Abstract
Purpose
Rose bengal is an organic anionic dye used to assess damage of the ocular surface epithelium in ocular surface disease. It has been proposed that mucins have a protective role, preventing rose bengal staining of normal ocular surface epithelial cells. The current study was undertaken to evaluate rose bengal staining in a human corneal-limbal epithelial (HCLE) cell line known to produce and glycosylate membrane-associated mucins.
Methods
HCLE cells were grown to confluence in serum-free medium and switched to DMEM/F12 with 10% serum to promote differentiation. Immunolocalization of the membrane-associated mucins MUC1 and MUC16 and the T-antigen carbohydrate epitope was performed with the monoclonal antibodies HMFG-2 and OC125 and jacalin lectin, respectively. To assess dye uptake, cultures were incubated for 5 minutes with 0.1% rose bengal and photographed. To determine whether exclusion of negatively charged rose bengal requires a negative charge at the cell surface, cells were incubated with fluoresceinated cationized ferritin. The effect of hyperosmotic stress on rose bengal staining in vitro was evaluated by increasing the ion concentration (Ca+2 and Mg+2) in the rose bengal uptake assay.
Results
The cytoplasm and nucleus of confluent HCLE cells cultured in media without serum, lacking the expression of MUC16 but not MUC1, as well as human corneal fibroblasts, which do not express mucins, stained with rose bengal. Culture of HCLE cells in medium containing serum resulted in the formation of islands of stratified cells that excluded rose bengal. Apical cells of the stratified islands produced MUC16 and the T-antigen carbohydrate epitope on their apical surfaces. Colocalization experiments demonstrated that fluoresceinated cationized ferritin did not bind to these stratified cells, indicating that rose bengal is excluded from cells that lack negative charges. Increasing the amounts of divalent cations in the media reduced the cellular area protected against rose bengal uptake.
Conclusions
These results indicate that stratification and differentiation of corneal epithelial cells, as measured by the capacity to produce the membrane-associated mucin MUC16 and the mucin-associated T-antigen carbohydrate on their apical surfaces provide protection against rose bengal penetrance in vitro and suggest a role for membrane-associated mucins and their oligosaccharides in the protection of ocular surface epithelia.
Rose bengal, the 4,5,6,7-tetrachloro 2′,4′,5′,7′-tetraiodo derivative of fluorescein (see Fig. 1), is an organic anionic dye that has been clinically used for many decades to assess damage to the ocular surface epithelium in ocular surface disease.1 Application of rose bengal to the ocular surface of patients with dry eye results in patches of superficial punctate staining, the frequency and intensity of which has been used to characterize the disease, assess its severity, and monitor the clinical response to therapy.2
Figure 1.
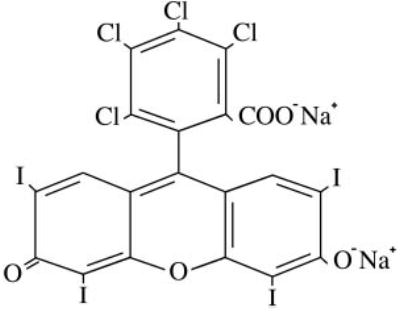
Molecular structure of rose bengal.
Although initial reports speculated that rose bengal stains desquamated, degenerated, or dead cells,3–5 recent evidence has shown that it can stain healthy human and rabbit corneal epithelial cells grown in vitro.6,7 It has been hypothesized that rose bengal staining occurs when there is poor protection of the surface epithelium by the preocular tear film rather than by lack of cell viability. Tseng et al.8 have shown that rose bengal binds to several tear components: albumin, lactoferrin, transferrin, and lysozyme. However, the total maximum binding capacity of the nonmucin proteins in normal tears is too small to explain the lack of rose bengal staining on the normal ocular surface.8 These experiments also showed that precoating rabbit corneal epithelial cells with porcine stomach mucin blocks the entry of rose bengal, indicating that mucins are an effective barrier to rose bengal diffusion.
Mucins are highly O-glycosylated, high-molecular-weight glycoproteins that constitute a major component of the protective biofilm on the surface of all epithelial cells.9 Based on structural characterization, two different types of mucins have been described: secreted mucins, which can be subclassified as small soluble and gel-forming, and membrane-associated mucins,10 which have a hydrophobic domain that anchors the mucin in the plasma membrane, contributing to the molecular composition of the cell surface and to the glycocalyx.11–14 At the human ocular surface, goblet cells in the conjunctiva produce the gel-forming mucin MUC5AC, whereas the stratified epithelia produce the membrane-associated mucins MUC1, −4, and −16.12–14
In this study, we evaluated rose bengal staining in the recently characterized human corneal-limbal epithelial (HCLE) cell line known to produce and glycosylate the membrane-associated mucins MUC1, −4, and −16 but not the goblet cell mucin MUC5AC.15 We hypothesize that it is the cell surface of differentiated cells that is the barrier to rose bengal penetrance and that membrane-associated mucins are a component of the barrier.
Methods
Cell Cultures
Telomerase-immortalized human corneal-limbal epithelial (HCLE) cells and human corneal fibroblasts were plated at a seeding density of 5 × 104 cells/cm2 on culture chamber slides (Laboratory-Tek, Naperville, IL) and maintained at 37°C in 5% CO2. Characterization of HCLE cell cultures has been reported.15 HCLE cultures were grown in a medium optimized for proliferation of keratinocytes (keratinocyte serum-free medium; Invitrogen-Gibco Corp., Grand Island, NY) to achieve confluence. After reaching confluence, cells were switched to DMEM/F12 supplemented with 10% calf serum and 10 ng/mL EGF for 7 days, which promotes stratification of corneal cells and differentiation.15 Primary cultures of human corneal fibroblasts, obtained from James Zieske (Schepens Eye Research Institute, Boston, MA), were grown in DMEM/F12 plus antibiotic-antimycotic 200 mM l-glutamine and 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO).
Cellular Uptake of Rose Bengal
Rose bengal (Aldrich Chemical Co., Milwaukee, WI) was dissolved in PBS (phosphate-buffered saline, Ca+2- and Mg+2-free; pH 7.4), at a concentration of 0.1%. Rose bengal uptake by cell cultures was determined using a modified version of the protocol described by Feenstra et al.16 Briefly, after growing the cells under different conditions, the culture medium was aspirated and the cells rinsed three times with PBS, followed by incubation with rose bengal solution. After 5 minutes of incubation, the rose bengal was aspirated and the cell layer photographed using an inverted microscope (Eclipse TS100; Nikon, Tokyo, Japan). Pictures were taken with a digital camera (SPOT Insight Fire Wire; Diagnostic Instruments, Inc., Sterling Heights, MI).
Divalent cations could associate with charged anionic surface molecules such as mucins17,18 and affect rose bengal uptake. To determine the effect of Ca+2 and Mg+2 ions on rose bengal uptake, cultures of stratified HCLE cells were rinsed three times with PBS, followed by incubation for 5 minutes with 0 to 10 mM CaCl2 or MgCl2 in PBS. HCLE cells were then incubated with rose bengal dissolved in the corresponding CaCl2 or MgCl2 concentration. After removal of the medium, two different areas on each culture chamber were photographed as indicated earlier. Rose bengal-negative areas within the cell culture were quantified using the camera software (SPOT 4.0.1; Diagnostic Instruments, Inc.).
Fluorescence Microscopy
To determine the presence of membrane-associated mucins on HCLE cultures grown at different time points, cells in culture chamber slides were fixed in 4% paraformaldehyde for en face immunofluorescence microscopy, as described elsewhere.15 Briefly, cultures were rinsed in PBS and blocked with PBS with 1% bovine serum albumin (BSA). Cultures were incubated for 1 hour at room temperature with primary antibodies to membrane-associated mucins. The primary antibodies used were HMFG-2 (dilution 1:100; Biodesign, Saco, ME) to a peptide from MUC1, and clone OC125 (dilution 1:50) to a peptidic epitope on MUC16 (Dako Corp., Carpinteria, CA). Cultures were then incubated for 1 hour at room temperature with the fluorescein-conjugated secondary antibody. Slides were coverslipped with fluorescence mounting medium plus propidium iodide (Vectashield; Vector Laboratories, Burlingame, CA) and viewed under a fluorescence microscope.
To determine the presence of O-glycans on membrane-associated mucins on HCLE cultures, cells in culture chamber slides were examined by lectin histochemistry using jacalin, a lectin that specifically recognizes the T-antigen (Galβ1-3GalNAc∝1-Ser/Thr) present on O-glycans.19,20 Cells grown on chamber slides were rinsed in PBS, fixed in 100% methanol for 15 minutes at room temperature, and returned to fresh PBS. Fixed cells were then incubated in blocking buffer consisting of 1% BSA in PBS for 30 minutes. Cultures were then incubated with fluorescein-conjugated jacalin (dilution 1:100) for 1 hour at room temperature, washed in PBS, coverslipped with mounting medium plus propidium iodide, and viewed under a fluorescence microscope. As control, jacalin was preincubated with its hapten-blocking sugar at a concentration of 0.2 M for 20 minutes before application to sections as previously described.21
Cationized Ferritin Binding
Cationized ferritin (CF; Electron Microscopy Sciences, Hatfield, PA) was labeled with fluorescein isothiocyanate (FITC) using the FITC labeling kit (Calbiochem, La Jolla, CA) according to the manufacturer’s instructions. The resultant molar FITC-to-CF ratios obtained varied between 1.8 and 3.0. CF-FITC binding to HCLE cells was determined with a previously described protocol.22 Basically, HCLE cells were incubated for 1 hour at 4°C with 100 μg/mL of CF-FITC in 500 μL of culture medium. After they were washed twice with PBS, the cells were fixed in 4% paraformaldehyde for 30 minutes, washed with PBS, coverslipped with mounting medium plus propidium iodide (Vectashield, Vector Laboratories, Inc.) and photographed.
For MUC16 and CF-FITC colocalization experiments, HCLE cells in culture chamber slides were washed with PBS and incubated for 1 hour at 37°C with a mixture containing the OC125 antibody (dilution 1:25) and CF-FITC (100 μg/mL) in DMEM/F12. Cells were then washed with PBS, fixed in 4% paraformaldehyde, blocked with PBS with 1% BSA and incubated for 1 hour with the rhodamine-conjugated secondary antibody. Slides were coverslipped with antifade medium (Vectashield, Vector Laboratories, Inc.) and viewed under a fluorescence microscope.
Results
Exclusion of Rose Bengal Dye from Stratified Islands of HCLE Cells
Rose bengal uptake was examined in HCLE cells at three different stages during culture: (1) subconfluent, (2) confluent in the absence of serum, and (3) confluent after 7 days of serum supplement. Experiments with cultures of human corneal fibroblasts, which lack the expression of membrane-associated mucins, were performed simultaneously to those of HCLE cells. In subconfluent HCLE cells and corneal fibroblasts, addition of rose bengal (0.1% wt/vol) resulted in rapid staining of the cell cytoplasm and nucleus of all cells (Fig. 2). In both cell lines, the intensity of rose bengal staining in the nucleus was stronger than in the cytoplasm. Rose bengal staining was also observed in all cells in confluent cultures of HCLE cells and corneal fibroblasts before the addition of serum to the culture medium. After culture in medium containing 10% calf serum for 7 days, rose bengal did not penetrate islands of stratified HCLE cells. Although the size and shape of these rose bengal-negative areas was variable, they were predominantly rounded with diameters varying ~50 to 400 μm. As determined by phase-contrast microscopy, cultures of corneal fibroblasts grown for 7 days after confluence in serum also stratified, as evidenced by super imposition of elongated cells running perpendicular to one another (Fig. 2). However, rose bengal penetrated all cells, indicating that corneal fibroblasts lack the protective barrier present in HCLE cells after 7 days in culture in the presence of serum.
Figure 2.
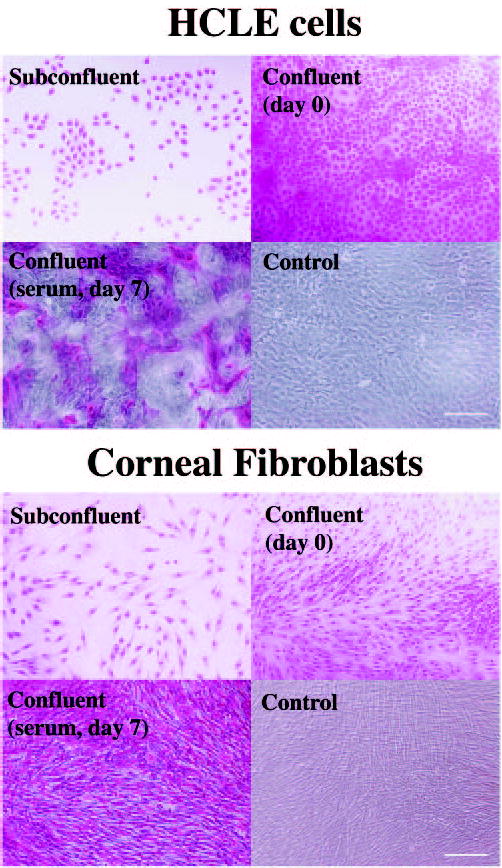
Rose bengal staining in HCLE cells and human corneal fibroblasts at different stages during culture. Islands of rose bengal-negative cells appeared on HCLE cells after 7 days of becoming confluent and after addition of serum. All corneal fibroblasts were rose bengal-positive 7 days after becoming confluent. Control micrographs are phase-contrast images of confluent cells grown in serum for 7 days. Scale bars, 200 μm.
Membrane-Associated Mucins in HCLE Cell Cultures
To determine whether the appearance of islands that excluded rose bengal in HCLE cells correlated with the appearance of glycosylated membrane-associated mucins, the expression of MUC1, MUC16, and the O-linked carbohydrate epitope T-antigen was evaluated in cell cultures grown at different stages. As demonstrated by immunofluorescence, proliferating HCLE cells grown in keratinocyte serum-free medium did not produce MUC1 or MUC16 (Fig. 3A). Jacalin, a lectin recognizing the T-antigen, bound intracellularly to a perinuclear region corresponding to the Golgi apparatus. Once confluent, but in the absence of serum, binding of HMFG-2 to MUC1 was detected to various degrees to some, but not all, round-shaped corneal cells. MUC16 was not detected at this stage, and the T-antigen was observed in the perinuclear region of all cells in culture. After addition of serum for 7 days, MUC1, MUC16, and the T-antigen were detected on the cell surfaces (Fig. 3A). The relative intensity of HMFG-2 binding of MUC1 was decreased compared with the no-serum condition. This could be explained by the susceptibility of the HMFG-2 antibody to sialylation and the fact that stratification and differentiation of HCLE cells may induce MUC1 glycosylation. Treatment with neuraminidase has been shown to increase binding of MUC1 antibodies.23 As demonstrated with the OC125 antibody and jacalin, MUC16 and the T-antigen appeared on the cell surface in islands of flat, stratified HCLE cells, in a pattern similar to those areas in which rose bengal staining was excluded. Antibody to MUC16 bound to the apical cell membranes of these scattered apical cells of the stratified cultured epithelia, as demonstrated by confocal imaging of the cells in cross-section (Fig. 3B). These results on mucin expression are in agreement with those recently reported by Hori et al.,24 who used a telomerase-immortalized human conjunctival epithelial cell line (HCjE). That study demonstrated by Western blot analysis the presence of MUC1 protein before serum addition. MUC16 protein, which was not detected until 12 hours after serum treatment, increased thereafter.24
Figure 3.
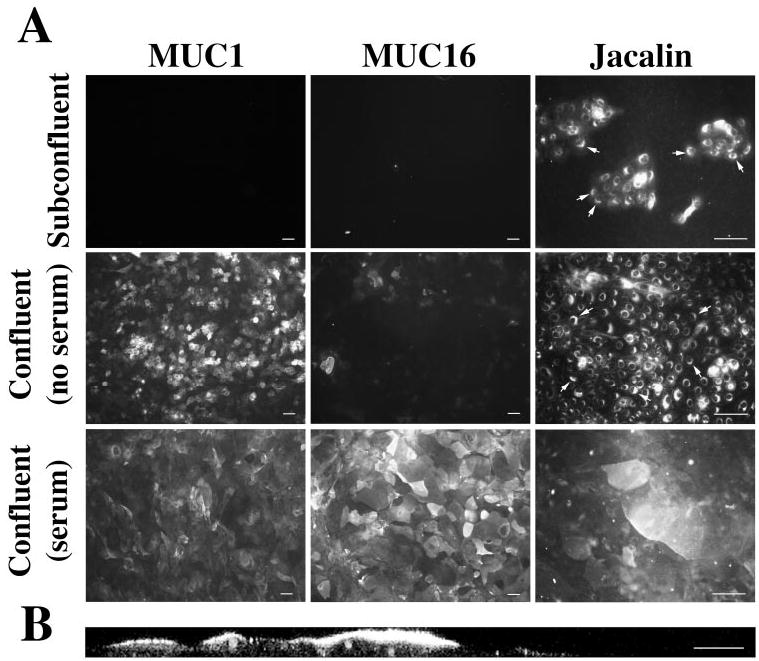
(A) Immunofluorescence analysis of MUC1, MUC16, and the T-antigen in HCLE cells at different stages of differentiation. In confluent HCLE cultures treated for 7 days with serum, MUC16 appeared in islands of stratified cells, in a pattern similar to those areas in which rose bengal staining was excluded. Arrows: indicate perinuclear binding of jacalin recognizing the T-antigen epitope (propidium iodide was included in the mounting medium, to localize the position of the nuclei of cells in culture). (B) Proof that MUC16 is on stratified apical cells is demonstrated by reconstruction of vertical sections from stacked confocal images of the labeled cultures (B is reprinted with permission from Gipson et al. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496 –2506. © Cadmus Professional Communications). Scale bars: (A) 200 μm; (B) 25 μm.
Repeated attempts to colocalize rose bengal staining and membrane-associated mucins in HCLE cells grown for 7 days in serum have been unsuccessful. Not only does rose bengal have excitation and emission spectra that overlap with most commonly used fluorochromes, but rose bengal diffuses out of the cells several minutes after the initial incubation. This diffusion interferes with visualization of the binding of the antibodies to MUC16.
Cationized Ferritin Binding to HCLE Cells and Its Colocalization with MUC16
To determine whether the exclusion of the negatively charged rose bengal dye from HCLE cells after serum addition arises from changes in the anionic character of the cell surface, cultures were labeled with fluoresceinated cationized ferritin (CF-FITC). CF-FITC is a probe commonly used to label anionic sites at the cell surface,25,26 which has been shown to distinguish the poorly differentiated edge cells in islands of airway epithelial cell cultures, containing more negative surface charge, from the central, well-differentiated cells.22 In this work, CF-FITC-binding to HCLE cells was determined at different stages of differentiation and compared to the pattern of rose bengal and mucin staining. All proliferating, nondifferentiated, HCLE cells stained positively for CF-FITC (Fig. 4IA). In these cells, punctate CF-FITC binding appeared at the perinuclear region toward the periphery of the cell. Confluent cultures in the absence of serum displayed a homogeneous CF-FITC binding on the cell surface, indicating the cell’s overall negative charge (Fig. 4IB). Addition of serum for 7 days resulted in a lack of CF-FITC binding to islands of cells, indicating a decrease in anionic sites in these areas (Fig. 4IC). The pattern of CF-FITC binding to HCLE cells at different stages of differentiation was comparable to that of rose bengal staining, suggesting that rose bengal penetrates undifferentiated, negatively charged HCLE cells, and that the expression of the membrane-associated mucin MUC16 in differentiated cells may confer protection against rose bengal uptake.
Figure 4.
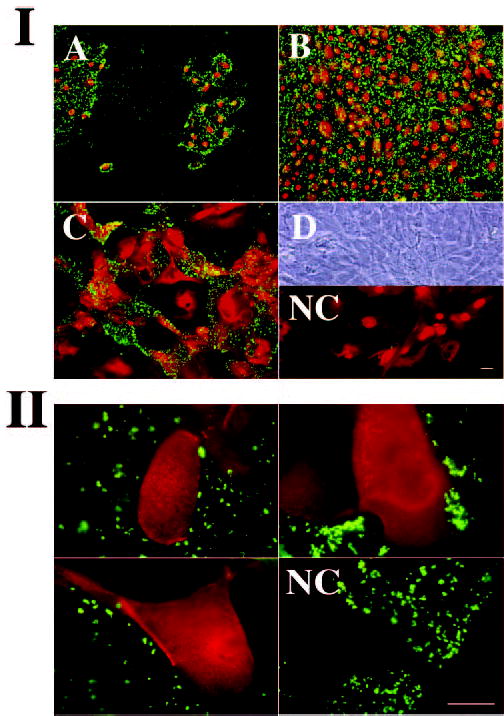
(I) Binding of CF-FITC (green) to HCLE cells at different stages of differentiation. (A) Subconfluent cells; (B) confluent cells (serum-free). (C) Confluent cells (7 days in serum). (D) Phase-contrast corresponding to confluent cells grown under conditions in (C). NC, Negative control (no CF-FITC). Propidium iodide (red) was included in the mounting medium to stain cell nuclei. (II) Colocalization experiments in three different areas of HCLE cell cultures demonstrating exclusion of CF-FITC binding (green) in cells producing the membrane-associated mucin MUC16 (red). NC, negative control (no OC125 antibody). Scale bars, 100 μm.
Colocalization experiments were performed to test whether the HCLE cells cultured 7 days in serum that did not bind CF-FITC, were differentiated cells producing the membrane-associated mucin MUC16. As shown in Figure 4II, when mixtures of the OC125 primary antibody to MUC16 and CF-FITC were applied to HCLE cultures, cells producing the membrane-associated MUC16 mucin in their apical surfaces did not bind CF-FITC. Not all areas in the cell culture that did not bind the OC125 antibody bound CF-FITC, indicating that other mucins or glycoproteins on the cell surface may also prevent CF-FITC binding. These results indicate that MUC16 mucin production is a marker of differentiation in HCLE cells, and that MUC16 expression contributes to protect differentiated corneal cells against rose bengal uptake at the ocular surface.
Effect of Ca+2 and Mg+2 Ions on Rose Bengal Uptake
We took advantage of the ability of HCLE cells to differentiate in culture to determine the effect of increased Ca+2 and Mg+2 ion concentration on rose bengal uptake. After washing the cells with PBS in the absence of CaCl2 or MgCl2, the percentage of area protected against rose bengal uptake in HCLE cell cultures was 26.7% ± 7.4%. Addition of increasing amounts of CaCl2 resulted in a dose-dependent reduction in the area protected against rose bengal uptake (Fig. 5A). Addition of MgCl2 to cell cultures also resulted in reduced protection, although less efficiently than the addition of CaCl2. At 1 mM concentrations, the area protected for CaCl2 and MgCl2 was 2.8% ± 2.3% and 13.2% ± 2.2%, respectively (Fig. 5). The differential effect of Ca+2 and Mg+2 ions on rose bengal uptake may be related to their unique interaction with surface molecules on the cell surface glycocalyx in HCLE cells. Calcium ions have been shown to bind and affect the physicochemical characteristics of mucins.17,18
Figure 5.
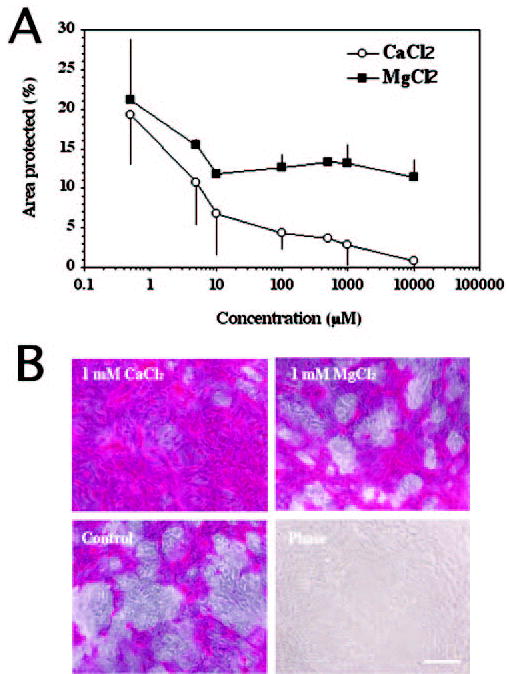
Effect of different concentrations of CaCl2 and MgCl2 on rose bengal uptake. In the absence of CaCl2 or MgCl2, the total area protected was 26.7% ± 7.4%. (A) Quantification of the area protected (%) in HCLE cells against rose bengal uptake after addition of increasing amounts of CaCl2 and MgCl2. Error bars, SEM. (B) Phase contrast images of representative areas after addition of 1 mM CaCl2 and MgCl2. Scale bar, 200 μm.
Discussion
In this study, we have analyzed the factors modulating the penetration of rose bengal into human corneal epithelial cells using a cell culture model that can be manipulated to produce specific membrane-associated mucins and their O-linked carbohydrates. Stratified islands of corneal cells producing membrane-associated mucins, but not human corneal fibroblasts, have the capacity to exclude the rose bengal dye. The appearance of areas of cells that exclude the rose bengal dye after serum addition correlates with the synthesis in their apical surface of the membrane-associated MUC16 mucin and T-antigen carbohydrate, suggesting that this mucin, with its O-linked carbohydrates, plays a role in the protection and control of the local microenvironment at the ocular surface.
Several investigators have suggested that loss of apical glycocalyx and mucin network from discrete areas in patients with dry eye leads to tear film instability and rose bengal staining (for review, see Khan-Lim and Berry27). Two studies in which the monoclonal antibody H185, which recognizes a carbohydrate epitope on MUC16,28,29 was used have shown that the biosynthesis and/or glycosylation of this membrane-associated mucin on the ocular surface is altered in patients with superior limbic keratoconjunctivitis30 and non-Sjögren’s, aqueous-deficient patients with dry eye.31 In the latter study, the researchers found a positive correlation between decreased H185 antibody binding in apical conjunctival epithelial cells and higher rose bengal staining scores in patients with dry eye, indicating that H185-binding pattern was closely related to the severity of dry eye. Using an antibody to a membrane-associated mucin in the glycocalyx, Pflugfelder et al.32 also found a positive correlation between decreased transmembrane mucin expression and increased rose bengal staining in conjunctival impression cytology specimens from patients with aqueous tear deficiency. Although the transmembrane mucin was not identified, the investigators suggested that membrane-associated mucins are necessary for the normal differentiation of ocular surface epithelia and that lack of this class of mucins may contribute to the development of squamous metaplasia.
MUC5AC is the major secreted mucin at the ocular surface, produced by goblet cells in the conjunctiva.33 Several reports have shown that the density of goblet cells and the levels of MUC5AC mucin are significantly reduced in patients with dry eye.32,34 The lack of MUC5AC may contribute to loss of protection of the ocular surface epithelium and to rose bengal staining. The hypothesis that rose bengal staining could be blocked by tear components such as secreted mucin and albumin was tested in rabbit corneal epithelial cells grown in vitro that stain with rose bengal.7 Using Sepharose 4B purified porcine stomach mucin (PSM) in rose bengal assays, Tseng and Zhang8 showed that precoating rabbit corneal epithelial cell layers with PSM blocks rose bengal uptake. Although these experiments suggest that secreted mucin may influence the extent of rose bengal staining, the authors did not evaluate whether the rabbit epithelial cells produced membrane-associated mucins in their apical glycocalyx and whether the concentrations of PSM used in the assay were equivalent to levels of secreted mucin found in human tears. Our results using differentiated HCLE cells, which do not produce MUC5AC, indicate that surface molecules in the glycocalyx are sufficient to establish an intrinsic diffusional barrier that protects the ocular surface epithelia against rose bengal uptake.
The mechanism by which rose bengal crosses cell membranes in mucin-producing epithelial cells is not well understood.27 Poorly differentiated HCLE cells have an overall negative surface charge that binds cationized ferritin. Despite the anionic nature of rose bengal, it diffuses efficiently through this negatively charged surface. It has been hypothesized that negatively charged molecules are repelled and pass quickly through the anionic cell membrane, compared with positively charged molecules, which are attracted to the charge and retained.11 Lack of binding of CF-FITC and the appearance of MUC16 and mucin O-glycans on the apical surface of HCLE cells indicate a relatively late stage of differentiation of HCLE cells. Our data show that MUC16 is produced when areas of rose bengal-negative cells appear in stratified islands of apical cells. MUC16 contains a long glycosylated extracellular amino terminal domain35 that may extend above other membrane-associated mucins and glycoproteins in the glycocalyx, serving as a component of the diffusion barrier that prevents rose bengal penetrance. Treatment with cytochalasin B, which blocks the endocytosis of fluid phase markers by inducing the depolymerization of the actin cytoskeleton,22,36,37 did not affect rose bengal uptake (data not shown), suggesting that its uptake is governed by a passive transport mechanism. This mechanism seems to be different from fluorescein, a structurally related dye. Fluorescein, which has been shown to be a vital dye,16 did not penetrate poorly differentiated HCLE cells (data not shown) compared with rose bengal, indicating that exclusion of rose bengal requires the proper differentiation and maintenance of the integrity of the cell surface.
Osmotic stress caused by an increased extracellular osmolarity is a common feature of dry eye conditions and is the consequence of an increase in tear film evaporation or a decrease in tear secretion.38 Several investigators have attempted to determine whether elevated tear film osmolarity could be correlated with ocular surface disease.38,39 Gilbard and Farris reported a significant positive correlation between tear film osmolarity and rose bengal staining in a group of patients with dry eye disease before and after treatment with isotonic and one-half isotonic saline.40 A recent report has shown in human corneal epithelial cells that hyperosmotic stress stimulates the production of matrix metalloproteinases, which may promote inflammation by cleavage of precursors of proinflammatory factors into their active forms, suggesting that hyperosmotic stress may cause inflammation.41 However, the mechanism(s) by which hyperosmolarity affect surface integrity in ocular surface epithelial cells remains unclear. Compared with Na+ (133.2 ± 0.2 mM), the concentration of Ca+2 (0.80 ± 0.04 mM) and Mg+2 (0.61 ± 0.03 mM) in normal tears is relatively small.38 We hypothesize that an alteration in the balance of Ca+2 and Mg+2 ions in tears produces damage to the ocular surface epithelia as a result of the ions’ specific interactions with the HCLE glycocalyx components, independently of the effect of hyperosmolarity itself. As shown by Gilbard and Rossi,38 lacrimal gland disease results in a 3.5% increase in tear electrolytes, including Ca+2 and Mg+2. The data in the present study show that increased Ca+2 and Mg+2 concentration has an adverse effect on the protection of HCLE cells, perhaps altering the distribution of cell surface components, including the protective membrane-associated mucin MUC16. Several studies have shown that divalent cations interact noncovalently with secreted mucins and that this interaction can alter the structure of mucins.42– 45 Using light-scattering analysis, Varma et al.46 have shown that calcium ions affect the conformation of porcine submaxillary mucin, resulting in a more contracted gel. These calcium-dependent interactions with mucins can also alter the diffusion of molecules through mucus networks, as shown with saliva and respiratory mucin.47,48 At the ocular surface, interaction of cations with membrane-associated mucins may alter the glycocalyx architecture, resulting in damage and reduced tear film stability.
In conclusion, these results suggest a mechanistic link between staining with rose bengal in patients with ocular surface disorders and the pathologic process. As determined in vitro, stratification and differentiation of corneal epithelial cells, as measured by the capacity to produce the membrane-associated MUC16 mucin and the T-antigen O-carbohydrate, provide protection against rose bengal penetrance and suggest a role for membrane-associated mucins and their oligosaccharides in the protection of ocular surface epithelia. On the ocular surface of patients with dry eye, we hypothesize that rose bengal staining occurs in areas of cells either lacking or having an alteration (i.e., glycosylation) of these hydrophilic, membrane-tethered mucins.
Footnotes
Supported by National Eye Institute Grants R01EY014847 (PA) and R01EY03306 (IKG).
Disclosure: P. Argüeso None; A. Tisdale, None; S. Spurr-Michaud, None; M. Sumiyoshi, None; I.K. Gipson, None
References
- 1.Kim J. The use of vital dyes in corneal disease. Curr Opin Ophthalmol. 2000;11:241–247. doi: 10.1097/00055735-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640 – 650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Passmore JW, King JH., Jr Vital staining of conjunctiva and cornea: review of literature and critical study of certain dyes. Arch Ophthalmol. 1954;53:568 –574. [PubMed] [Google Scholar]
- 4.Norn MS. Vital staining of cornea and conjunctiva. Acta Ophthalmol (Copenh) 1962;40:389 – 401. doi: 10.1111/j.1755-3768.1962.tb02387.x. [DOI] [PubMed] [Google Scholar]
- 5.Norn MS. Dead, degenerate, and living cells in conjunctival fluid and mucous thread. Acta Ophthalmol (Copenh) 1969;47:1102–1115. doi: 10.1111/j.1755-3768.1969.tb02509.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Foulks GN. Evaluation of the effect of lissamine green and rose bengal on human corneal epithelial cells. Cornea. 1999;18:328 –332. doi: 10.1097/00003226-199905000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Feenstra RP, Tseng SC. What is actually stained by rose bengal? Arch Ophthalmol. 1992;110:984 –993. doi: 10.1001/archopht.1992.01080190090035. [DOI] [PubMed] [Google Scholar]
- 8.Tseng SC, Zhang SH. Interaction between rose bengal and different protein components. Cornea. 1995;14:427– 435. doi: 10.1097/00003226-199507000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607– 634. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 10.Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6:D1192–D1206. doi: 10.2741/moniaux. [DOI] [PubMed] [Google Scholar]
- 11.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45– 60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 12.Gipson IK, Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1– 49. doi: 10.1016/s0074-7696(03)31001-0. [DOI] [PubMed] [Google Scholar]
- 13.Gipson IK, Hori Y, Argueso P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surface. 2004;2:131–148. doi: 10.1016/s1542-0124(12)70149-0. [DOI] [PubMed] [Google Scholar]
- 14.Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78:379 –388. doi: 10.1016/s0014-4835(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 15.Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496 –2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- 16.Feenstra RP, Tseng SC. Comparison of fluorescein and rose bengal staining. Ophthalmology. 1992;99:605– 617. doi: 10.1016/s0161-6420(92)31947-5. [DOI] [PubMed] [Google Scholar]
- 17.Espinosa M, Noe G, Troncoso C, Ho SB, Villalon M. Acidic pH and increasing (Ca(2+)) reduce the swelling of mucins in primary cultures of human cervical cells. Hum Reprod. 2002;17:1964 –1972. doi: 10.1093/humrep/17.8.1964. [DOI] [PubMed] [Google Scholar]
- 18.Kuver R, Lee SP. Calcium binding to biliary mucins is dependent on sodium ion concentration: relevance to cystic fibrosis. Biochem Biophys Res Commun. 2004;314:330 –334. doi: 10.1016/j.bbrc.2003.12.088. [DOI] [PubMed] [Google Scholar]
- 19.Peumans WJ, Van Damme EJ. Plant lectins: specific tools for the identification, isolation, and characterization of O-linked glycans. Crit Rev Biochem Mol Biol. 1998;33:209 –258. [PubMed] [Google Scholar]
- 20.Arockia Jeyaprakash A, Jayashree G, et al. Structural basis for the energetics of jacalin-sugar interactions: promiscuity versus specificity. J Mol Biol. 2005;347:181–188. doi: 10.1016/j.jmb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe H, Gipson IK. Detection of blood group differences in human corneal epithelium using a monoclonal antibody and lectins. Arch Ophthalmol. 1994;112:667– 673. doi: 10.1001/archopht.1994.01090170111032. [DOI] [PubMed] [Google Scholar]
- 22.Matsui H, Johnson LG, Randell SH, Boucher RC. Loss of binding and entry of liposome-DNA complexes decreases transfection efficiency in differentiated airway epithelial cells. J Biol Chem. 1997;272:1117–1126. doi: 10.1074/jbc.272.2.1117. [DOI] [PubMed] [Google Scholar]
- 23.Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK. Human corneal and conjunctival epithelia express MUC1 mucin. Invest Ophthalmol Vis Sci. 1995;36:1818 –1827. [PubMed] [Google Scholar]
- 24.Hori Y, Spurr-Michaud S, Russo CL, Argueso P, Gipson IK. Differential regulation of membrane-associated mucins in the human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2004;45:114 –122. doi: 10.1167/iovs.03-0903. [DOI] [PubMed] [Google Scholar]
- 25.King CA, Preston TM. Fluoresceinated cationised ferritin as a membrane probe for anionic sites at the cell surface. FEBS Lett. 1977;73:59 – 63. [PubMed] [Google Scholar]
- 26.Danon D, Goldstein L, Marikovsky Y, Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972;38:500 –510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- 27.Khan-Lim D, Berry M. Still confused about rose bengal? Curr Eye Res. 2004;29:311–317. doi: 10.1080/02713680490516864. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe H, Fabricant M, Tisdale AS, Spurr-Michaud SJ, Lindberg K, Gipson IK. Human corneal and conjunctival epithelia produce a mucin-like glycoprotein for the apical surface. Invest Ophthalmol Vis Sci. 1995;36:337–344. [PubMed] [Google Scholar]
- 29.Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003;44:2487–2495. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe H, Maeda N, Kiritoshi A, Hamano T, Shimomura Y, Tano Y. Expression of a mucin-like glycoprotein produced by ocular surface epithelium in normal and keratinized cells. Am J Ophthalmol. 1997;124:751–757. doi: 10.1016/s0002-9394(14)71691-5. [DOI] [PubMed] [Google Scholar]
- 31.Danjo Y, Watanabe H, Tisdale AS, et al. Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci. 1998;39:2602–2609. [PubMed] [Google Scholar]
- 32.Pflugfelder SC, Tseng SC, Yoshino K, Monroy D, Felix C, Reis BL. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology. 1997;104:223–235. doi: 10.1016/s0161-6420(97)30330-3. [DOI] [PubMed] [Google Scholar]
- 33.Argueso P, Gipson IK. Epithelial mucins of the ocular surface: structure, biosynthesis and function. Exp Eye Res. 2001;73:281–289. doi: 10.1006/exer.2001.1045. [DOI] [PubMed] [Google Scholar]
- 34.Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004 –1011. [PubMed] [Google Scholar]
- 35.O’Brien TJ, Beard JB, Underwood LJ, Shigemasa K. The CA 125 gene: a newly discovered extension of the glycosylated N-terminal domain doubles the size of this extracellular superstructure. Tumour Biol. 2002;23:154 –169. doi: 10.1159/000064032. [DOI] [PubMed] [Google Scholar]
- 36.Pratten MK, Lloyd JB. Pinocytosis and phagocytosis: the effect of size of a particulate substrate on its mode of capture by rat peritoneal macrophages cultured in vitro. Biochim Biophys Acta. 1986;881:307–313. doi: 10.1016/0304-4165(86)90020-6. [DOI] [PubMed] [Google Scholar]
- 37.Gottlieb TA, Ivanov IE, Adesnik M, Sabatini DD. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993;120:695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbard JP, Rossi SR. Changes in tear ion concentrations in dry-eye disorders. Adv Exp Med Biol. 1994;350:529 –533. doi: 10.1007/978-1-4615-2417-5_89. [DOI] [PubMed] [Google Scholar]
- 39.Gilbard JP, Farris RL, Santamaria J., II Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch Ophthalmol. 1978;96:677– 681. doi: 10.1001/archopht.1978.03910050373015. [DOI] [PubMed] [Google Scholar]
- 40.Gilbard JP, Farris RL. Tear osmolarity and ocular surface disease in keratoconjunctivitis sicca. Arch Ophthalmol. 1979;97:1642–1646. doi: 10.1001/archopht.1979.01020020210003. [DOI] [PubMed] [Google Scholar]
- 41.Li DQ, Chen Z, Song XJ, Luo L, Pflugfelder SC. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:4302– 4311. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- 42.Forstner JF, Forstner GG. Calcium binding to intestinal goblet cell mucin. Biochim Biophys Acta. 1975;386:283–292. doi: 10.1016/0005-2795(75)90270-6. [DOI] [PubMed] [Google Scholar]
- 43.Marriott C, Shih CK, Litt M. Changes in the gel properties of tracheal mucus induced by divalent cations. Biorheology. 1979;16:331–337. doi: 10.3233/bir-1979-164-507. [DOI] [PubMed] [Google Scholar]
- 44.Steiner CA, Litt M, Nossal R. Effect of Ca++ on the structure and rheology of canine tracheal mucin. Biorheology. 1984;21:235–252. doi: 10.3233/bir-1984-211-226. [DOI] [PubMed] [Google Scholar]
- 45.Paz HB, Tisdale AS, Danjo Y, Spurr-Michaud SJ, Argueso P, Gipson IK. The role of calcium in mucin packaging within goblet cells. Exp Eye Res. 2003;77:69 –75. doi: 10.1016/s0014-4835(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 46.Varma BK, Demers A, Jamieson AM, Blackwell J, Jentoft N. Light scattering studies of the effect of Ca2+ on the structure of porcine submaxillary mucin. Biopolymers. 1990;29:441– 448. doi: 10.1002/bip.360290214. [DOI] [PubMed] [Google Scholar]
- 47.Raynal BD, Hardingham TE, Sheehan JK, Thornton DJ. Calcium-dependent protein interactions in MUC5B provide reversible cross-links in salivary mucus. J Biol Chem. 2003;278:28703–28710. doi: 10.1074/jbc.M304632200. [DOI] [PubMed] [Google Scholar]
- 48.Verdugo P, Aitken M, Langley L, Villalon MJ. Molecular mechanism of product storage and release in mucin secretion. II. The role of extracellular Ca++ Biorheology. 1987;24:625– 633. doi: 10.3233/bir-1987-24615. [DOI] [PubMed] [Google Scholar]


