Abstract
The Clostridium acetobutylicum ATCC 824 spo0A gene was cloned, and two recombinant strains were generated, an spo0A inactivation strain (SKO1) and an spo0A overexpression strain [824(pMPSOA)]. SKO1 was developed by targeted gene inactivation with a replicative plasmid capable of double-crossover chromosomal integration—a technique never used before with solventogenic clostridia. SKO1 was severely deficient in solvent formation: it produced only 2 mM acetone and 13 mM butanol, compared to the 92 mM acetone and 172 mM butanol produced by the parental strain. After 72 h of growth on solid media, SKO1 formed long filaments of rod-shaped cells that failed to septate. SKO1 cells never achieved the swollen clostridial form typical of the parental strain and did not form endospores. No spo0A transcripts were detected in SKO1, while transcription of two solvent formation operons (aad-ctfA-ctfB and adc; both containing 0A boxes in their promoter regions) was limited. Strain 824(pMSPOA) produced higher butanol concentrations than the control strain [824(pIMP1)] and dramatically elevated spo0A transcript levels and displayed a bimodal pattern of spo0A transcription similar to that of B. subtilis. Microscopic studies indicated that sporulation was both enhanced and accelerated due to spo0A overexpression compared to that of both the 824(pIMP1) and parental strains. Consistent with that, expression of the key solvent formation genes (aad-ctfA-ctfB and adc) and three sporulation-specific genes (spoIIGA, sigE, and sigG) was observed earlier in strain 824(pMSPOA) than in the plasmid control. These data support the hypothesis that Spo0A is a transcriptional regulator that positively controls sporulation and solvent production. Its effect on solvent formation is a balancing act in regulating sporulation versus solvent gene expression: its overexpression apparently tips the balance in favor of accelerated and enhanced sporulation at the expense of overall solvent production.
The series of complex morphological changes that occur during the sporulation process in Bacillus subtilis has been extensively studied. Differentiation into endospores involves more than 125 genes, the transcription of which is temporally and spatially controlled by six RNA polymerase sigma factors (σA, σH, σF, σE, σG, and σK), and four DNA binding proteins (Spo0A, AbrB, Hpr, and Sin) (38). Spo0A controls the initiation of sporulation, the development of competence for DNA uptake, and many other stationary-phase-associated processes. In response to environmental, cell cycle, and metabolic signals, the phosphorelay two-component signal transduction system is responsible for phosphorylating Spo0A (Spo0A∼P), thus activating its function. Once activated, Spo0A is able to activate or repress transcription at the promoters that it controls (13). The consensus DNA binding site for Spo0A∼P is a 7-bp sequence (5′-TGNCGAA-3′, with a preference for N = T) called a 0A box (when the opposite orientation is observed, the sequence is referred to as a reverse 0A box).
Morphological and molecular studies have long suggested that B. subtilis and Clostridium acetobutylicum employ similar mechanisms for sporulation even though the environmental triggers of sporulation differ in these two organisms (32, 46). To date, four genes encoding homologues to B. subtilis sigma factors σA, σE, σG, and σK have been cloned and studied in C. acetobutylicum (32, 34, 35, 46). The genes spoIIGA, sigE, and sigG are clustered on the C. acetobutylicum chromosome (32, 46). The three homologous genes in B. subtilis, spoIIGA, spoIIGB (sigE), and spoIIIG (sigG), form a gene cluster with two different promoters, a σA-specific promoter upstream of spoIIGA and a σH-dependent promoter upstream of sigG. Primer extension analysis (32) confirmed that this is also the case in C. acetobutylicum DSM 1731 (which is virtually identical [15] to the type strain C. acetobutylicum ATCC 824). Despite these similarities between the two organisms, the recently published genome sequence of C. acetobutylicum ATCC 824 (25) suggests that many of the identified B. subtilis sporulation genes are missing in C. acetobutylicum. It is likely, then, that in C. acetobutylicum the control of sporulation and other associated stationary-phase events is substantially different from that in B. subtilis.
Specific to the transition to the stationary phase in C. acetobutylicum is a global response including a metabolic shift to solvent formation, initiation of sporulation, granulose accumulation, and expression of heat shock proteins (1). The molecular nature of the common and separate control mechanisms of these phenomena is still largely unknown (1, 19, 33). Spo0A-mediated regulation of solvent formation has been documented in C. beijerinckii NCIMB 8052 (2, 28) with an spo0A mutant generated by an integration of plasmid pSRW44, which contains an internal fragment (ca. 540 bp long) of the spo0A gene (44). This asporogenous mutant (AA243) contains several copies—estimated to be five—of pSRW44 (the sequence of the locus of integration has not been reported). AA243 produces extremely low levels of solvents (<3 mM butanol and no acetone), even under the most favorable conditions (butyrate and acetate addition in controlled-pH fermentations [28]). Ravagnani and coworkers (28) used gel retardation assays to show that the C-terminal domains of both the C. beijerinckii and B. subtilis Spo0A proteins bind to the C. beijerinckii ptb gene promoter (which contains two 0A boxes). ptb codes for the phosphotransbutyrylase (PTB) protein, which catalyzes the first step in the conversion of butyryl coenzyme A (CoA) to butyrate. They also examined the C. acetobutylicum adc gene, whose promoter region contains three 0A boxes. adc codes for acetoacetate decarboxylase, which catalyzes the formation of acetone from acetoacetate. Because DNase I footprinting failed to show protection of the 0A boxes, they carried out in vivo gene expression experiments with the parental C. beijerinckii strain with the gusA reporter system to show that Spo0A binds to both ptb and adc promoter 0A boxes, downregulates ptb expression, and upregulates adc expression (28).
The spo0A gene was previously mapped to the C. acetobutylicum ATCC 824 chromosome (3) and has been assigned position CAC2071 on the chromosome (25). Here we report the cloning and characterization of this spo0A gene by both inactivating it and overexpressing it in this organism. To our knowledge, overexpression of spo0A has not been previously reported in any microbial system. We carried out morphological, fermentation, and Northern analyses to examine the role of spo0A in the regulation of both solventogenesis and sporulation in C. acetobutylicum ATCC 824. C. beijerinckii NCIMB 8052 and C. acetobutylicum ATCC 824 are two very different organisms with different chromosome sizes (6.5 versus 4 Mb, respectively [25, 43]). They show very little DNA similarity (15) and have different phenotypic characteristics (e.g., C. beijerinckii degenerates must faster than C. acetobutylicum and displays different degeneration characteristics). The two organisms contain different solvent formation enzymes (e.g., no aad/adhE1 in C. beijerinckii) and have different product formation gene arrangements. In fact, in C. acetobutylicum, all of the essential solvent formation genes are located on the pSOL1 megaplasmid (whose loss leads to strain degeneration [4]), while there is no such plasmid in C. beijerinckii. Thus, control of sporulation and solvent formation may involve different mechanisms in these two organisms.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Table 1 lists the bacterial strains and plasmids used in this study.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Bacterial strains | ||
| C. acetobutylicum | ||
| ATCC 824 | ATCCb | |
| SKO1 | ATCC 824 spo0A::MLSr | This study |
| E. coli ER2275 | recA lacZ mcrBC | NEBc |
| Plasmids | ||
| pAN1 | Cmr; carries the φ3TI gene | 20 |
| pIMP1 | Apr | 22 |
| pMSPOA | Apr MLSr; carries spo0A | This study |
| pJC4 | MLSr Tcr | 17 |
| pGEM-4Z | Apr | Promegad |
| pTHAAD | Apr Cmr; carries aad | 18 |
| pCLH2 | Cmr | This study |
| pSCLH2 | pCLH2 with 0.58-kb internal spo0A fragment | This study |
| pESCLH2 | pSCLH2 with MLSr | This study |
| pETSPO | Derivative of pESCLH2 | This study |
| pFNK6 | Apr MLSr; carries adc ctfA ctfB | 21 |
| pHXS5 | Apr; carries orf1 aad ctfA ctfB | 23 |
Abbreviations: Cmr, chloramphenicol resistant; φ3TI, φ3TI methyltransferase; Apr, ampicillin resistant; Tcr, tetracycline resistant.
ATCC, American Type Culture Collection, Manassas, Va.
New England Biolabs.
Promega, Madison, Wi.
Growth and maintenance of strains and fermentation analysis.
Escherichia coli strains were grown and maintained as previously described (24). All of the recombinant clostridial strains examined in this study were derived from C. acetobutylicum ATCC 824. All clostridial strains were grown anaerobically at 37°C either in liquid cultures in 10-ml tubes of clostridium growth medium (CGM [42]) or on agar (15 g/liter) plates of reinforced clostridial medium (Difco Laboratories, Detroit, Mich.). For electroporation, C. acetobutylicum was grown under anaerobic conditions at 37°C in liquid 2XYTG (pH 5.2) supplemented with 100 μg of erythromycin (ERM) per ml as needed (22). Transformants were grown on 2XYTG plates (pH 5.8) with the appropriate antibiotics (40 μg of ERM per ml or 25 μg of thiamphenicol [TH] per ml) after electroporation. CBM plates were used to grow cells for morphological studies (26). For long-term storage, C. acetobutylicum strains were frozen at −85°C in CGM with 15% glycerol. Controlled pH 5 bioreactor fermentations (2- or 5-liter total volume with clarithromycin, a pH-stable derivative of ERM, used for selection of recombinant strains) and glucose and fermentation product analyses were carried out as previously described (9, 24).
Amylase activity assay.
The presence of the pSOL1 megaplasmid (4) in C. acetobutylicum strains was detected by monitoring amylase activity on 2XYTGMA plates (P. Soucaille, personal communication). This was possible due to the presence of two pSOL1-encoded α-amylase genes that are not subject to catabolite repression. The components of 2XYTGMA plates (per liter) were 16 g of Tryptone, 10 g of yeast extract, 2 g of NaCl, 4 g of glucose, 5 g of soluble starch, 41.9 g of morpholinepropanesulfonic acid (MOPS), and 15 g of agar. The pH of 2XYTGMA was adjusted to 6.5 since this pH was optimal for α-amylase activity. After 36 to 48 h of growth on 2XYTGMA plates, 100 μl of a 5% iodine solution was spread over the surface of the plate. Bacterial colonies with amylase activity (thus derived from cells carrying the pSOL1 megaplasmid) degraded the starch surrounding the colonies, resulting in an unstained halo around them following treatment with iodine.
Phase-contrast microscopy.
Strains of C. acetobutylicum were grown on CBM plates. After 24, 48, and 72 h of growth, cells were suspended in CGM liquid medium and 2 μl of the cell suspension was placed on a poly-l-lysine-coated glass slide. The cells were examined at 1,260-fold magnification in a phase-contrast light microscope (Zeiss Axiophot; Zeiss, Thornwood, N.Y.) equipped with a Zeiss AxioCam camera.
E. coli DNA isolation and manipulation.
Isolation of plasmids from E. coli by the alkaline lysis method and further manipulation of E. coli plasmid DNA were performed with standard protocols (18). Restriction enzymes were purchased from New England Biolabs (Beverly, Mass.) or Pharmacia Biotech Inc. (Piscataway, N.J.) and used under recommended conditions. T4 DNA ligase and alkaline phosphatase were also purchased from Pharmacia. DNA fragments were purified from agarose gels with activated DEAE-cellulose membranes (31).
C. acetobutylicum plasmid and genomic DNA isolation.
Plasmid DNA isolation was carried out as previously described (11). Chromosomal DNA was prepared as previously described (22) with minor improvements (9).
Bacterial transformations.
DNA was introduced into E. coli strains by electroporation with the BTX Electro Cell Manipulator in the high-voltage mode (2.5 kV/Ω) with a resistance of 129 Ω and a charging voltage of 2.44 kV. Electrotransformation of C. acetobutylicum was performed as previously described (22). Prior to transformation into C. acetobutylicum, plasmids were methylated in E. coli ER2275 carrying the methylating plasmid pAN1 (20).
DNA sequencing analysis.
DNA sequencing analysis was carried out on an ABI Prism 3100 sequencer by capillary electrophoresis with the ABI Prism dGTP Big Dye Terminator Ready Reaction kit (Applied Biosystems, Foster City, Calif.). Database searches were conducted with the WWW Blast server (www.ncbi.nlm.nih.gov) and the Argonne National Laboratory-WIT site (http://wit.mcs.anl.gov/WIT2/). The University of Wisconsin Genetics Computer Group sequence analysis software package was used to align amino acid sequences of Spo0A homologues.
DNA amplification by PCR.
Plasmid DNA was amplified by PCR with 50 ng of DNA in a final volume of 100 μl containing 300 μM each dATP and dTTP, 200 μM each dGTP and dCTP, 100 μg of nonacetylated bovine serum albumin per ml, 0.01 μg of each nucleotide primer per ml, and 0.05 U of Vent DNA polymerase (New England Biolabs) per μl. Amplification reactions were performed in a Perkin-Elmer GeneAmp PCR System 2400 thermocycler programmed for touchdown PCR: 5 min at 94°C, followed by 30 cycles of a 1-min denaturing step at 94°C, 1 min of annealing at a variable temperature (depending on the melting temperature of the PCR primers [Table 2]), and a 72°C extension step for a variable time (ca. 1 min/kb of length). The PCR programs concluded with a 7-min incubation at 72°C before the reaction mixture was cooled to 4°C.
TABLE 2.
Oligonucleotides used for PCR and sequencinga
| Primer name | Oligonucleotide sequence (5′-3′) | Use |
|---|---|---|
| SPOA5UP | GGAGTTTATATTGAATGgATCCTTAAAAG | PCR spo0A gene |
| 3SPOA3DN | TTACTATTCCTTGGTgATCATTAAAGAAA | PCR spo0A gene |
| BSPOFP5′ | atggatccGCCTGACCTTGTTGTTCTCGATA | PCR spo0A fragment |
| BSPOFN3′ | cgggatccaCGTGACCATGCAACTTCAATAG | PCR spo0A fragment |
| UPERM | cgtggaagcttGTGCTCTACGACCAAAAG | PCR MLSr gene |
| DSERM | aggaagcttGTGAATGCGCAAAAGACAT | PCR MLSr gene |
| 5′NSKO | GGTGGGATAGTTCAAGGAATG | PCR SKO1 |
| 3′NSKO | CATCGTAACTCCCTTGGATAC | PCR SKO1 |
| PIMPSEQ | TCCCAGTCACGACGTTGTAA | Sequencing |
| SPOSEQ2 | GACCATGCAACTTCAATAGC | Sequencing |
| SEQ3SPO | TACATCCATATCAAAAGGTT | Sequencing |
| SEQDSPO | CGACAAAACTATTATTTATC | Sequencing |
| PTB-DO | CTATTGGCTTTAAGAGTCCGC | PCR ptb probe |
| PTB-UP | GCTGTGGATGGAGTTAAGTCAG | PCR ptb probe |
| THIOL-3E | CATGATTTTAAGGGGGGTACCATATGCA | PCR thl probe |
| THIOL-5B | GTTATTTTTAAGGATCCTTTATAGCAC | PCR thl probe |
| 5′SIGE | GCAATGAGCTTAGAGAGCCTAT | PCR sigE-sigG probe |
| 3′SIGG | ACTTCCATGGACTTCATAGCA | PCR sigE-sigG probe |
Lowercase letters in the oligonucleotide sequences indicate nucleotide substitutions used to generate useful restriction sites near the termini of PCR products. Restriction sites are underlined.
Genomic DNA was amplified with rTth DNA polymerase XL (Applied Biosystems) along with the PCR Optimization Kit (Invitrogen, Carlsbad, Calif.). Typically, 0.5 to 1 μg of chromosomal DNA was used in a 50-μl reaction mixture.
Cloning of the spo0A gene and construction of pMSPOA.
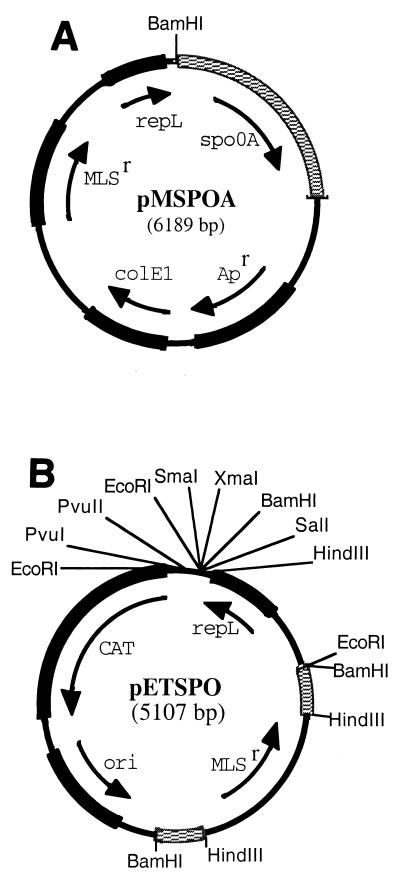
The spo0A gene (843 nucleotides [nt]), along with 43 nt from the upstream spoIVB gene, 364 nt of the upstream intergenic region (which includes the spo0A promoter), and 277 nt from the downstream intergenic region (a total of 1.5 kb), was amplified by PCR from the chromosome of C. acetobutylicum ATCC 824 with primers SPOA5UP and 3SPOA3DN (Table 2). Single-nucleotide substitutions created a BamHI restriction site in SPOA5UP and a BclI site restriction site in 3SPOA3DN. After a BamHI-BclI double digestion, the 1.5-kb spo0A fragment was ligated into BamHI-linearized pIMP1. The resulting plasmid was designated pMSPOA (Fig. 1A). The spo0A region of pMSPOA was sequenced with primers PIMPSEQ, SPOSEQ2, SEQ3SPO, and SEQDSPO (Table 2).
FIG. 1.
Partial restriction maps of plasmids pMSPOA (A) and pETSPO (B). In pETSPO, the spo0A fragments are checkered.
Construction of pETSPO.
E. coli vector pGEM-4Z was double digested with DraI and PvuII, generating a 937-bp fragment containing the gram-negative origin of replication (ori). The ori fragment was then ligated to the 1.5-kb EcoRI-HindIII fragment of pTHAAD (6) carrying the gene for chloramphenicol acetyltransferase expression, which confers resistance to both chloramphenicol and TH. The product of the ligation was plasmid pCLH2 (2.44 kb). A 580-bp internal spo0A fragment was PCR amplified from chromosomal DNA with primers BSPOFP5′ and BSPOFN3′ (Table 2). These primers introduced BamHI restriction sites on the ends of this internal spo0A fragment in which 147 bp from the 5′ end and 116 bp from the 3′ end of the structural gene were absent. After EcoRI linearization of plasmid pCLH2, the ends were filled and this vector was ligated to the internal spo0A fragment, yielding plasmid pSCLH2 (3.03 kb). A 1.05-kb fragment containing the macrolide-lincosamide-streptogramin B resistance (MLSr)-encoding gene was then PCR amplified from pIMP1 with primers UPERM and DSERM (Table 2). These primers introduced HindIII restriction sites on the ends of the MLSr-encoding fragment. After digestion of the PCR product with HindIII, the fragment was ligated into the HindIII site located in the center of the internal spo0A fragment of pSCLH2. The product of the ligation was plasmid pESCLH2 (4.06 kb). A gram-positive origin of replication (repL) was obtained on a 1.04-kb DNA fragment after digestion of pIMP1 with FspI. EcoRI linkers were added to the ends of the repL fragment, and the product was ligated into pESCLH2 at the unique EcoRI site to construct pETSPO (Fig. 1B).
Targeted inactivation of spo0A.
Methylated pETSPO (Fig. 1B) was introduced into C. acetobutylicum ATCC 824 by electroporation. A liquid culture of strain 824(pETSPO) was then used to confluently streak agar (15 g/liter) plates of reinforced clostridial medium containing 40 mg of ERM per liter. The cultures were transferred to fresh plates containing no antibiotics every 24 h for 5 consecutive days with replica plating. Cells that grew on ERM but not on TH were selected by comparing cultures that were replica plated onto medium containing TH (25 mg/liter) and ERM (40 mg/liter). These isolates were grown in liquid medium containing 40 mg of ERM per liter, and the cultures were used for plasmid DNA isolation, chromosomal DNA isolation, and frozen stock preparation. The liquid cultures were also plated onto starch-containing 2XYTGMA plates to confirm amylase activity and thus the presence of pSOL1. The criteria for further screening were absence of a replicating plasmid, presence of pSOL1, sensitivity to TH (chloramphenicol acetyltransferase), and resistance to ERM (MLSr). Isolates that met these criteria were further analyzed by PCR amplification and sequencing.
C. acetobutylicum RNA isolation.
RNA was isolated from 5 to 15 ml of fermentation culture. Following centrifugation (5 min, 4°C, 4,000 × g), the supernatant was suspended in 200 μl of SET solution (25% sucrose, 0.05 M Tris-HCl, 0.05 M EDTA) containing 20 mg of lysozyme per ml. After a 5-min incubation at 37°C, 1 ml of RNA STAT-60 (Tel-Test Inc., Friendswood, Tex.) was added to the mixture and the lysed cells were frozen at −85°C. Upon thawing, samples were processed in accordance with the manufacturer's (Tel-Test) instructions. Total RNA was quantified by spectrophotometric analysis at 260 nm. The quality of the RNA preparations was assessed by the spectrophotometric 260-nm/280-nm ratio, which was ≥1.7 for all of the samples used for Northern analysis. Furthermore, RNA integrity and loading were determined by first separating 15 μg of all RNA samples on 1% agarose denaturing gels and then staining by ethidium bromide in order to visualize the rRNA bands (9). Only good-quality RNA was used for Northern analysis.
mRNA probe preparation for Northern analysis.
Probes were prepared for detection of the following six mRNA transcripts: spo0A, thiolase (thl), PTB-butyrate kinase (ptb-buk), aldehyde/alcohol dehydrogenase-acetoacetyl-CoA:acetate-butyrate:CoA transferase (aad-ctfA-ctfB), acetoacetate decarboxylase (adc), solR (24), and sigE-sigG. The solR and aad-ctfA-ctfB probes were prepared with plasmid pHXS5. After AccI-EcoRI double digestion of pHSX5, one of the resulting fragments (1.8 kb) contained the entire solR gene and a second one (4.9 kb) contained the entire sol operon (aad-ctfA-ctfB). The adc probe was a 1.2-kb fragment resulting from AccI digestion of pFNK6. AccI-EcoRI double digestion of pMSPOA (Fig. 1A) resulted in a 1.5-kb fragment containing the spo0A gene. PCR amplification from chromosomal DNA was used to obtain probes for the remaining three transcripts, thl, ptb-buk, and sigE-sigG. The thiolase PCR primers were THIOL-5B and THIOL-3E (Table 2), and they were used to amplify a 1.25-kb fragment. Primers PTB-UP and PTB-DO (Table 2) were used to amplify a 1.3-kb fragment containing only the ptb gene to be used as a probe for the ptb-buk transcript. The sigE and sigG genes were amplified together as a 2.1-kb fragment with primers 5′SIGE and 3′SIGG (Table 2). Double-stranded DNA probes were purified from agarose gels with activated DEAE-cellulose membranes (31). The probes were labeled with [α-32P]dCTP (3,000 Ci/mmol; ICN Biomedicals, Inc., Costa Mesa, Calif.) with the Prime-It II Random Primer Labeling Kit from Stratagene (La Jolla, Calif.). Unincorporated radiolabeled deoxynucleoside triphosphate, [α-32P]dCTP, was removed with Nuc Trap columns (Stratagene).
Northern analysis.
Fifteen-microgram RNA samples were used for Northern analysis as previously described (24), with small modifications (9). InstantImager Electronic Autoradiography (Packard Instrument Company, Meriden, Conn.) was used to image all blots. The InstantImager software was used to quantify the hybridization signals, and the values reported are total counts. Since the same membrane was probed for each of the seven messages, it was necessary to strip the blot after each round of hybridization and autoradiography. Membranes were stripped in a 55% formamide solution containing 2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) and 1.0% sodium dodecyl sulfate at 65°C for 60 min, followed by washing for 15 min at 65°C in 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% sodium dodecyl sulfate. In order to be able to compare Northern blot data for the two pairs of strains [C. acetobutylicum ATCC 824 (wild-type or parental strain) versus SKO1 and 824(pMSPOA) versus 824(pIMP1)], the blots for each pair and each probe were probed, imaged, and stripped simultaneously. The probes used to detect each transcript were prepared at the same time and used in the same amounts.
RESULTS
DNA sequence-based bioinformatic analysis.
The B. subtilis and C. acetobutylicum Spo0A proteins show 53% identity and 72% conservation over the entire polypeptide length. The N-terminal domains of the B. subtilis and C. acetobutylicum Spo0A homologues show 49% identity and 71% conservation over 122 amino acids (aa), with 73% identity and 89% conservation over the 115 aa comprising the Spo0A-specific C-terminal domain. The C-terminal effector or activator domain of Spo0A has three regions (CI, CII, and CIII) (2). Region CII contains the portion of the helix-turn-helix motif (12 aa long: SRVERAIRHAIE) comprising the recognition helix as part of a 22-aa sequence (the putative site of protein-DNA interaction) that is perfectly conserved in B. subtilis and C. acetobutylicum (9). In view of the very high level of protein homology between the Spo0A proteins from C. acetobutylicum, C. beijerinckii, and B. subtilis (2, 9) and the demonstrated binding of the Spo0A protein on the DNA 0A boxes not only in B. subtilis but also in C. beijerinckii (28), we assumed that Spo0A binds to such 0A boxes in C. acetobutylicum as well.
Two promoters have been identified upstream of the spo0A structural gene in B. subtilis (39). A vegetative promoter recognized by EσA is responsible for maintaining a low level of Spo0A during exponential growth. A second promoter, recognized by EσH, is responsible for the increased expression of spo0A during the transition to the stationary phase. As in B. subtilis, the C. acetobutylicum spo0A promoter region contain sequences that match promoter consensus sequences recognized by EσA and EσH. There are three 0A boxes upstream of B. subtilis and C. beijerinckii 8052 spo0A. In contrast, only one 0A box—it overlaps the −10 element of the EσH promoter—was found in the promoter regions of the C. acetobutylicum spo0A. As in B. subtilis (37), there are two likely 0A boxes in the promoter region of the spoIIGA-sigE operon but none in front of the sigG gene. However, all three genes may be transcribed from the spoIIGA-sigE promoter (8).
The sol operon (5) on the pSOL1 megaplasmid (4), which contains the three genes encoding aldehyde/alcohol dehydrogenase (aad [23] or adhE1 [5]) and the two CoA transferase subunit genes (ctfA and ctfB), has a single reverse 0A box located 5′ of its distal promoter (28). The monocistronic adc gene contains two 0A boxes and one reverse 0A box in its promoter region (28). The chromosomal thiolase (thl) gene (27) contains no 0A boxes in its promoter region. Unlike the ptb gene in C. beijerinckii (28), there are no 0A boxes in the promoter region of the ptb-buk operon (responsible for butyrate formation). There are also no 0A boxes in the promoter region of the pta-ack operon (responsible for acetate formation) or in that of the putative regulatory gene solR (10, 24).
Inactivation of spo0A.
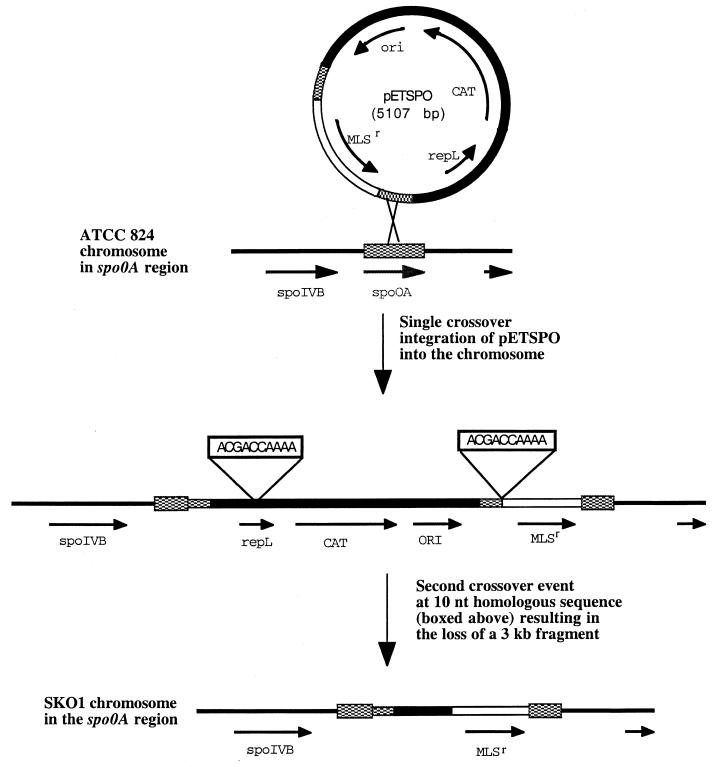
After several attempts to generate an spo0A knockout with nonreplicative vectors (7) failed, we developed a method with a replicating plasmid—a technique never before used with solventogenic clostridia—in order to inactivate the spo0A gene as discussed in Materials and Methods. One isolate, SKO1, that satisfied the criteria for spo0A disruption with the MLSr-encoding gene was selected for further study. To confirm that the MLSr-encoding gene was inserted by double-crossover recombination events into the spo0A gene in SKO1, chromosomal DNA was used as the template for PCR amplification with primers 5′NSKO and 3′NSKO (Table 2). When these primers were used in a PCR with the parent strain chromosomal DNA serving as the template, the product was, as expected, a 2.45-kb fragment. The expected double-crossover event in which only the MLSr-encoding gene was inserted into spo0A would have resulted in the amplification of a 3.47-kb fragment. Instead, when chromosomal DNA from isolate SKO1 was used as the PCR template, a 4.5-kb PCR product was amplified. Sequencing disclosed the precise genetic arrangement in the SKO1 spo0A region (9). From this information, the putative crossover events that occurred during spo0A inactivation in SKO1 were determined (Fig. 2). First, the entire 5.1-kb pETSPO plasmid was apparently integrated into the chromosome at the spo0A gene. This resulted in a strain that was resistant to both TH and ERM. Since the 583-nt internal spo0A fragment was duplicated in the chromosome of this single-crossover event strain, a second homologous recombination event at the spo0A site would be expected. If this had occurred in the spo0A sequence, the result would have been either reversion to the parental genotype or deletion of plasmid DNA except for the MLSr marker, an ideal double crossover. This expected second crossover event in the spo0A gene apparently did not take place. Instead, a crossover event occurred between two 10-nt homologous sequences (5′-ACGACCAAAA-3′) that were present in the 3′ end of the repL structural gene and upstream of the MLSr marker (Fig. 2). Loss of the 3-kb fragment between these 10-nt-long homologous sequences resulted in the generation of SKO1, whereby the spo0A gene was inactivated through insertion of a 2.1-kb fragment containing the MLSr-encoding gene.
FIG. 2.
Putative crossover events during spo0A gene inactivation. spo0A sequences are checkered, and the MLSr-encoding gene is represented by an open segment. All other sequences are shown as black lines or filled segments.
In addition to confirming the presence of the pSOL1 megaplasmid in strain SKO1 by detection of α-amylase activity, PCR was used to amplify the aad gene located on the pSOL1 megaplasmid. Results of both of these experiments (9) indicated that SKO1 did, indeed, contain the pSOL1 megaplasmid. After heating of several SKO1 colonies to 70°C for 10 min in liquid culture (a standard procedure used to induce vegetative growth of sporulated C. acetobutylicum strains [30]), lack of growth indicated that mature spores were not formed by this recombinant strain. This was in contrast to the behavior of the parental (wild-type) strain, Spo0A-overexpressing strain 824(pMSPOA), and plasmid control strain 824(pIMP1).
Phase-contrast microscopy.
In order to investigate possible differences in cell morphology among the parental strain, SKO1, C. acetobutylicum ATCC 824(pIMP1), and 824(pMSPOA), cells from cultures grown on CBM plates were examined by phase-contrast light microscopy. Cells from all four strains examined after 24 h of growth had similar shapes and sizes (9). After 48 h, parental-strain cells were swollen in the typical cigar-shaped clostridial form (16) with a small percentage (10 to 20%) of the cells possessing phase-bright endospores. SKO1 cells grown for 48 h were not swollen, and their shape was similar to that observed after 24 h of growth. After 48 h of growth, more than 50% of the 824(pMSPOA) cells contained phase-bright endospores or had released the endospores, resulting in cell debris and phase-bright free spores, and the rest were in the swollen clostridial form. After 48 h, control strain 824(pIMP1) had fewer cells in the swollen clostridial form or containing endospores than did 824(pMSPOA) but more than did the parent strain. After 72 h of growth, debris was prevalent in cell suspensions of all four strain cultures. The intact parental-strain cells were usually in the swollen clostridial form, but some free spores were also present. There was no detectable endospore formation in SKO1 cultures, the cells did not swell, and a large fraction of the cells formed filaments of connected rods. This SKO1 morphology is consistent with that observed when the spo0A gene was inactivated in B. anthracis and C. beijerinckii (2). After 72 h of growth, more than half of the intact 824(pMSPOA) cells photographed contained endospores and there were also a large number of free spores in cell suspensions of the 824(pMSPOA) strain. These numbers were smaller (ca. 40% of the intact cells contained endospores) in plasmid control strain 824(pIMP1).
Effect of spo0A inactivation on solvent production.
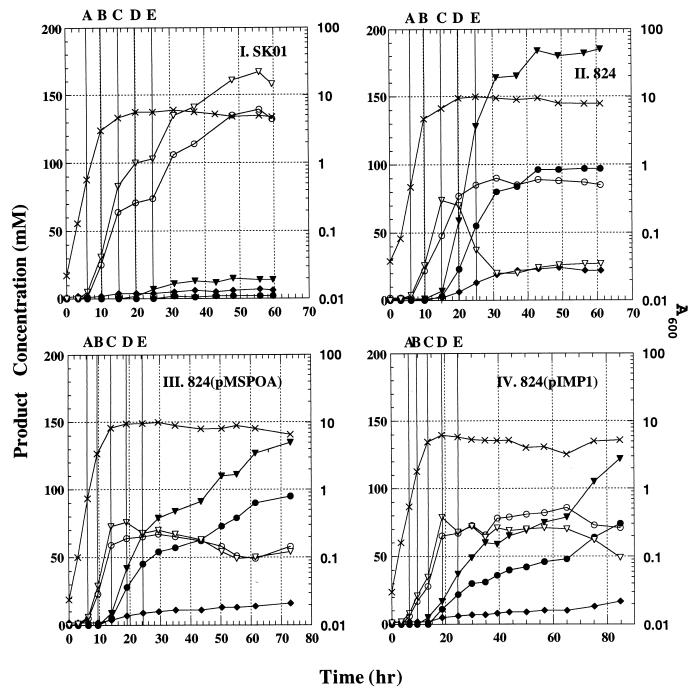
Batch bioreactor SKO1 cultures (n = 4) grew slower (by 31% [doubling times were 1.10 ± 0.06 h for the parental strain and 1.44 ± 0.07 h for the SKO1 strain]; all reported differences are based on mean values and are statistically significant [P < 0.05] unless otherwise noted) and to a lower density (by 30%) than parental-strain cultures (n = 4) (Fig. 3). Fermentations of both strains were complete (no further changes in product concentration) within 60 h. Both acetate and butyrate accumulated to much higher levels (by ca. 50 and 500%, respectively, at the end of the fermentations), and very small amounts were reutilized in the SKO1 cultures compared to the parental-strain fermentations. Accumulation of solvents was very low in SKO1 fermentations (mean values of 2 mM acetone, 13 mM butanol, and 5 mM ethanol) compared to parental-strain fermentations (92 mM acetone, 172 mM butanol, and 21 mM ethanol).
FIG. 3.
Representative fermentation kinetics from bioreactor experiments of strain SKO1 (I), the parental strain (II), strain 824(pMSPOA) (III), and strain 824(pIMP1) (IV). Samples from these experiments were used for the Northern analysis data presented in Fig. 4 and 5. The five sampling points (A, B, C, D, and E) for each experiment are indicated by solid vertical lines. Symbols: ×, A600; ▾, butanol concentration; •, acetone concentration; ♦, ethanol concentration; ▿, butyrate concentration; ○, acetate concentration.
Effect of spo0A overexpression on product formation.
Batch fermentations (Fig. 3) of strain 824(pMSPOA) (n = 4) and plasmid control strain 824(pIMP1) (n = 2) showed that neither of these two types of fermentation was complete, even after 70 h, both showing continuing solvent production. Strain 824(pMSPOA) grew slightly faster and to much greater (by ca. 50%) cell densities than strain 824(pIMP1). Maximum and final concentrations of acids were similar in the 824(pMSPOA) and 824(pIMP1) fermentations. Final solvent concentrations in the 824(pMSPOA) fermentations were 20% higher than those in 824(pIMP1) fermentations. Strain 824(pMSPOA) produced 25% less butanol than the parental strain but the same levels of acetone and ethanol. Solvent formation was initiated at about the same time for all of the strains [824, 824(pMSPOA), and 824(pIMP1)], but strain 824 produced solvents at much higher rates during the first 40 to 50 h (Fig. 3). In contrast, strains 824(pMSPOA) and 824(pIMP1) produced solvents at lower rates but for a prolonged time, even after 70 h. It has been documented that the swollen clostridial forms of the cells are the active producers of solvents (spores and forespore-forming cells do not produce solvents) (16, 29). Thus, these fermentation data should be interpreted as mean values from a mixed population of solvent-producing and non-solvent-producing cells, with the fraction of these two types of cells varying substantially (as revealed by the morphological studies described above) among strains 824, 824(pMSPOA), and 824(pIMP1). Finally, liquid cultures of strain 824(pMSPOA) were quite viscous within 24 h of growth in both tubes and the bioreactor. Culture viscosity decreased when high solvent levels accumulated at the end of the fermentation period.
Northern analysis.
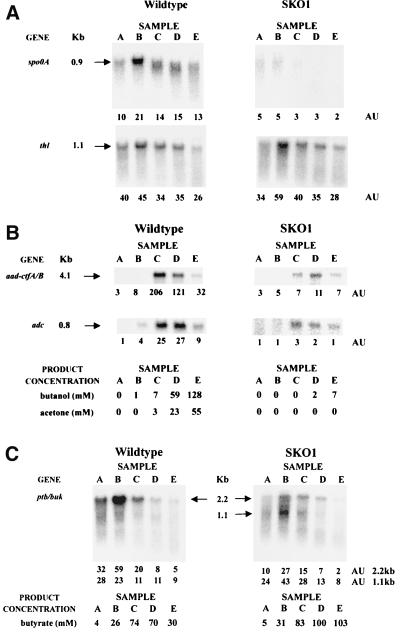
Radiolabeled probes were designed to detect transcripts corresponding to genes with (spo0A, aad-ctfA-ctfB, adc, and spoIIGA-sigE-sigG) or without (thl, ptb-buk, and solR) 0A boxes in their promoter regions. In the parental strain, the spo0A message was detectable in cells from all stages of the culture examined (samples A through E; an additional late-stationary-phase sample [F] was also analyzed, but the results are not shown here due to excessive RNA degradation) (Fig. 4A). The message was most abundant at the end of the exponential growth phase (sample B), when it was twice as abundant as during the mid-exponential growth phase (sample A) and 50% more abundant than during the stationary phase. That the level of spo0A transcripts peaked just prior to the transition to the stationary phase correlates well with its putative role as a transcriptional regulator responsible for controlling changes in gene expression that occur as the organism ceases exponential growth. As expected, no spo0A message was detected in strain SKO1 (Fig. 4A).
FIG. 4.
Parental (wild-type) and SKO1 strain Northern analyses with the spo0A and thl probes (A), the aad-ctfA/B and adc probes (B), and the ptb-buk probe (C). RNA samples collected during the exponential growth phase (samples A [h 6 and 6.3; A600 of 0.57 and 0.47 for the SKO1 and parental strains, respectively] and B [h 10 and 9.5; A600 of 3 and 4.7 for the SKO1 and parental strains, respectively]), the transition state (samples C [h 15; A600 of 4.6 and 6.7 for the SKO1 and parental strains, respectively]), and the stationary phase (samples D [h 20; A600 of 5.6 and 9.5 for the SKO1 and parental strains, respectively] and E [h 25; A600 of 5.6 and 9.9 for the SKO1 and parental strains, respectively]) of fermentations (Fig. 3) were electrophoretically resolved and probed with 32P-labeled probes. The blots were electronically imaged with InstantImager Electronic Autoradiography, and the total radioactive counts were measured after 10 min of imaging for spo0A, aad-ctfA-ctfB, and adc and 20 min of imaging for thl and ptb-buk. The mRNA levels are reported in arbitrary units (AU), which are the total counts divided by 1,000.
The thl gene encodes a central metabolic pathway enzyme that has significant activity throughout batch fermentations (12, 40). The levels and patterns of thiolase gene expression were similar in the parental and SKO1 strains (Fig. 4A). The highest thl transcript level was detected at the end of exponential growth (sample B) in the fermentations of both strains, a pattern that correlates well with previous observations that thiolase activity reaches its peak at the end of the exponential growth phase (12, 40). These results indicate that spo0A inactivation does not significantly alter the level of thiolase transcription, and this is consistent with the fact that there are no 0A boxes upstream of the thl gene.
For both strains, only background levels of the 4.1-kb aad-ctfA-ctfB transcript were detected during exponential cell growth (samples A and B) (Fig. 4B). In the parental strain, the maximum level of aad-ctfA-ctfB was detected during the transition from exponential growth to the stationary phase of fermentation (sample C). The levels of the aad-ctfA-ctfB transcript detected in SKO1 were much lower than in the parental strain. In fact, the maximum SKO1 aad-ctfA-ctfB transcript level during the stationary phase of fermentation (sample D) was about 20 times lower than the maximum parental-strain aad-ctfA-ctfB transcript level. A drastic decrease in the abundance of the 0.8-kb adc transcript upon inactivation of spo0A was also observed (Fig. 4B). The highest levels of SKO1 adc transcripts, detected in the transition from exponential growth to stationary phase or in stationary phase (samples C and D), were about 10 times lower than parental-strain levels.
An attempt was made to detect a sigE-sigG message in the parental and SKO1 strains, but none was reliably detected in any of the samples analyzed. A substantial amount of a degraded low-molecular-weight message hybridized to the radioactive sigE-sigG probe in stationary-phase samples E and F of the parental strain, but none did so in the SKO1 strain (data not shown).
The ptb gene is the first gene in a bicistronic message also containing the buk gene. Together, these genes encode the enzymes responsible for the conversion of butyryl-CoA to butyrate. In the parental strain, this 2.2-kb ptb-buk signal was strongest during the exponential growth phase (samples A and B) and was significantly decreased in stationary phase (samples D and E) (Fig. 4C). This pattern of the 2.2-kb ptb-buk signals was similar in strain SKO1 (Fig. 4C), but the transcript levels were lower (by 50 to 300% at points A and B). A second, shorter message is quite apparent in the SKO1 samples, so we analyzed the data for the full (2.2-kb) and short (1.1-kb) transcripts separately. For consistency, we did so also for the parental strain, although the short message was not as clearly identifiable in this strain. The probe we used for Northern analysis of the ptb-buk operon is made up of a large fragment from the ptb gene, the intergenic region and a 40-bp fragment from the buk gene. This 40-bp fragment shows a high percentage of homology (90%) with a second buk gene (bukII), a monocistronic chromosomal gene with a high level of homology to the buk gene of the ptb-buk operon (14). Thus, our Northern probe would likely also capture any bukII transcript that has a calculated size of about 1.2 kb. Therefore, the smaller 1.1-kb transcript could represent this bukII transcript or a truncated ptb-buk transcript. We noted that Huang et al. (14) reported very low BKII expression levels in C. acetobutylicum. We also noted that bukII contains a 0A box in its promoter region and a reverse 0A box just inside its coding region. The ptb-buk transcript levels and butyrate concentrations corresponded well to the PTB activity previously reported in parental-strain batch fermentations (12) and to β-galactosidase activity during fermentations of a strain with a β-galactosidase reporter gene under the control of the ptb promoter (40). We also noted that the SKO1 strain produced higher levels of butyrate despite the lower ptb-buk transcript levels.
Collectively, these data show that spo0A inactivation decreased the transcription of solvent formation genes more than 10-fold but did not significantly alter the transcription of the thiolase gene. In fact, the thl gene appears to behave like a housekeeping gene within the time points examined in this study, and this is consistent with the results from our reporter system study (40). The uncertainty about the two transcripts (Fig. 4C) makes it impossible to draw any conclusions regarding the effect of Spo0A on ptb-buk operon expression.
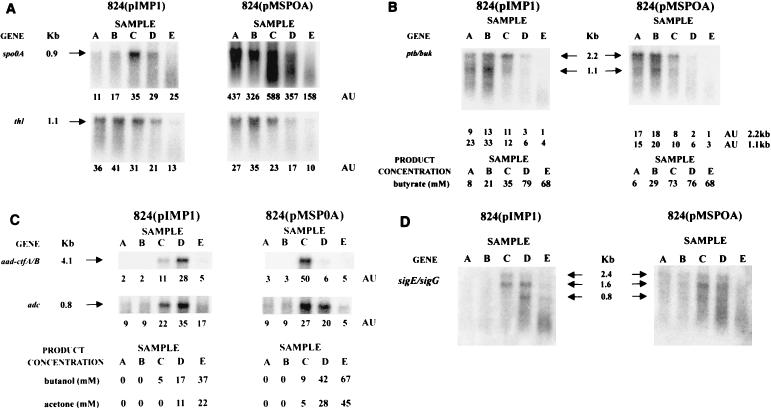
Levels of spo0A mRNA in the spo0A-overexpressing strain C. acetobutylicum ATCC 824(pMSPOA) were up to 40 times greater than those in strain 824(pIMP1) throughout the fermentation period (Fig. 5A). The maximum spo0A transcript level detected was observed during the transition from exponential to stationary phase (sample C). The spo0A message appeared to be shorter in samples B, C, and D of strain 824(pMSPOA), but this was not the case for strain 824(pIMP1). The parental strain Northern data also show shorter transcripts in samples C, D, and E (Fig. 4A). These shorter messages may correspond to transcripts from the two different promoters (recognized by EσA and EσH) upstream of spo0A, which are 90 nt apart. These data suggest for the parental and 824(pMSPOA) strains a bimodal expression of spo0A, similar to what was observed in B. subtilis, whereby spo0A is transcribed first from the weaker vegetative promoter and later from the sporulation-specific promoter (39). These results show that the presence of plasmid-encoded spo0A results in a significant increase in the number of spo0A transcripts. The thl and ptb-buk transcript levels were similar in strains 824(pMSPOA) and 824(pIMP1) (Fig. 5A and B) and thus were apparently not affected by spo0A overexpression.
FIG. 5.
Northern analysis of strains 824(pIMP1) and 824(pMSPOA) with the spo0A and thl probes (A), the ptb-buk probe (B), the aad-ctfA-ctfB and adc probes (C), and the sigE-sigG probe (D). RNA samples collected during the exponential growth phase {samples A [h 6.3 and 6.5; A600 of 0.74 and 0.54 for the 824(pMSPOA) and 824(pIMP1) strains, respectively] and B [h 9.5 and 9.5; A600 of 3.4 and 1.8 for the 824(pMSPOA) and 824(pIMP1) strains, respectively]}, the transition state {samples C [h 14 and 13.5; A600 of 8.2 and 4.9 for the 824(pMSPOA) and 824(pIMP1) strains, respectively]}, and the stationary phase {samples D [h 19 and 18.8; A600 of 9.4 and 6.2 for the 824(pMSPOA) and 824(pIMP1) strains, respectively] and E [h 24.3 and 24.8; A600 of 9.6 and 5.8, for the 824(pMSPOA) and 824(pIMP1) strains, respectively]} of fermentations (Fig. 3) were electrophoretically resolved and probed with 32P-labeled probes. The blots were electronically imaged with InstantImager Electronic Autoradiography, and the total radioactive counts were measured after 10 min of imaging for spo0A, aad-ctfA-ctfB, and adc and 20 min of imaging for thl, ptb-buk, and sigE-sigG. The mRNA levels are reported in arbitrary units (AU), which are the total counts divided by 1,000.
Signals for the aad-ctfA-ctfB probe were detected during the transition to stationary phase and in the early stationary-phase samples (C and D, respectively) of both strains 824(pIMP1) and 824(pMSPOA) (Fig. 5C). In both strains, and in contrast to the parental strain (Fig. 4B), the aad-ctfA-ctfB signals show an abrupt downregulation. This is also in contrast to the slower downregulation of the adc signals in both strains 824(pIMP1) and 824(pMSPOA) (Fig. 5C) and suggests a differential regulation of these sol locus genes. Similar levels of adc transcripts were detected after the transition to stationary phase (samples C, D, and E) in both strains 824(pIMP1) and 824(pMSPOA), although, as in the case of the aad-ctfA-ctfB transcripts, the points of maximal transcript levels were different. Higher levels of both aad-ctfA-ctfB and adc transcripts appear to be expressed earlier in strain 824(pMSPOA) than in strain 824(pIMP1), and this is consistent with the earlier expression of spo0A in 824(pMSPOA) (Fig. 5A) and the faster rate of solvent formation early in the fermentation of strain 824(pMSPOA) than in that of strain 824(pIMP1) (Fig. 3). The ratio of maximal (aad-ctfA-ctfB)/adc signals in strain 824(pMSPOA) was approximately twice that in strain 824(pIMP1) but fourfold lower than that in the parental strain (Fig. 4B). This again suggests a differential regulation of the aad-ctfA-ctfB operon versus the adc gene.
A 2.1-kb radioactive probe containing both the sigE and sigG genes was used to examine the expression of these two sigma factor genes. Three different transcripts were detected in RNA samples from both 824(pIMP1) and 824(pMSPOA) strains with the radioactive sigE-sigG probe (Fig. 5D). These transcripts (2.4, 1.6, and 0.8 kb) correspond to messages containing spoIIGA-sigE-sigG, spoIIGA-sigE, and sigG, respectively. In strain 824(pIMP1), no sigE-sigG message was detected during exponential growth (samples A and B), the 2.4- and 1.6-kb transcripts were detected in the transition to stationary phase (sample C), and all three transcripts were detected in early stationary phase (sample D). In strain 824(pMSPOA), two faint transcripts (2.4 and 1.6 kb) were detected in samples A and B and all three transcripts were detected in samples C and D. Degradation made detection of bands difficult in samples E from both 824(pIMP1) and 824(pMSPOA). However, in samples E (Fig. 5D) and F (data not shown) from both of these strains, a substantial amount of a low-molecular-weight message hybridized to the sigE-sigG probe, similar to that observed in the parental strain Northern analysis, as discussed above. The sigE-sigG data of Fig. 5D should be contrasted to our inability to detect any sigE-sigG messages in the SKO1 strain and only a very low-level and degraded message in the parental strain. These results indicate that overexpression of the plasmid-encoded spo0A gene results in overexpression of a functional Spo0A protein, which stimulates earlier and greater expression of the spoIIGA, sigE, and sigG genes.
We also probed for differential expression of solR. The signals were very weak, and no differences could be detected. Thus, we are not able to conclude that solR is or is not under Spo0A control. We should note, however, that there are no 0A boxes in the solR promoter region or inside the coding region.
DISCUSSION
The morphological and fermentation (Fig. 3) data suggest that spo0A inactivation leads to an asporogenous phenotype without complete abolition of solvent formation. In contrast, spo0A inactivation in C. beijerinckii completely abolished acetone formation and allowed only very low levels of butanol formation (<3 mM) (28). Strain SKO1 also produces much higher concentrations of both butyrate (by ca. 5-fold) and acetate (by ca. 1.5-fold) compared to the parental strain. In C. beijerinckii, spo0A inactivation results in ca. 20% less acetate formation but ca. 35% higher butyrate levels (28). We believe that the reduced cell densities of strain SKO1 (Fig. 3) is not a direct effect of the spo0A inactivation but rather an indirect one due to the accumulation of the two acids as a result of the inability of the cells to take up butyrate and acetate. We base this interpretation on the fact that two other degenerate mutants (M5 and DG1) resulting from loss of the pSLO1 plasmid show almost identical growth and acid formation characteristics (reference 4 and data not shown). These two mutants produce no solvents, except for some ethanol, but apparently still express the chromosomal spo0A gene. The inability of these two mutants to sporulate and produce solvents is due to the loss of pSOL1 genes. C. beijerinckii contains no such plasmid. These findings suggest that spo0A expression has different effects on product formation by C. acetobutylicum and C. beijerinckii.
Overexpression of spo0A in strain 824(pMSPOA) appears to accelerate both the sporulation process and solvent formation compared to those in plasmid control strain 824(pIMP1). Strain 824(pMSPOA) [and, to a lesser extent, strain 824(pIMP1)] show dramatically accelerated and increased sporulation compared to that of the parental strain. Fermentations of both strains 824(pMSPOA) and 824(pIMP1) show a slower rate of solvent formation than the parental strain, and this is consistent with the lower levels of the aad-ctfA-ctfB transcripts (Fig. 5C). However, these results should be reinterpreted on the basis of the actual fraction of cells producing solvents, i.e., cells in the clostridial form versus nonproducing forespore-forming cells and spores (16, 29). In view of the fact that 824(pMSPOA) [and, to a lesser extent, 824(pIMP1)] contain a much larger fraction [by at least 50% for strain 824(pMSPOA)] of forespore-forming cells and spores than does the parental strain, the fermentation data suggest that the ability of clostridial-form cells of strain 824(pMSPOA) to produce solvents is as great as and likely greater than that of those of the parental strain. Similarly, if the Northern analysis data could be presented on the basis of the cells that contain the solvent formation gene transcripts, they might show that such transcripts are indeed more abundant and present at higher concentrations in clostridial-form cells of the 824(pMSPOA) strain, as would have been expected from spo0A overexpression. Taken together, these data suggest that the regulation by Spo0A of two related but very different processes (sporulation versus solvent production) is a balancing act when viewed from the solvent production point of view. Very little or no expression of Spo0A would not be sufficient for induction of solvent formation genes, while high-level expression of Spo0A would result in accelerated sporulation and commitment into differentiated cellular forms that do not produce solvents. Thus, optimal production of solvents may require a low to medium level of Spo0A expression.
We have previously reported host-plasmid interactions (including those from plasmid pIMP1) resulting in positive effects on solvent production in low-pH (4.5) fermentations (41). Here, at pH 5.0, the effect on solvent production was not positive overall, although the sustained solvent production by both strains 824(pMSPOA) and 824(pIMP1) might have eventually resulted in solvent concentrations similar to or higher than that produced by the parental strain. The effect of plasmid pIMP1 was, however, positive in accelerating the sporulation process compared to that of the parental strain.
Although the aad-ctfA-ctfB transcript levels in strain 824(pMSPOA) were higher than those in strain 824(pIMP1), there were no significant changes in the maximum level of adc transcripts (Fig. 5C). Expression of the solvent formation genes aad-ctfA-ctfB and adc and sporulation-specific genes sigE-sigG occurred earlier in strain 824(pMSPOA) than in plasmid control strain 824(pIMP1). These data and the fact that lack of spo0A expression in SKO1 does not completely abolish either sol locus gene (aad-ctfA-ctfB and adc) expression (Fig. 4B) or solvent production (Fig. 3) suggest that Spo0A/Spo0A∼P may be one of many regulators of solvent gene expression. Examples of this type of multiregulator control of gene expression are known in many organisms, notably in B. subtilis. The activating effect of Spo0A∼P has to counteract several negative regulators, like SinR and Hpr, that prevent sporulation. In fact, most abrB-repressed B. subtilis genes are also controlled by one or more regulators that allow the cell to respond to multiple physiological conditions (36).
The delayed expression of sigG compared to that of sigE (Fig. 5D) is consistent with primer extension analysis experiments (32). The presence of low levels of a 2.4-kb transcript (Fig. 5D) early in the culture possibly containing all three genes (spoIIGA-sigE-sigG) is contrary to reported primer extension analysis data (32) but similar to what has been reported in B. subtilis (8). In B. subtilis, the SigG protein is apparently translated not from this long message but rather from the delayed short sigG message (8). Inability to detect any intact sigE-sigG message in the parental strain is probably because spoIIAG-sigE and sigG are expressed late and at low levels. In fact, previous attempts to detect these messages by Northern analysis in C. acetobutylicum DSM 1731 were not successful and necessitated the use of primer extension analysis (32). Lack of any detectable expression of a sigE-sigG message in SKO1 may, indeed, reflect the effect of spo0A inactivation. These data also further support the conclusion that Spo0A overexpression does, indeed, accelerate and enhance sporulation.
A second thiolase gene (thlB) (45) in C. acetobutylicum is located on the pSOL1 plasmid and shows very low expression as part of a three-gene operon yielding large-size (1.7- and 2.7-kb) transcripts. In contrast, the chromosomal monocistronic gene thl we used in this study produces a 1.4-kb transcript. Thus, there is no ambiguity regarding the interpretation of our thl data.
The high viscosity of 824(pMSPOA) cultures might be due to earlier and increased accumulation (as suggested by the morphological studies) of granulose, a storage polysaccharide produced during the stationary phase of parental-strain cultures. Solvent accumulation dissolves this material and prevents an increase in culture viscosity.
The gene inactivation method used in this study to create SKO1 was conceptualized and implemented without difficulty. In fact, SKO1 was isolated during the first use of the technique. More than two dozen colonies where obtained that met the antibiotic resistance requirements for a potential gene inactivation product. Of these isolates, three were used in PCR experiments to determine the size of the spo0A region. All three of the selected isolates showed evidence that a genetic change (an insertion) occurred in the spo0A region based on the size of the PCR products. Only one of these three isolates (SKO1) was used in sequencing reactions to determine the precise nature of the genetic change. The targeted gene inactivation technique presented in this work would be beneficial to genetic studies with clostridia, and efforts to use it to inactivate other genes are warranted.
The findings presented here provide support for the hypothesis that Spo0A acts as a multifunctional regulatory protein that is crucial for transcription of solvent formation and sporulation genes. Our data suggest that its effect on solvent formation is a balancing act in regulating sporulation versus solvent gene expression. Northern analysis data show that the regulation of spo0A expression is similar to that in B. subtilis, despite the different numbers of 0A boxes found in the promoter regions of the corresponding spo0A genes. Our data also show that Spo0A apparently regulates other sporulation genes (here the spoIIGA, sigE, and sigG genes) in a manner similar to that in B. subtilis.
Acknowledgments
This work was supported by a National Science Foundation grant (BES-9905669) and a National Institutes of Health Predoctoral Biotechnology Training Grant (GM 08449).
We thank Abbott Laboratories for the donation of clarithromycin, G. N. Bennett for useful suggestions during the preparation of the manuscript, Wouter Hendriksen for additional replicate experiments, and Seshu Tummala and Chris Tomas for technical assistance.
REFERENCES
- 1.Bahl, H., H. Muller, S. Behrens, H. Joseph, and F. Narberhaus. 1995. Expression of heat shock genes in Clostridium acetobutylicum. FEMS Microbiol. Rev. 17:341-348. [DOI] [PubMed] [Google Scholar]
- 2.Brown, D. P., L. Ganova-Raeva, B. D. Green, S. R. Wilkinson, M. Young, and P. Youngman. 1994. Characterization of spo0A homologues in diverse Bacillus and Clostridium species reveals regions of high conservation within the effector domain. Mol. Microbiol. 14:411-426. [DOI] [PubMed] [Google Scholar]
- 3.Cornillot, E., C. Croux, and P. Soucaille. 1997. Physical and genetic map of the Clostridium acetobutylicum ATCC 824 chromosome. J. Bacteriol. 179:7426-7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornillot, E., R. V. Nair, E. T. Papoutsakis, and P. Soucaille. 1997. The genes for butanol and acetone formation in Clostridium acetobutylicum ATCC 824 reside on a large plasmid whose loss leads to degeneration of the strain. J. Bacteriol. 179:5442-5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer, R. J., J. Helms, and P. Dürre. 1993. Cloning, sequencing, and molecular analysis of the sol operon of Clostridium acetobutylicum, a chromosomal locus involved in solventogenesis. J. Bacteriol. 175:6959-6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green, E. M., and G. N. Bennett. 1998. Genetic manipulation of acid and solvent formation in Clostridium acetobutylicum ATCC 824. Biotechnol. Bioeng. 58:217-221. [DOI] [PubMed] [Google Scholar]
- 7.Green, E. M., Z. L. Boynton, L. M. Harris, F. B. Rudolph, E. T. Papoutsakis, and G. N. Bennett. 1996. Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology 142:2079-2086. [DOI] [PubMed] [Google Scholar]
- 8.Haldenwang, W. G. 1995. The sigma factors of Bacillus subtilis. Microbiol. Rev. 59:1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris, L. M. 2001. Ph.D. dissertation. Northwestern University, Evanston, Ill.
- 10.Harris, L. M., L. Blank, R. P. Desai, N. E. Welker, and E. T. Papoutsakis. 2001. Analysis of recombinant strains of Clostridium acetobutylicum with an inactivated solR gene. J. Ind. Microbiol. Biotechnol. 27:322-328. [DOI] [PubMed] [Google Scholar]
- 11.Harris, L. M., R. P. Desai, N. E. Welker, and E. T. Papoutsakis. 2000. Characterization of recombinant strains of the Clostridium acetobutylicum butyrate kinase inactivation mutant: need for new physiological models for solventogenesis and butanol inhibition? Biotechnol. Bioeng. 67:1-11. [PubMed] [Google Scholar]
- 12.Hartmanis, M. G. N., and S. Gatenbeck. 1983. Intermediary metabolism in Clostridium acetobutylicum: levels of enzymes involved in formation of acetate and butyrate. Appl. Environ. Microbiol. 47:1277-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoch, J. A. 1993. spoO genes, the phosphorelay, and the initiation of sporulation, p. 747-755 In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 14.Huang, K.-X., S. Huang, F. B. Rudolph, and G. N. Bennett. 2000. Identification and characterization of a second butyrate kinase from Clostridium acetobutylicum ATCC 824. J. Mol. Microbiol. Biotechnol. 2:33-38. [PubMed] [Google Scholar]
- 15.Johnson, J. L., J. Toth, S. Santiwatanakul and J.-S. Chen. 1997. Cultures of “Clostridium acetobutylicum” from various collections comprise Clostridium acetobutylicum, Clostridium beijerinckii, and two other distinct types based on DNA-DNA reassociation. Int. J. Syst. Bacteriol. 47:420-424. [DOI] [PubMed] [Google Scholar]
- 16.Jones, D. T., A. van der Westhuizen, S. Long, E. R. Allcock, S. J. Reid, and D. R. Woods. 1982. Solvent production and morphological changes in Clostridium acetobutylicum. Appl. Environ. Microbiol. 43:1434-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, S. Y., G. N. Bennett, and E. T. Papoutsakis. 1992. Construction of Escherichia coli-Clostridium acetobutylicum shuttle vectors and transformation of Clostridium acetobutylicum strains. Biotechnol. Lett. 14:427-432. [Google Scholar]
- 18.Lee, S. Y., and S. Rasheed. 1990. A simple procedure for maximum yield of high quality plasmid DNA. BioTechniques 9:676-679. [PubMed] [Google Scholar]
- 19.Long, S., D. T. Jones, and D. R. Woods. 1984. The relationship between sporulation and solvent production in Clostridium acetobutylicum P262. Biotechnol. Lett. 6:529-534. [Google Scholar]
- 20.Mermelstein, L. D., and E. T. Papoutsakis. 1993. In vivo methylation in Escherichia coli by Bacillus subtilis phage φ3T I methyltransferase to protect plasmids from restriction upon transformation of Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 59:1077-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mermelstein, L. D., E. T. Papoutsakis, D. J. Petersen, and G. N. Bennett,. 1993. Metabolic engineering of Clostridium acetobutylicum for increased solvent production by enhancement of acetone formation enzyme activities using a synthetic acetone operon. Biotechnol. Bioeng. 42:1053-1060. [DOI] [PubMed] [Google Scholar]
- 22.Mermelstein, L. D., N. E. Welker, G. N. Bennett, and E. T. Papoutsakis. 1992. Expression of cloned homologous fermentative genes in Clostridium acetobutylicum ATCC 824. Bio/Technology 10:190-195. [DOI] [PubMed] [Google Scholar]
- 23.Nair, R. V., G. N. Bennett, and E. T. Papoutsakis. 1994. Molecular characterization of an aldehyde/alcohol dehydrogenase gene from Clostridium acetobutylicum ATCC 824. J. Bacteriol. 176:871-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair, R. V., E. M. Green, G. N. Bennett, and E. T. Papoutsakis. 1999. Regulation of the sol locus genes for butanol and acetone formation in Clostridium acetobutylicum ATCC 824 by a putative transcriptional repressor. J. Bacteriol. 181:319-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nölling, J., G. Breton, M. V. Omelchenko, et al. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183:4823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien, R. W., and J. G. Morris. 1971. Oxygen and the growth and metabolism of Clostridium acetobutylicum. J. Gen. Microbiol. 68:307-318. [DOI] [PubMed] [Google Scholar]
- 27.Petersen, D. J., and G. N. Bennett. 1991. Cloning of the Clostridium acetobutylicum ATCC 824 acetyl coenzyme A acetyltransferase (thiolase; EC 2.3.1.9) gene. Appl. Environ. Microbiol. 57:2735-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravagnani, A., K. C. Jennert, E. Steiner, R. Grunberg, J. R. Jefferies, S. R. Wilkinson, D. I. Young, E. C. Tidswell, D. P. Brown, P. Youngman, J. G. Morris, and M. Young. 2000. Spo0A directly controls the switch from acid to solvent production in solvent-forming clostridia. Mol. Microbiol. 37:1172-1185. [DOI] [PubMed] [Google Scholar]
- 29.Reardon, K. F., and J. E. Bailey. 1992. Activity of regeneration of continuous Clostridium acetobutylicum bioconversions of glucose. Biotechnol. Prog. 8:316-326. [Google Scholar]
- 30.Roos, J. W., K. McLaughlin, and E. T. Papoutsakis. 1985. The effect of pH on nitrogen supply, cell lysis, and solvent production in fermentations of Clostridium acetobutylicum. Biotechnol. Bioeng. 27:681-694. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Santangelo, J. D., A. Kuhn, A. Treuner-Lange, and P. Dürre. 1998. Sporulation and time course expression of sigma-factor homologous genes in Clostridium acetobutylicum. FEMS Microbiol. Lett. 161:157-164. [DOI] [PubMed] [Google Scholar]
- 33.Sauer, U., and P. Dürre. 1995. Differential induction of genes related to solvent formation during the shift from acidogenesis to solventogenesis in continuous culture of Clostridium acetobutylicum. FEMS Microbiol. Lett. 125:115-120. [Google Scholar]
- 34.Sauer, U., J. D. Santangelo, A. Treuner, M. Buchholz, and P. Dürre. 1995. Sigma factor and sporulation genes in Clostridium. FEMS Microbiol. Rev. 17:331-340. [DOI] [PubMed] [Google Scholar]
- 35.Sauer, U., A. Treuner, M. Buchholz, J. D. Santangelo, and P. Dürre. 1994. Sporulation and primary sigma factors homologous genes in Clostridium acetobutylicum. J. Bacteriol. 176:6572-6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonenshein, A. L. 2000. Control of sporulation initiation in Bacillus subtilis. Curr. Opin. Microbiol. 3:561-566. [DOI] [PubMed] [Google Scholar]
- 37.Spiegelman, G. B., T. H. Bird, and V. Voon. 1995. Transcription regulation by the Bacillus subtilis response regulator Spo0A, p. 159-179 In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. American Society for Microbiology, Washington, D.C.
- 38.Stragier, P., and R. Losick. 1996. Molecular genetics of sporulation in Bacillus subtilis. Annu. Rev. Genet. 30:297-341. [DOI] [PubMed] [Google Scholar]
- 39.Strauch, M., K. Trach, J. Day, and J. Hoch. 1992. Spo0A activates and represses its own synthesis by binding at its dual promoters. Biochimie 74:619-626. [DOI] [PubMed] [Google Scholar]
- 40.Tummala, S. B., N. E. Welker, and E. T. Papoutsakis. 1999. Development and characterization of a gene expression reporter system for Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 65:3793-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walter, K. A., L. D. Mermelstein, and E. T. Papoutsakis. 1994. Host-plasmid interactions in recombinant strains of Clostridium acetobutylicum ATCC 824. FEMS Microbiol. Lett. 123:335-342. [Google Scholar]
- 42.Wiesenborn, D. P., E. T. Papoutsakis, and F. B. Rudolph. 1988. Thiolase from Clostridium acetobutylicum ATCC 824 and its role in the synthesis of acids and solvents. Appl. Environ. Microbiol. 54:2717-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilkinson, S. R., and M. Young. 1993. Wide diversity of genome sizes among different strains of Clostridium acetobutylicum. J. Gen. Microbiol. 139:1069-1076. [Google Scholar]
- 44.Wilkinson, S. R., and M. Young. 1994. Targeted integration of genes into the Clostridium acetobutylicum chromosome. Microbiology 140:89-95. [Google Scholar]
- 45.Winzer, K., K. Lorenz, B. Zickner, and P. Dürre. 2000. Differential regulation of the two thiolase genes from Clostridium acetobutylicum DSM 792. J. Mol. Microbiol. Biotechnol. 2:531-541. [PubMed] [Google Scholar]
- 46.Wong, J., C. Sass, and G. N. Bennett. 1995. Sequence and arrangement of genes encoding sigma factors in Clostridium acetobutylicum. Gene 153:89-92. [DOI] [PubMed] [Google Scholar]