngeal supraglottal afferents contained in the internal branch of the superior laryngeal nerve encode changes in airflow, pressure changes on the laryngeal mucosa, and laryngeal muscle tension in the cat and dog (25, 27). Laryngeal subglottal afferents in the recurrent laryngeal nerve are activated during evoked vocalization and may reflect changes in vocal fold length and tension and subglottal pressure (11). Recently, rapid changes in vocal fold position during voicing in humans were found to produce changes in voice pitch, suggesting that intrinsic laryngeal muscle feedback may play a role in voice control (26, 29).
In most striated muscle groups, changes in muscle length are mediated by 1a afferents from muscle spindles. The presence of muscle spindles in the intrinsic laryngeal muscles in humans is controversial (19, 23). One report found muscle spindles in the interarytenoid (28), others reported spindles in the thyroarytenoid muscle (4, 5, 24), whereas a more recent study failed to confirm the presence of spindles in the thyroarytenoid muscle (8).
When servomotor-controlled displacements were applied to the thyroid cartilage during voicing in humans, rapid changes occurred in voice fundamental frequency and were accompanied by short-latency muscle responses recorded using surface electromyography (EMG) on the neck (26). The fundamental frequency changes in the voice demonstrated changes in the length and/or tension of the vocal folds. Because the surface EMG responses occurred 30–35 ms following the onset of displacement but before the voice changes at 55–65 ms, the authors suggested that the EMG responses were possibly muscle stretch responses induced by changes in vocal fold length/ tension. The muscle(s) that produced the EMG response could not be identified, however, because only surface electrodes were used. In another study that used a servomotor to displace the thyroid cartilage, a response occurred ~25 ms in the cricothyroid muscle following cartilage compression in two subjects and no response in the thyroarytenoid muscle. The basis for the cricothyroid response is not clear, given limited evidence of muscle spindles in this muscle (19, 23). Other explanations could be a response to cricothyroid joint receptors (7), or a stretch of the overlying strap muscles, which contain muscles spindles and may have been picked up from electrodes in the underlying cricothyroid muscle (16).
Our purpose was to test the hypothesis that muscle responses to thyroid cartilage displacement originate in the intrinsic laryngeal muscles of normal speaking adults (26, 29). We expected that a rapid force would move the thyroid cartilage posteriorly, exciting stretch receptors, which may be present in the cricothyroid muscle. Furthermore, we hypothesized that, when the force is released from the thyroid cartilage, the vocal folds will lengthen, stretching the thyroarytenoid muscle and raising the fundamental frequency. Such a mechanical lengthening could excite stretch receptors that might be present in the thyroarytenoid (24), producing a muscle response that would decrease the fundamental frequency by shortening the vocal folds.
In addition, to determine whether the effects of mechanical force on laryngeal muscles depend on ongoing laryngeal muscle activity, we applied the mechanical perturbation in six conditions: high-pitch phonation, which would increase intrinsic muscle activity; phonation at the person’s typical pitch; pressed phonation (which increases thyroarytenoid muscle activity); high-pitch whisper (vocal fold lengthening without closure); effortful closure or Valsalva (vocal fold closure and shortening); and quiet breathing.
METHODS
Subjects
Ten normal volunteers, seven men, gave informed consent to participate in the study approved by the Internal Review Board of the National Institute of Neurological Disorders and Stroke (mean age 42 yr; range 23–69 yr). None of the participants had current or previous histories of neurological or laryngeal disorders or were taking medications that would affect responses to laryngeal stimulation. All had normal findings on nasolaryngoscopy by an otolaryngologist and were without symptoms of a voice disorder, upper respiratory tract infection, or gastroesophageal reflux on the day of testing. All subjects were naive; none of the experimenters participated in the study.
EMG
After a ground electrode was attached, the subjects were placed in a supine position with the neck extended. A small amount of 1% lidocaine was injected subcutaneously over the cricothyroid membrane to minimize discomfort during electrode insertions. Following standard techniques (13), bipolar needle EMG electrodes (27-gauge, 37.5 mm) were used to locate a laryngeal muscle before placing bipolar hooked wire electrodes in the muscle. Electrode placements in the thyroarytenoid muscles were verified by increased activation during phonation and effortful glottic closure without prominent onset and offset bursts indicative of lateral cricoarytenoid activity. Verification of the sternothyroid muscle included increased activation at the low end of a decreasing pitch glide and during head turning and head lowering. Placement in the cricothyroid was verified by increased activation during the high end of a pitch glide, but without activation during head rising to demonstrate that strap muscle activity was not included in the recording field. The bipolar hooked wire electrodes (0.0002-in. diameter with 1 mm of insulation removed at the tip) were placed in the thyroarytenoid and sternothyroid bilaterally and in the cricothyroid unilaterally. Surface EMG electrodes were placed bilaterally over the thyroid cartilage to replicate the previous laryngeal area surface electrode placement of Sapir et al. (26). On each side of the cartilage, the upper electrodes were placed ~1 cm from midline and ~1 cm below the thyroid notch bilaterally, and the lower electrodes were placed over the cricoid cartilage medial to the sternocleidomastoid on each side. The skin was cleaned, and conductive gel was applied to ensure optimal EMG recordings.
After electrode placements, maximal activation gestures were recorded, including the Valsalva maneuver, water swallow, and throat clear for thyroarytenoid activity, high pitch /i/ during an ascending scale for cricothyroid, and effortful chin tuck for sternothyroid. The EMG signals were band-pass filtered between 30 and 3,000 Hz before digitization. All acoustic, EMG, and stimulus signals were anti-alias filtered digitally on a 64 times oversampled signal using a brick-wall filter with linear phase at 5 kHz before the signal was downsampled to 12 kHz. The effective sampling rate was 12,000 samples/s for each channel.
Servomotor stimuli
The mechanical stimuli were delivered to the skin overlying the thyroid cartilage using a servomotor probe operating under force feedback control. A small curved 2 × 1 cm plate was attached at the end of the servomotor shaft to fit over the thyroid prominence. The direction of the probe displacement was adjusted to push the thyroid cartilage posteriorly toward the spine and somewhat rostrally. The stimuli had a rise and fall time of 20 ms and plateau duration of either 250 or 500 ms with a force level of between 0.9 and 1.6 N (mean = 1.4 N) to produce the largest displacement without discomfort. In three subjects, the force output saturated without producing discomfort, and the stimulus level was set just below the saturation point.
For the male participants, the probe rested on the thyroid prominence. For the female subjects, a midline position was chosen where a perceptible change in fundamental frequency could be produced. To ensure that the thyroid cartilage was effectively displaced by the stimulus on each trial, a consistent contact between the stimulus probe and the midline of the skin over the thyroid cartilage was maintained using a slight contact pressure and two-sided tape. Adjustments were made to the height of the probe to accommodate changes in laryngeal height (e.g., low-pitch condition) for each block of trials. The probe was monitored to ensure displacement of the thyroid cartilage toward the spine at stimulus onset with a clear rebound at offset.
To indicate cartilage displacement, a piezoelectric movement transducer was taped on the skin over the thyroid cartilage. The servomotor force and movement signals occurred abruptly in opposite directions at stimulus onset and offset (Fig. 1A). Movement artifacts frequently occurred at stimulus onset and offset in the EMG signals in opposite directions during thyroid cartilage displacement and rebound.
Fig. 1.
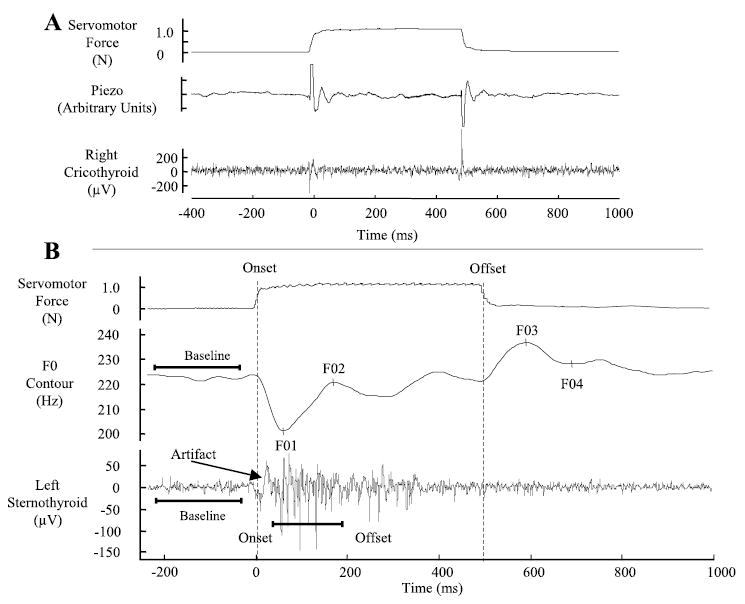
A: example waveforms showing the effect of the perturbation on thyroid cartilage position from a single trial in the rest condition. The top tracing shows the servomotor force signal to indicate stimulus onset and offset points. The second tracing shows the piezoelectric acceleration signal. The third tracing shows the effect of the stimulus on the cricothyroid muscle recording. A movement artifact of opposite polarity is present at stimulus onset and offset in the muscle recording. B: example waveforms showing the servomotor force signal, fundamental frequency (F0) contour, and sternothyroid (ST) electromyographic (EMG) signal from a single trial in the high-pitch condition. The servomotor force signal is in Newtons (N). The F0 contour in Hertz (Hz) has the four F0 events labeled as F01–F04. The ST recording is in microvolts (μV).
We used fiber-optic nasolaryngoscopy to visualize the effects of cartilage displacement on laryngeal position in a subset of trials in each subject. The force onset displaced the larynx as a whole in the posterior direction with an anterior shift at stimulus offset.
Task description
The mechanical perturbations were applied during six phonatory and nonphonatory conditions. The high-pitch phonation condition replicated Sapir et al. (26). We first measured the fundamental frequency of each subject’s typical speaking pitch on extended phonation of the vowel /i/. The subjects were then trained to produce the vowel /i/ at a test pitch that was one-half to two-thirds octave higher than their typical speaking pitch. Before each trial, a sinusoidal tone at the test frequency was presented, and the subject was asked to phonate at a matching pitch. The displacement was applied by the experimenter ~1–2 s after phonation was initiated. The two stimulus durations of 250 and 500 ms were applied in a randomized order in the high-pitch condition. The 250-ms duration replicated Sapir et al. (26). The 500-ms stimulus duration was used to measure possible long-latency responses that might occur >250 ms after stimulus onset. In the second condition, servomotor displacements were applied while subjects produced the vowel at their typical speaking pitch. The “pressed” voice condition was used in four subjects taught to produce a strained voice that would increase the levels of thyroarytenoid muscle contraction.
The nonphonatory conditions included rest, high-pitch whisper, and Valsalva. In the rest condition, the displacement was applied during exhalation by monitoring the subject and the combined abdomen/ribcage Respitrace signal. In the high-pitch whisper condition, subjects were instructed to whisper the vowel /i/ while approximating the high-pitch condition, to increase cricothyroid muscle activation. The Valsalva condition was used to assess whether muscle responses to displacement differed with active contraction of the thyroarytenoid muscle. The servomotor stimulus duration for the nonphonatory tasks was 500 ms.
The majority of trials were in the phonation condition to adequately replicate Sapir et al. (26) and randomly vary stimulus duration. Approximately 60–80 high-pitch phonation trials were recorded per subject, and 10–15 trials were used for the other conditions. The conditions were presented in five trial blocks in random order. Approximately 15% additional sham trials were interspersed during the experiment to prevent the subject from anticipating the displacement. On sham trials, a sound mimicking the stimulus was presented without thyroid displacement.
Analysis
The digitized signals were visually inspected and marked with custom routines written in MATLAB (version 6.5) to identify the onset, duration, and amplitude of the voice and muscular responses. Force onset and offset were defined as the points at which the force signal was 50% of the mean amplitude during the stimulus period (Fig. 1B). The fundamental frequency contour of the voice signal was obtained by applying a low-pass, digital filter (8th-order, zero-phase, Butterworth filter) and a custom zero-crossing algorithm that computed the inverse of the zero-crossing intervals to give fundamental frequency over time. The filter cutoff was ~50 Hz below the first formant of the vowel.
The peaks of the fundamental frequency responses were labeled as F01–F04 (Fig. 1B) and correspond to the fundamental frequency responses discussed by Sapir et al. (26). The latencies and amplitudes of the four points relative to stimulus onset were computed automatically with the use of custom pick-peaking algorithms (Matlab, version 6.5). The amplitudes for each fundamental frequency (F0) change were defined as follows: for F01, the difference (Hz) between the F0 at the F01 minima and the mean F0 over the 200-ms baseline period; for F02, the difference (Hz) between the F0 at the F01 minima and the F02 peak; for F03, the difference (Hz) between the F0 at the F03 peak and the F0 at stimulus offset; and for F04, the difference (Hz) between the F0 at the F03 peak and the F04 minima (Fig. 1B).
To remove direct current offset and movement artifacts due to the servomotor force impulse, the EMG signals were high-pass filtered >20 Hz with a fourth-order, zero-phase, Butterworth filter. The EMG traces from each trial were visually inspected to identify stimulus-related changes relative to the baseline period, 200 ms before stimulation. A baseline deflection >5 ms after the force stimulus onset was marked as the onset of a muscle response (Fig. 1B). Response offset was marked as the point at which the EMG signal returned to baseline and remained stable for at least 10 ms. An initial deflection occurring at stimulus onset was labeled as a stimulus-related movement artifact. The EMG measures obtained for each muscle response included 1) response latency (ms); 2) response duration (ms); and 3) mean amplitude (μV). The frequency of responses was computed for each muscle in each subject as a percentage of the total number of trials.
Planned statistical analyses compared voice and muscle responses across the experimental conditions. To determine whether different servomotor stimulus durations affected the fundamental frequency events (F01–F04), paired t-tests (P < 0.05) were used to compare the latency and amplitude of the four F0 events between the two durations with a Bonferroni correction for the eight tests (P = 0.05/8 = 0.00625). If significant voice changes occurred across conditions, then the EMG data were compared statistically; otherwise, the two duration conditions were combined for both the voice and EMG data. Paired t-tests (P < 0.05 with Bonferroni correction) compared the amplitude and latencies of the F0 events between the high-pitch and low-pitch conditions. The EMG data from the two pitch conditions were compared statistically, if voice differences occurred (P < 0.05). To determine whether the F0 events were related to muscle responses, separate Pearson correlation coefficients (P = 0.05 with Bonferroni correction) related the latencies and amplitudes of the first two F0 events with initial muscle response latency in subjects with reliable muscle responses.
RESULTS
Fundamental frequency (F0) changes
Reliable voice F0 changes occurred on every trial in the high- and low-pitch conditions in all 10 subjects (see Fig. 2 for voice responses from two subjects). In all subjects in the high-pitch condition, the F0 decrease began at stimulus onset and reached the F01 minima at 71 ms (SD 19.12) and averaged 29.25 Hz (SD 32.25). The second peak, F02, averaged 21.8 Hz (SD 15.7) and occurred at 173.3 ms (SD 20.66). At offset, the F0 increased by a mean of 20.5 Hz (SD 19.77) at 70.58 ms (SD 11.38), labeled F03 peak, followed by a latency decrease of 11.4 Hz (SD 7.69) at 203.36 ms (SD 50.24 ms), labeled F04. The initial F0 changes at both onset and offset demonstrated that the mechanical displacement altered the length and tension of the vocal folds. F0 changes did not occur in the sham trials, demonstrating that the F0 changes were due to the mechanical stimulus rather than subject anticipation of the stimulus. As expected, the latencies and magnitudes of the voice responses (F01–F04) in the 250- and 500-ms duration conditions did not differ; none of the paired t-tests comparing the amplitude and latencies of the F0 responses approached statistical significance across the nine subjects, with data from both conditions [F01 latency: t(8) = −0.45, P = 0.66; F02 latency: t(8) = −1.78, P = 0.11; F03 latency: t(8) = 0.51, P = 0.62; F04 latency: t(8) = 0.36, P = 0.72; F01 amplitude: t(8) = −1.17, P = 0.27; F02 amplitude: t(8) = 1.55, P = 0.16; F03 amplitude: t(8) = 0.53, P = 0.61; and F04 amplitude: t(8) = −0.76, P = 0.47]. The acoustic and muscle data from the two duration conditions were combined for each subject.
Fig. 2.
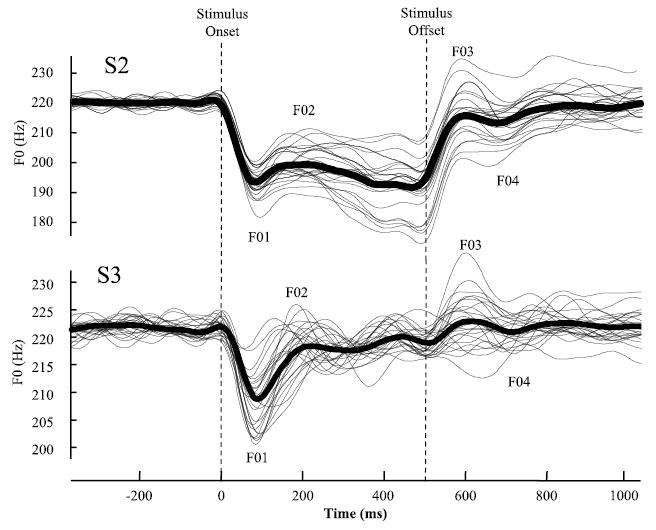
Fundamental frequency (F0) contours in Hz from 2 subjects (S2 and S3) in the high-pitch condition. At stimulus onset, there is a rapid, large magnitude decrease in F0 to the F01 point followed by a more gradual but still considerable increase to F02. The opposite pattern occurs at stimulus offset with a rapid increase to F03 followed by a gradual decrease to F04. Note that the F0 generally returns to the same baseline F0 level after stimulus offset. The average waveform (bold line) follows the same pattern as the individual traces.
The F0 responses in the high-pitch and low-pitch conditions showed a similar pattern of F0 changes but lower amplitudes and longer latencies in the low-pitch condition (Fig. 3). Negative values denoted a decrease in F0. The differences between the pitch conditions were not statistically significant for the seven subjects who participated in both conditions: F01 latency: t(6) = 1.18, P = 0.28; F02 latency: t(6) = −0.71, P = 0.50; F03 latency: t(6) = 0.46, P = 0.66; F04 latency: t(6) = 0.05, P = 0.96; F01 amplitude: t(6) = −2.29, P = 0.06; F02 amplitude: t(6) = −1.92, P = 0.10; F03 amplitude: t(6) = − 0.46, P = 0.66; and F04 amplitude: t(6) = −1.24, P = 0.26.
Fig. 3.
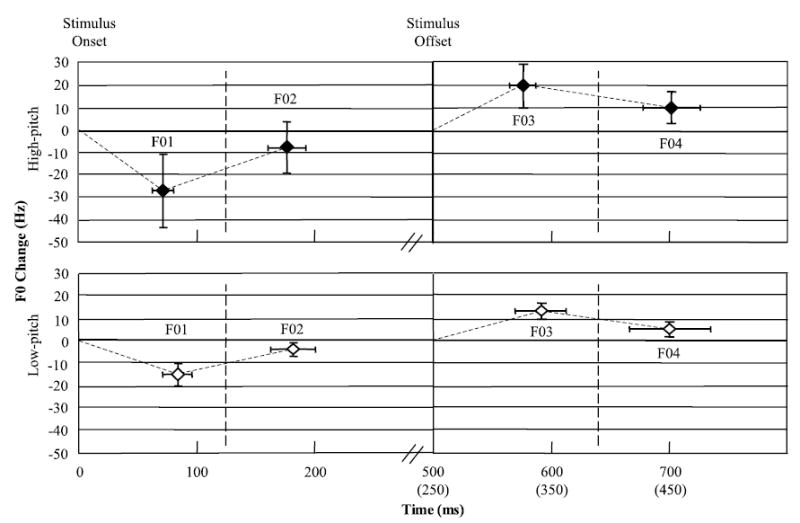
The average change in Hz and latency in fundamental frequency (F0) from the 10 subjects for the high-pitch and low-pitch condition collapsed across the 250- and 500-ms duration conditions.
EMG responses during phonation
Only the sternothyroid muscle showed responses to the force application; no responses were seen in the cricothyroid or thyroarytenoid muscles in any conditions or subjects (Fig. 4). Sternothyroid responses occurred in 5 of the 10 subjects, with 3 subjects showing highly consistent responses at a mean latency of 33.1 ms (SD 7.9) (Table 1). The latency of the intramuscular sternothyroid responses was similar to the surface responses in three subjects (S2, S5, and S9) with a mean latency of 27.7 ms (SD 4.3). Neither the left sternothyroid nor the left surface EMG recordings showed baseline activation, indicating that these muscles were not active for high-pitch phonation, but had responses at 30 ms following stimulus onset. In contrast, the left thyroarytenoid and right cricothyroid recordings were active during phonation but had no responses.
Fig. 4.
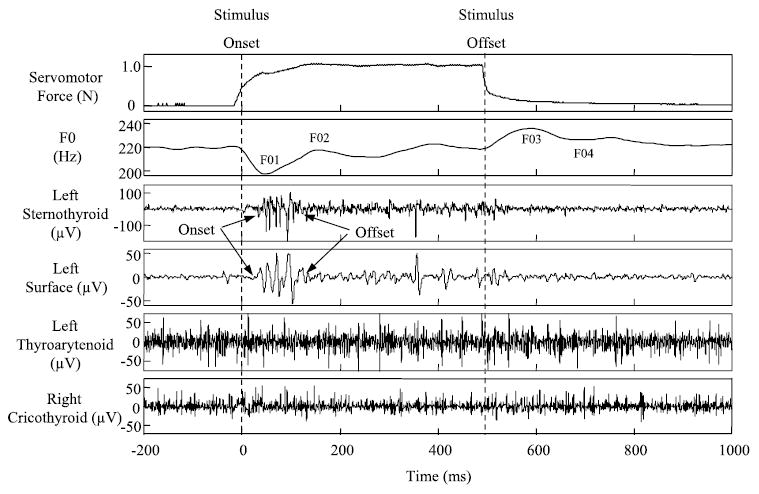
Muscle responses to the servomotor stimulus from a single subject in the high-pitch condition. The servomotor and fundamental frequency (F0) signals are above the muscle responses, which are in μV. A similar response occurs at the same latency in the left ST and left surface EMG recording. Muscle responses to the stimulus are not present in the left thyroarytenoid and right cricothyroid signals.
Table 1.
Latencies and response frequencies of the muscle and F0 responses in the phonation conditions according to subject and age
| High-Pitch Condition
|
Low-Pitch Condition
|
F0 Responses (High-Pitch Condition)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects
|
Latency, ms
|
Response frequency, %
|
Latency, ms
|
Response frequency, %
|
Latency, ms
|
Amplitude, Hz
|
||||||||
| No. | Age, yr | Gender | ST | Surface | ST | Surface | ST | Surface | ST | Surface | F01 | F02 | F01 | F02 |
| 1 | 69 | F | 117 | 60 | 82 | 161 | −94 | 46 | ||||||
| 2 | 33 | M | 27 | 31 | 95 | 79 | 26 | 30 | 75 | 63 | 65 | 185 | −14 | 13 |
| 3 | 34 | M | 73 | 174 | −25 | 8 | ||||||||
| 4 | 44 | M | 98 | 177 | −12 | 6 | ||||||||
| 5 | 37 | M | 30 | 29 | 89 | 87 | 25 | 25 | 75 | 56 | 65 | 184 | −15 | 26 |
| 6 | 56 | F | 57 | 53 | 50 | 158 | −14 | 30 | ||||||
| 7 | 43 | M | 53 | 122 | −2 | 3 | ||||||||
| 8 | 26 | F | 64 | 210 | −19 | 13 | ||||||||
| 9 | 51 | M | 23 | 71 | 20 | 69 | 71 | 178 | −26 | 20 | ||||
| 10 | 25 | M | 41 | 94 | 35 | 39 | 87 | 211 | −49 | 24 | ||||
F, female; M, male; ST, sternothyroid; F0, fundamental frequency.
Representative sternothyroid recordings from four subjects are presented in Fig. 5. The top two subjects, S2 and S5, had consistent sternothyroid responses. The bottom two subjects, S1 and S6, had inconsistent responses with a mean of 86.9 ms (SD 42.6). Three subjects (S5, S7, and S10) also showed late sternothyroid responses, with an average latency of 102 ms (SD 19) (see S5 in Fig. 5). Although the sternothyroid responses usually occurred following the thyroid cartilage force application, two subjects also had muscle responses following stimulus offset that were less consistent than their onset responses (see S1 and S6 in Fig. 5). Because the offset responses were inconsistent, these were not analyzed further. The movement artifacts seen in S6 illustrate that the servomotor stimulus occurred before muscle responses. No muscle responses occurred on any of the sham trials.
Fig. 5.
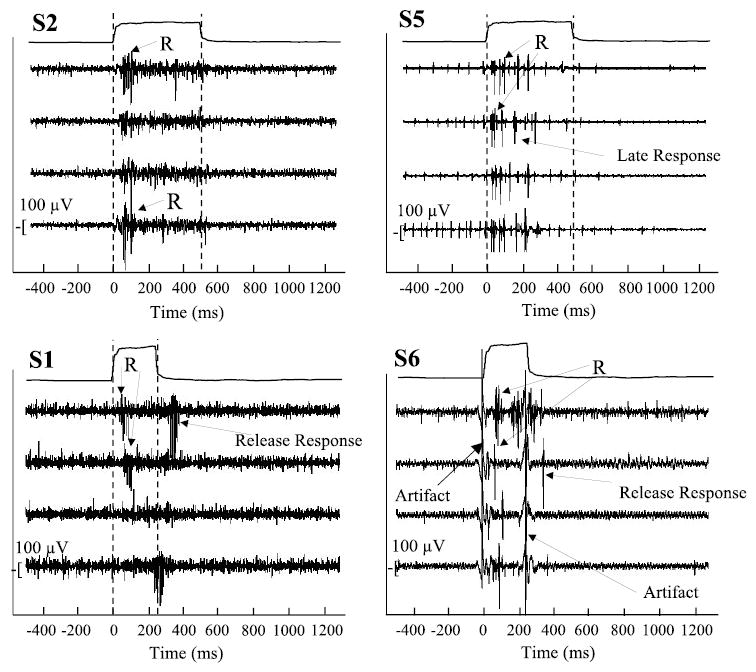
A cascade of ST muscle responses (R) from single trials from 4 subjects with ST responses (high-pitch condition). Two subjects (S2 and S5) had consistent ST responses with reliable onset latencies. The ST responses from the other 2 subjects (S1 and S6) showed more variable latencies and also postrelease responses. The stimulus artifacts in S6 clearly show the onset and offset of the servomotor stimuli.
The latencies of the intramuscular sternothyroid and surface recordings were similar across the different voiced and nonvoiced conditions in subjects with consistent responses (Fig. 6A). The latency of the sternothyroid and surface responses was between 25 and 35 ms and preceded the F01 and F02 voice changes. The frequency of the sternothyroid and surface responses was more consistent in the high-pitch condition than in other conditions (Fig. 1B and Table 1).
Fig. 6.
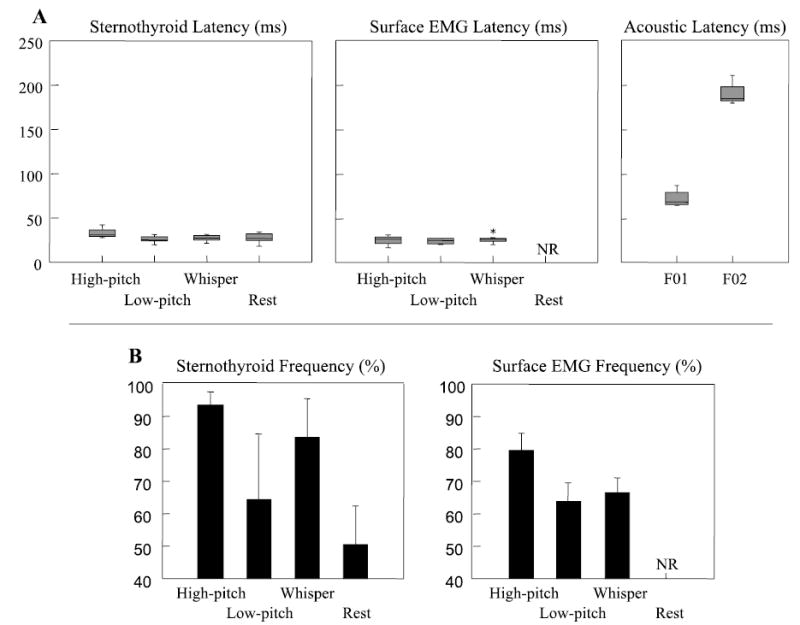
A: latency in milliseconds (ms) of intramuscular ST, surface laryngeal recordings (Surface), and changes in voice fundamental frequency (F01 and F02) for subjects 2, 5, 9, and 10 combined. The box plots show the quartile distribution around the median. B: response frequency (%) for subjects 2, 5, 9, and 10 (combined) is shown for ST and surface responses (Surface). The histographs show the mean frequency and 1 SD. NR, no response.
To determine whether the changes in voice fundamental frequency were related to the muscle responses, we computed Pearson correlation coefficients between the latencies and amplitudes of the muscle responses and the changes in fundamental frequency at F01 or F02 with a Bonferroni correction for the number of tests (P = 0.05/8 = 0.00625) (Table 2). None of the resulting r values were statistically significant (Table 1), demonstrating that neither the latency nor the magnitude of the F0 changes was related to the sternothyroid muscle responses.
Table 2.
Correlation matrix (Pearson r) of the linear associations between the latency of the first sternothyroid response and the latency and amplitude of the fundamental frequency (F0) events
| Subject
|
||||||
|---|---|---|---|---|---|---|
| S1 19 Observations | S2 37 Observations | S5 45 Observations | S6 15 Observations | S9* 42 Observations | S10 55 Observations | |
| F01 latency | −0.12 | 0.14 | 0.05 | −0.12 | −0.24 | 0.53 |
| F02 latency | 0.03 | −0.13 | −0.06 | 0.06 | 0.08 | −0.32 |
| F01 amplitude | −0.31 | 0.09 | −0.02 | 0.14 | 0.17 | 0.14 |
| F02 amplitude | 0.44 | −0.03 | −0.39 | 0.23 | 0.01 | 0.25 |
S1–S10, subjects 1–10.
Surface electromyographic results are only available for subject 9. None of the correlations were significant at P < 0.05 after Bonferroni correction.
The absence of EMG responses in four subjects permitted a comparison of whether the presence of an EMG response affected the amplitude or latency of the F0 events (26). The latencies and amplitudes of the F01 and F02 voice response from the six subjects with EMG responses were compared with those of the four subjects without EMG responses using separate t-tests with Bonferroni correction (4 tests). The amplitude of the F02 response was significantly higher in subjects with either a sternothyroid response or surface EMG response [t(8) =− 3.1, P = 0.05], but the F01 latency, F01 amplitude, and F02 latency did not differ between groups. To determine whether the presence of a sternothyroid response influenced voice latencies or amplitudes in the two subjects with inconsistent sternothyroid responses (S1 and S6), separate t-tests (4 tests) with Bonferroni correction compared the amplitude and latency of the F01 and F02 events between trials, with and without muscle responses. No significant differences were found for any of the comparisons.
The pattern of EMG results was similar in the low-pitch and high-pitch conditions. Responses occurred in the low-pitch condition in the sternothyroid and surface EMG recordings in the four subjects who also had reliable sternothyroid responses in the high-pitch condition. The latencies of the sternothyroid and surface EMG were similar in both pitch conditions (Fig. 6A and Table 1), but the frequency of both responses tended to decrease in the low-pitch condition, particularly for the surface EMG. Statistical comparisons between trials with and without a muscle response (sternothyroid or surface) found no differences in the latency or amplitude of voice changes for the low-pitch condition.
The F0 changes observed in the high- and low-pitch conditions were not seen in the pressed phonation condition (4 subjects). Muscle responses were only observed in two subjects, with one subject (S10) showing sternothyroid responses and the other subject (S9) showing surface responses. Responses were not observed in the thyroarytenoid and cricothyroid, although both muscles were active before and during the stimulus. The sternothyroid responses tended to be more variable in the pressed phonation condition than in the other voiced conditions. The latency and frequency of surface responses in S10 were 29 ms (±3 ms) and 93%, respectively, however, which were similar to this subject’s responses in the other pitch conditions (Table 1).
Nonphonation conditions
We compared responses across conditions that varied vocal fold position (29). During Valsalva, the vocal folds are closed and shortened, whereas, during high-pitch whisper, they are open and lengthened. During exhalation at rest, the glottis is open and the vocal folds are relaxed. No responses to force occurred in the thyroarytenoid or cricothyroid muscles in these conditions. Only the two subjects (S2 and S5) who had reliable sternothyroid responses during high-pitch phonation had sternothyroid responses in the whisper and rest conditions. The latencies of the sternothyroid responses in the nonphonation conditions were similar to those in both pitch conditions (Fig. 6A), although fewer responses occurred in the nonphonation conditions (Fig. 6B). Sternothyroid muscle responses were infrequent after cartilage release in the whisper condition. Surface EMG responses were less frequent in the low-pitch and whisper conditions and did not occur during quiet exhalation. No muscle responses occurred in the Valsalva condition.
DISCUSSION
Mechanical displacement of the thyroid cartilage during phonation did not elicit responses in the thyroarytenoid and cricothyroid muscles, despite robust effects of mechanical perturbation on the fundamental frequency of the voice. Force applied to the thyroid cartilage reduced the length and tension of the vocal folds, resulting in an initial F0 lowering, the F01 response, while the release of force increased F0, the F03 response. These rapid F0 changes demonstrate that the servo-motor force pulses induced the expected changes in vocal fold length. Posterior displacement of the thyroid cartilage to stretch the cricothyroid muscle, however, did not elicit cricothyroid responses. Similarly, the release from force, to stretch the thyroarytenoid muscle, failed to elicit thyroarytenoid responses.
Instead, the only muscle responses occurred in the sternothyroid muscle and were most likely a result of thyroid cartilage displacement in a posterior and upward direction, which would stretch this extrinsic strap muscle. The surface EMG recordings had similar latencies as the intrinsic sternothyroid responses, indicating that the surface electrodes most likely picked up signals from the sternothyroid. We used the same surface electrode placements as Sapir et al. (26) and found comparable EMG onset latencies, suggesting that their surface EMG responses may also have originated from the sternothyroid.
One difference between the studies is that all 19 subjects had muscle responses in the Sapir et al. study (26), whereas only 6 of our 10 subjects had EMG responses. Sapir et al. only recruited young male subjects with large thyroid prominences, which may have enhanced muscle response elicitation in that study. In contrast, our subject group included three women who did not have a laryngeal prominence. However, two of our female subjects had sternothyroid responses, whereas two male subjects with evident thyroid prominences did not, indicating that servomotor placement on a prominent thyroid cartilage was not essential for eliciting muscle responses. The lack of a sternothyroid muscle response in four of our subjects, despite consistent voice responses, also demonstrates that a sternothyroid response was not required for a voice response.
The voice responses elicited by force application were very similar in the two studies, although our F0 changes were greater in amplitude and duration (26). This is likely due to the higher force levels and displacements used in our study, 1.4 N and 8.5 mm, compared with 0.7 N and 3.3 mm, respectively, in Sapir et al. (26). Our initial F0 decrease lasted for 71 ms and was longer than theirs of 24 ms, most likely because of higher forces being applied in our study. Despite the use of greater forces, no thyroarytenoid or cricothyroid responses were detected in our study.
The later F02 response peaked at 80 ms in Sapir et al. (26) and at 176 ms in this study. Sapir and colleagues suggested that the F02 voice change was mediated by a muscle response occurring at 34 ms. In our study, each subject showed an F02 voice response, but only six had sternothyroid responses, suggesting that the F02 voice change was not dependent on a sternothyroid muscle response. Rather, the F02 and F04 voice changes may be mechanical rebound responses, because they both occurred in the opposite directions from the initial F01 and F03 voice responses to mechanical displacement. Although the F02 increase was greater in subjects with sternothyroid responses than in subjects without muscle responses, the F0 increase is not commensurate with sternothyroid contractions that typically depress the thyroid cartilage and lower the voice (14, 20). Furthermore, because the F02 change occurred in the absence of a muscle response, this voice change is more likely produced by passive rebound of the laryngeal tissue.
On the other hand, the F02 and F04 voice responses might correspond to the auditory pitch-shift reflex (9, 18, 30). The subjects in our study felt and heard the servomotor stimulus, so the later F02 and F04 voice changes may have been compensatory responses, causing the subjects to shift their pitch in the opposite direction from the perceived error. The latency of the F02 and F04 responses in this study occurred ~100 ms after the mechanically induced F01 and F03 voice changes, which approximates the latencies reported by Burnett et al. (9). Future studies are needed to examine how the laryngeal muscular system responds during the pitch-shift reflex.
In contrast with Titze et al. (29), who reported bilateral cricothyroid responses in both of their subjects, no cricothyroid responses occurred in our 10 subjects. A possible explanation is that the EMG recordings of Titze et al. included electrical pickup from sternothyroid muscles overlying the cricothyroid electrodes. We checked that our cricothyroid recordings did not increase during a chin-tuck or head-raising gesture to demonstrate that we were not picking up activity from neighboring strap muscles. Such a precaution was not mentioned in the Titze et al. study. The latency of their cricothyroid response was ~30 ms (based on Fig. 6, p. 2278, Ref. 29), which is similar to our sternothyroid response latencies. The absence of cricothyroid and thyroarytenoid responses in our study does not support the role of intrinsic laryngeal stretch reflexes in vocal vibrato, as proposed by Titze et al. (29).
Sternothyroid responses
Reflexes in the sternothyroid muscles have not been reported previously, but muscle spindles have been identified in human fetal sternothyroid muscles and monkey preparations (16, 23), along with dense muscle spindle populations in the human sternohyoid (8). The 35- to 40-ms sternothyroid latency seems late for a monosynaptic stretch reflex, given that stretch reflexes in the masseter (21) and trapezius (1) have latencies of 6 and 12 ms in humans, respectively. Reflex latency, however, depends on the anatomical orientation of the intrafusal organ and whether the stimulus delivery effectively and rapidly lengthens the intrafusal fibers. The sternothyroid responses occurred most often in the high-pitch condition when the larynx is moved upward and stretches the sternothyroid, possibly increasing the stretch effects on the 1a afferents in this muscle when force was applied to the thyroid cartilage (22). In studies of whiplash and falling with a rapid head acceleration/deceleration response, onset latencies in the sternocleidomastoid from these indirect stretch stimuli vary between 22 and 35 ms (3, 15), similar to the 25- to 30-ms latencies found here.
Laryngeal proprioception
Although the voice changes in fundamental frequency clearly demonstrated that changes occurred in the length and tension of the vocal folds, our stimulus may not stretch the spindles previously reported in the vocalis portion of the thyroarytenoid (24). Another study (8), however, failed to find muscle spindles in the human thyroarytenoid. One the other hand, our servomotor stimulus did not elicit the laryngeal adductor response in the thyroarytenoid muscle, suggesting that our external servomotor stimulus did not deflect the mucosal mechanoreceptors previously shown to elicit thyroarytenoid responses in humans (6) and are capable of conveying sensory information related to thyroarytenoid muscle tension and vocal fold movement (2, 25). At this point, our study does not provide evidence that either the thyroarytenoid or cricothyroid muscles mediate short-latency stretch responses.
In conclusion, although other receptors may mediate laryngeal sensory feedback during vocalization, such as muscle spindles in the human interarytenoid muscle (28) and mechanoreceptors in the laryngeal mucosa (12), we speculate that the short-latency sternothyroid responses found here provide evidence that spindles in extrinsic laryngeal muscles might provide sensory feedback regarding changes in laryngeal height. Possibly the rapid changes in laryngeal height, which occur during pitch changes (14), could generate somatosensory feedback for laryngeal control during voice production.
Acknowledgments
The authors thank Kimberly Bidus and Lisa Crujido for assistance with data collection and Drs. Pamela Kearney and Eric A. Mann for assistance with laryngeal electromyography and nasolaryngoscopy.
References
- 1.Alexander CM, Harrison PJ. The bilateral reflex control of the trapezius muscle in humans. Exp Brain Res. 2002;142:418–424. doi: 10.1007/s00221-001-0951-2. [DOI] [PubMed] [Google Scholar]
- 2.Andreatta RD, Mann EA, Poletto CJ, Ludlow CL. Mucosal afferents mediate laryngeal adductor responses in the cat. J Appl Physiol. 2002;93:1622–1629. doi: 10.1152/japplphysiol.00417.2002. [DOI] [PubMed] [Google Scholar]
- 3.Aoki M, Matsunami K, Han XY, Yamada H, Muto T, Ito Y. Neck muscle responses to abrupt vertical acceleration in the seated human. Exp Brain Res. 2001;140:20–24. doi: 10.1007/s002210100804. [DOI] [PubMed] [Google Scholar]
- 4.Baken RJ. Neuromuscular spindles in the intrinsic muscles of a human larynx. Folia Phoniatr (Basel) 1971;23:204–210. doi: 10.1159/000263490. [DOI] [PubMed] [Google Scholar]
- 5.Baken RJ, Noback CR. Neuromuscular spindles in intrinsic muscles of a human larynx. J Speech Hear Res. 1971;14:513–518. doi: 10.1044/jshr.1403.513. [DOI] [PubMed] [Google Scholar]
- 6.Bhabu P, Poletto C, Mann E, Bielamowicz S, Ludlow CL. Thyroarytenoid muscle responses to air pressure stimulation of the laryngeal mucosa in humans. Ann Otol Rhinol Laryngol. 2003;112:834–840. doi: 10.1177/000348940311201002. [DOI] [PubMed] [Google Scholar]
- 7.Bradley RM. Sensory receptors of the larynx. Am J Med. 2000;108(Suppl 4a):47S–50S. doi: 10.1016/s0002-9343(99)00339-3. [DOI] [PubMed] [Google Scholar]
- 8.Brandon CA, Rosen C, Georgelis G, Horton MJ, Mooney MP, Sciote JJ. Staining of human thyroarytenoid muscle with myosin antibodies reveals some unique extrafusal fibers, but no muscle spindles. J Voice. 2003;17:245–254. doi: 10.1016/s0892-1997(03)00013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnett TA, Freedland MB, Larson CR, Hain TC. Voice F0 responses to manipulations in pitch feedback. J Acoust Soc Am. 1998;103:3153–3161. doi: 10.1121/1.423073. [DOI] [PubMed] [Google Scholar]
- 10.Busby PA, Clark GM. Pitch estimation by early-deafened subjects using a multiple-electrode cochlear implant. J Acoust Soc Am. 2000;107:547–558. doi: 10.1121/1.428353. [DOI] [PubMed] [Google Scholar]
- 11.Clark KF, Farber JP. Recording from afferents in the intact recurrent laryngeal nerve during respiration and vocalization. Ann Otol Rhinol Laryngol. 1998;107:753–760. doi: 10.1177/000348949810700903. [DOI] [PubMed] [Google Scholar]
- 12.Davis PJ, Nail BS. Quantitative analysis of laryngeal mechanosensitivity in the cat and rabbit. J Physiol. 1987;388:467–485. doi: 10.1113/jphysiol.1987.sp016625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano M, Ohala J. Use of hooked-wire electrodes for electromyography of the intrinsic laryngeal muscles. J Speech Hear Res. 1969;12:362–373. doi: 10.1044/jshr.1202.362. [DOI] [PubMed] [Google Scholar]
- 14.Honda M, Hiraim H, Masaki S, Shimada Y. Role of vertical larynx movement and cervical lordosis in F0 control. Lang Speech. 1999;42:401–411. doi: 10.1177/00238309990420040301. [DOI] [PubMed] [Google Scholar]
- 15.Ito Y, Corna S, vonBrevern M, Bronstein A, Gresty H. The functional effectiveness of neck muscle reflexes for head-righting in response to sudden fall. Exp Brain Res. 1997;117:266–272. doi: 10.1007/s002210050221. [DOI] [PubMed] [Google Scholar]
- 16.Jakubowicz M, Radziemski A, Kedzia A. Histological and quantitative studies of the muscle spindles in the human fetal infrahyoid muscles. Folia Morphol (Warsz) 1992;51:55–59. [PubMed] [Google Scholar]
- 17.Jones JA, Munhall KG. Perceptual calibration of F0 production: evidence from feedback perturbation. J Acoust Soc Am. 2000;108:1246–1251. doi: 10.1121/1.1288414. [DOI] [PubMed] [Google Scholar]
- 18.Larson CR, Burnett TA, Kiran S, Hain TC. Effects of pitch-shift velocity on voice F-0 responses. J Acoust Soc Am. 2000;107:559–564. doi: 10.1121/1.428323. [DOI] [PubMed] [Google Scholar]
- 19.Lucas Keene MF. Muscle spindles in the human laryngeal muscles. J Anat. 1961;95:25–29. [PMC free article] [PubMed] [Google Scholar]
- 20.Niimi S, Horiguchi S, Kobayashi N, Yamada M. Electromyographic study of vibrato and tremolo in singing. Annual Bulletin of the RILP. 1987;21:153–164. [Google Scholar]
- 21.Poliakov AV, Miles TS. Stretch reflexes in human masseter. J Physiol. 1994;476:323–331. doi: 10.1113/jphysiol.1994.sp020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polus BI, Patak A, Gregory JE, Proske U. Effect of muscle length on phasic stretch reflexes in humans and cats. J Neurophysiol. 1991;66:613–622. doi: 10.1152/jn.1991.66.2.613. [DOI] [PubMed] [Google Scholar]
- 23.Raman R, Devanandan MS. Muscle receptors: content of some of the extrinsic and intrinsic muscles of the larynx in the bonnet monkey (Macaca Radiata) Anat Rec. 1989;223:433–436. doi: 10.1002/ar.1092230413. [DOI] [PubMed] [Google Scholar]
- 24.Sanders I, Han Y, Wang J, Biller H. Muscle spindles are concentrated in the superior vocalis subcompartment of the human thyroarytenoid muscle. J Voice. 1998;12:7–16. doi: 10.1016/s0892-1997(98)80070-2. [DOI] [PubMed] [Google Scholar]
- 25.Sant’Ambrogio G, Mathew OP, Fisher JT, Sant’Ambrogio FB. Laryngeal receptors responding to transmural pressure, airflow and local muscle activity. Respir Physiol. 1983;54:317–330. doi: 10.1016/0034-5687(83)90075-0. [DOI] [PubMed] [Google Scholar]
- 26.Sapir S, Baker KK, Larson CR, Ramig LO. Short-latency changes in voice F0 and neck surface EMG induced by mechanical perturbations of the larynx during sustained vowel phonation. J Speech Lang Hear Res. 2000;43:268–276. doi: 10.1044/jslhr.4301.268. [DOI] [PubMed] [Google Scholar]
- 27.Shiba K, Miura T, Yuza J, Sakamoto T, Nakajima Y. Laryngeal afferent inputs during vocalization in the cat. Neuroreport. 1999;10:987–991. doi: 10.1097/00001756-199904060-00017. [DOI] [PubMed] [Google Scholar]
- 28.Tellis CM, Rosen C, Thekdi A, Sciote JJ. Anatomy and fiber type composition of human interarytenoid muscle. Ann Otol Rhinol Laryngol. 2004;113:97–107. doi: 10.1177/000348940411300203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Titze IR, Story B, Smith M, Long R. A reflex resonance model of vocal vibrato. J Acoust Soc Am. 2002;111:2272–2282. doi: 10.1121/1.1434945. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y, Larson CR, Bauer JJ, Hain TC. Compensation for pitch-shifted auditory feedback during the production of Mandarin tone sequences. J Acoust Soc Am. 2004;116:1168–1178. doi: 10.1121/1.1763952. [DOI] [PMC free article] [PubMed] [Google Scholar]


