Abstract
Purpose
Oncolytic replication-competent herpes simplex virus type-1 (HSV) mutants have the ability to replicate in and kill malignant cells. We have previously demonstrated the ability of replication-competent HSV to control bladder cancer growth in an orthotopic murine model. We hypothesized that a combination of a chemotherapeutic agent used for intravesical treatment - mitomycin-C (MMC) - and oncolytic HSV would exert a synergistic effect in the treatment of human transitional cell carcinoma (TCC).
Materials and Methods
We used the mutant HSV NV1066, which is deleted for viral genes ICP0 and ICP4 and selectively infects cancer cells, to treat TCC lines, KU19-19 and SKUB. Cell survival was determined by lactate dehydrogenase (LDH) assay for each agent as well as for drug-viral combinations from days 1 to 5. The isobologram method and the combination index method of Chou-Talalay were used to assess for synergistic effect.
Results
NV1066 enhanced MMC mediated cytotoxicity at all combinations tested for both KU19-19 and SKUB. Combination of both agents demonstrated a synergistic effect and allowed dose reduction by 12 and 10.4 times (NV1066) and by 3 and 156 times (MMC) in the treatment of KU19-19 and SKUB respectively, while achieving an estimated 90% cell kill.
Conclusion
These data provide the cellular basis for the clinical investigation of combined mitomycin-C and oncolytic HSV therapy in the treatment of bladder cancer.
Keywords: Mitomycin, bladder neoplams, gene therapy, herpes simplex virus 1 Human, drug synergism, carcinoma, transitinal cell
Background
Superficial bladder cancer patients are currently treated primarily by surgical means with adjuvant local chemotherapy or immune therapy for the prevention of local disease recurrence 1, 2. Nevertheless, much is still needed to be done to improve our current rate of cure for patients with bladder cancer. It is estimated that 57,400 new patients will be diagnosed each year in the USA alone and that 12,500 will die from their cancer 3. Of the newly diagnosed patients, 70% will suffer from superficial bladder cancer 4 with a recurrence rate of 50–80% and a progression rate of 10–20%, both of which necessitate a rigorous follow-up schedule.
Different therapeutic approaches have been developed in an effort to decrease the recurrence rate of patients diagnosed with superficial bladder cancer. Of the chemotherapeutic agents used, mitomycin-C (MMC), when applied locally as an immediate single dose treatment, has demonstrated the capability of reducing the rate of early recurrences after trans-urethral resection of bladder tumor (TURBT) 1, 5. However the incidence of early recurrence is high (22.2% recurrence in the first year) 1 even when combined treatment modality is applied. In an effort to lower recurrence rate research has been focused on novel therapies.
Non-replicating viruses such as Adeno and Adeno-associated viruses and herpes simplex viruses (HSV) along with retroviruses (murine leukemia, avian leucosis and spleen necrosis viruses) are one of the modalities tested 6. Unlike replication defective vectors they can efficiently disseminate within tumor tissue 7, however they may pose a safety concern. Among the viruses studied, HSV based therapy has several advantages including the high rate of previous exposure in the general population (up to 84%) 8, lowering the likelihood of a full systemic infection, as well as the present availability of effective antiviral treatment.
Replication-competent oncolytic HSV therapy has been demonstrated to be effective in limiting tumor growth in both in vitro and in vivo animal models of different human cancers 9–12. Recent reports demonstrated a synergistic effect in the treatment of non small cell lung oncolytic herpes simplex virus (HSV) and MMC12.
We therefore hypothesized that a combination of chemotherapeutic agents currently used for intravesical treatment - mitomycin-C (MMC) and an oncolytic HSV - would exert a synergistic effect in the treatment of human transitional cell carcinoma (TCC).
Materials and Methods
Cells
The human bladder cancer cell line SKUB was isolated and characterized at Memorial Sloan-Kettering Cancer Center from a primary transitional cell carcinoma of the bladder 11. The human transitional cell carcinoma cell line Ku-19-1913 was a kind gift from the laboratory of Professor Masaru Murai, Chairman of Department of Urology, Keio University School of Medicine. Both cell lines were grown in RPMI 1640 medium, supplemented with FBS, 100 U/ml penicillin and 100 mg/ml streptomycin.
Virus
NV1066 is a replication-competent attenuated herpes simplex-1 mutant virus provided by Medigene, Inc. (San Diego, CA), whose structure has been described elsewhere 14. NV1066 contains the enhanced green fluorescent protein (eGFP) sequence under the control of a cytomegalovirus (CMV) promoter. Virus stocks were generated using standard techniques 11, and the structure of the recombinant virus was confirmed by Southern blotting and sequencing (data not shown).
In vitro cytotoxicity assay
Two x 104 KU-19-19 and SKUB cells were plated in 12-well flat-bottom plates (Becton Dickinson, Franklin Lakes, NJ). The cells were exposed for 2 hours to MMC (0.36–10.0 μGr/ml, Bristol-Myers Squibb Oncology, Princeton, New Jersey 08543, USA). Following exposure, the medium was replaced and, subsequently, cells were infected with NV1066 at multiplicities of infection (MOI) of 0.05 to 1.0. Untreated cells and cells exposed only to virus or to MMC, served as the control.
At day 1 after infection and every day thereafter, cells were washed in PBS and lysed with a 1.35% Triton-X solution, and a lactate dehydrogenase (LDH) assay was performed. The cytotoxicity results of the three treatment arms of tumor cells are expressed as the surviving fraction, based on the percentage of the LDH released (conversion of a tetrazolium salt into a formazan product) as detected by the CytoTox96® (Promega Corporation, WI, USA), compared to that of untreated, control cells. A microplate reader (EL 312e: Bio-Tek Instruments, Winooski VT) was used to evaluate the presence of LDH as a color development measured by absorbance spectroscopy at 450 nm. Background optical density (OD) was subtracted from the OD readings of all samples. Cell viability was calculated by dividing the mean OD absorbance of the treated wells by the mean OD absorbance of the control wells. All samples were tested in triplicate.
Viral Titering
Two x104 cells were plated in 12-well plates and exposed to mitomycin-C 0.16 μGr/ml for two hours and then infected with NV1066 at MOI of 0.1. The supernatant was harvested from wells treated with combination therapy or virus alone every other day starting from day one after infection. Serial dilutions were made of the suspension, and viral plaques were grown and counted on confluent Vero cells in a standard viral plaque assay. All samples were tested in triplicate.
Statistical analysis
Data were analyzed using repeated measures multivariate analysis of variance. P<0.05 is considered statistically significant. All analyses were performed with Statistical Analysis Systems version 8.2 software (SAS Institute, Cary, NC).
Quantitative analysis of synergy
The multiple drug effect analysis of Chou and Talalay was used to determine the pharmacologic interaction between NV1066 and mitomycin-C. This analysis defines synergism or antagonism by determining how much the combination effect differs from the expected additive effect of the two therapeutic agents. The method has been described elsewhere 12, 15. Such an analysis involves plotting dose-effect curves for each therapy and multiplying diluted combinations of the therapies using the “median effect” equation.
A combination index (CI) is then determined. When the CI = 1, the interaction is considered additive. When CI <1 synergy is indicated and when CI >1 antagonism is indicated.
Data were also analyzed by the dose oriented isobologram technique. The axis on an isobologram represent the doses of each drug. Two points on the x and y axis are chosen that correspond to the doses of each drug necessary to generate that particular Fa value. The straight line (hypotenuse) drawn between these two points on the x- and y-axis corresponds to a strictly additive interaction between two therapeutic agents. If the point lies to the lower left of the hypotenuse, the effect is synergistic, and if the point lies to the upper right of the hypotenuse the effect is antagonistic. Another calculation available using the combination index method is the dose-reduction index (DRI). The DRI is a determination of the fold of dose reduction allowed for each drug when given in synergistic combination, as compared to the concentration of single agent that is needed to achieve the same effect level.
Vector Spread Assay
Vector propagation as analyzed by eGFP expression was determined by FACS analysis at a viral infective dose of MOI 0.01. One group was exposed to 0.1 μGr/ml mitomycin-C for 2 hours followed by infection with NV1066, while the other was only treated by NV1066. The GFP-positive live cells at 24 and 96 hours after treatment were expressed as percentage of all cells in the sample. Cells were harvested with 0.25% trypsin in PBS, centrifuged, washed in PBS, and brought up in 100 μl of PBS. Five μl of 7-amino-actinomycin (7-AAD, BD Pharmingen, San Diego, CA) was added as an exclusion dye for cell viability. Data for EGFP expression was acquired on a FACS Calibur machine equipped with Cell Quest software (Becton Dickinson, San Jose, CA). All samples were performed in triplicate.
Results
Cytotoxicity of mitomycin-C and NV1066
KU 1919 cells were exposed to 3 dosages of mitomycin-C (1,2,3 μGr/ml) and NV1066 at three different MOI’s (0.05, 0.1, 0.5) to evaluate dose dependent cytotoxicity (Figure 1A–C) . The cell kill for mitomycin-C and NV 1066 was noticeable starting at day 3. Repeated measures multivariate analysis of variance demonstrated statistically significant difference between treatment arms in all nine possible combination treatments at day 3–5 (Data shown in Figures 1C is most representative, a P value of less than 0.001 was obtained in all nine combinations tested).
Figure 1. Cytotoxicity of mitomycin-C and NV1066 in the treatment of KU19-19.

NV1066 in 3 different multiplicities of infection (MOI 0.05, 0.1, 0.5 Figure 1a) and 3 dosage of mitomycin-C (Figure 1b, MMC- 1,2,3 μGr/ml for 2 hours -2H) demonstrated dose-dependent cytotoxicity. The cell kill for mitomycin-C and NV1066 was notable from day 3. Repeated measures multivariate analysis of variance demonstrated statistically significant difference between treatment arms in all nine possible combination treatments in day 3 and 4 (Data shown in Figures 1C is most representative, however a P value of less than 0.001 was obtained in all 9 combinations tested).
Chou-Talalay analysis
The cell kill at day 4 demonstrated CI values of all nine possible combinations to be lower than 1 for both mitomycin (Table 1) and virus. Based on the study we determined that a ratio of 1:10 and 1:20 NV1066 to mitomycin-C would exert the strongest synergistic effect for cytotoxicity of the KU19-19 line.
Table- 1. Chou-Talalay analysis- Combination Index for 9 combination treatments.
Cell kill at day 4 (1- [cell survival in treated wells/cell survival in control wells]) demonstrated combination index (CI) values of all nine possible combination to be lower than 1 for both mitomycin-C (MMC. μGr/ml) and virus (NV1066, multiplicity of infection- MOI), however a combination of 1:10 (NV1066: MMC) demonstrated the lowest CI (0.35) and therefore the strongest synergistic effect.
| MMC (μgm/ml) | NV1066 (MOI) | Cell Kill | Combination Index |
|---|---|---|---|
| 1.0 | 0.05 | 0.67 | 0.41 |
| 1.0 | 0.1 | 0.75 | 0.35 |
| 1.0 | 0.5 | 0.84 | 0.43 |
| 2.0 | 0.05 | 0.71 | 0.71 |
| 2.0 | 0.1 | 0.75 | 0.64 |
| 2.0 | 0.5 | 0.84 | 0.62 |
| 3.0 | 0.05 | 0.73 | 0.95 |
| 3.0 | 0.1 | 0.78 | 0.83 |
| 3.0 | 0.5 | 0.84 | 0.83 |
In order to allow the construction of the isobologram plot, we tested the cytotoxicity of mitomycin-C (0.5, 1, 5 or 10 μGr/ml) and NV 1066 (MOI’s 0.05, 0.1, 0.5 or 1) alone, as well as with four combination treatments with a fixed dose ratio of 1:10 (NV1066: mitomycin-C, respectively). Cytotoxicity was determined at day 5. Combination therapy demonstrated better cell kill compared to either individual treatment (Figure 2A,B,C).
Figure 2. combination treatment of KU19-19 at a constant ratio of 1:10 (NV1066: mitomycin-C).

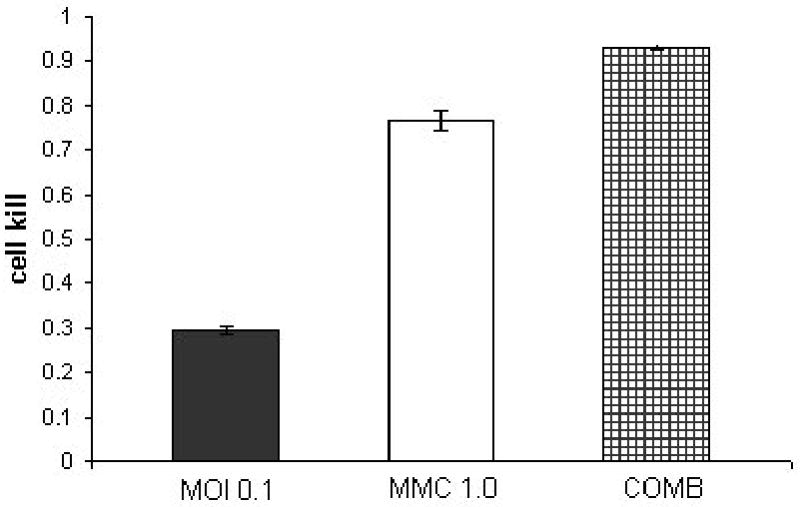
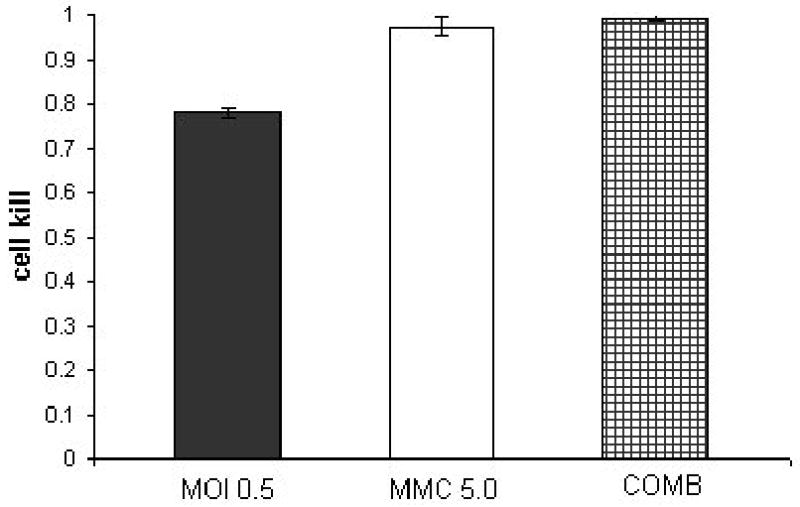
KU19-19 cell line treated with a combination of NV1066 in three different multiplicities of infection (MOI 0.05, 0.1, 0.5) and three dosages of mitomycin-C (MMC- 0.5,1,5 μGr/ml) at a constant dose ratio of 1:10. Y axis demonstrates the cell kill as calculated by 1% of control cell survival. The synergistic effect of the combined treatment (COMB) at day 5 after treatment is most notable in the lower doses of treatment.
With the experience we gained with KU19-19, we studied the effect of combined therapy for a second human TCC cell line (SKUB). Cytotoxicity of mitomycin-C (0.1, 0.5, 1 μGr/ml), NV 1066 (MOI’s 0.01, 0.05, 0.1) and all three combinations with a dose ratio of 1:10 (NV1066 to mitomycin-C respectively) were examined. Cytotoxicity and synergy were examined at two time points (Figure 3A,B,C present the data for day 4) Combined treatment proved to be superior to either virus or MMC treatment alone at both time points (day 3 and day 4).
Figure 3. Combination treatment of SKUB at a constant ratio of 1:10 (NV1066 : mitomycin-C).
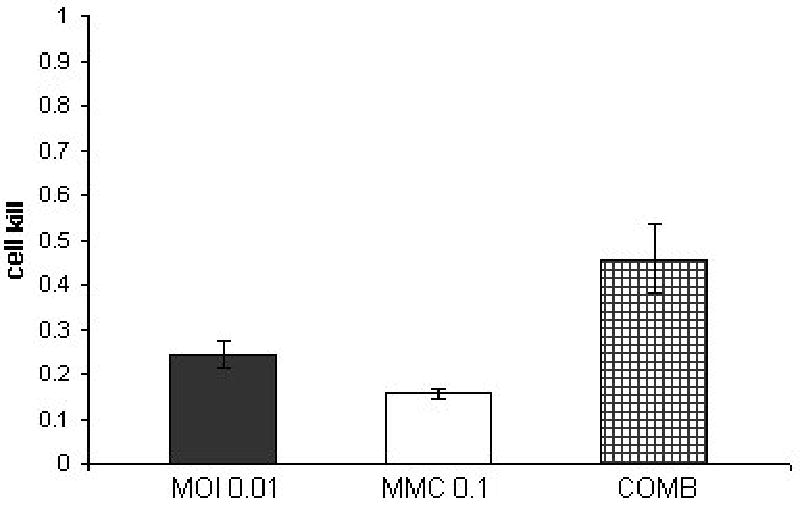
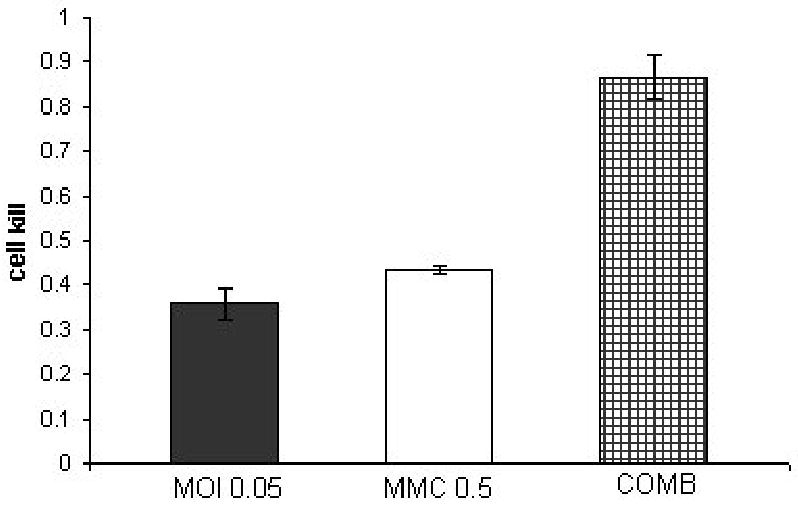

SKUB cell line treated with a combination of NV1066 in three different multiplicities of infection (MOI 0.01, 0.05, 0.1) and three dosages of mitomycin-C (MMC-0.1,0.5,1 μGr/ml) at a constant dose ratio of 1:10. Y axis demonstrates the cell kill as calculated by 1% of control cell survival. The synergistic effect of the combined treatment (COMB) at day 4 after treatment is better then each treatment alone.
In both cell lines a dose effect plot was created for each agent from which the lethal dose causing 50, 75, 90 and 95 and 97% cell kill was calculated. This allows the extrapolation of the CI – effect (Fa) curves for mitomycin-C and NV 1066 , both demonstrating that each agents when given in combination gain a CI value of lower than one and therefore demonstrating synergism. The isobologram demonstrated that a combination treatment achieved a synergistic effect form the expected cell kill from each agents (Figure 4). A drug reduction table demonstrates the amount of reduced dosage that can be achieved when a combination is used (Table 2).
Figure 4. Isobolograms demonstrate synergism of mitomycin-C and NV1066 for both KU19-19 (a) and SKUB (b) cell lines.
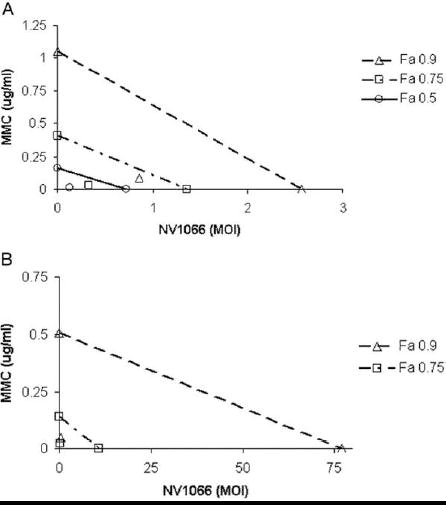
The doses of MMC (μGr/ml ) and NV1066 (multiplicity of infection- MOI) necessary to achieve 90% cell kill (open triangles) and 75% cell kill (open square) are plotted on the axes, and the connecting solid lines represent the expected additive effects for combination therapy. Experimental combination therapy doses necessary to generate actual LD values of 90% (open triangle FA=0.9), 75% (open squares FA=0.75) and 50% (open circle Fa=0.5) all lie to the lower left of the corresponding lines, indicating synergism. LD50 for SKUB cell line is not shown in the Figure B because of very low doses of each agent that can not be represented on the graph.
Table 2. Drug Reduction Index (DRI) for KU19-19 and SKUB cell lines.
Dose reduction index (DRI) is the –fold of dose reduction possible to achieve the same cell kill if mitomycin-C and virus are used in combination. Fraction affected (Fa) is the LDx = Lethal dosex, dose needed to produce cell kill of x%, MOI = multiplicity of infection, CI = combination index.
| DRI (fold) | ||||
|---|---|---|---|---|
| NV1066 (MOI) | MMC alone (μgm/ml) | NV1066 | MMC | |
| KU1-19 Fa: | ||||
| LD50 | 0.16 | 0.72 | 12.4 | 5.5 |
| LD75 | 0.41 | 1.36 | 12.4 | 4.1 |
| LD90 | 1.05 | 2.57 | 12.4 | 3.0 |
| LD97 | 3.1 | 5.37 | 12.3 | 2.1 |
| SKUB cell kill (% control): | ||||
| LD50 | 0.043 | 1.56 | 3.6 | 13 |
| LD75 | 0.14 | 10.9 | 6.1 | 45 |
| LD90 | 0.51 | 76.9 | 10.4 | 156 |
| LD97 | 2.15 | 743 | 19.1 | 662 |
Treatment with MMC did not reduce the viral replication as demonstrated by the viral growth curves (Figure 5) in both combined treatment and treatment with virus alone. Vector spread assay evaluating the expression of eGFP (by FACS), as a marker of viral infection, found a higher percentage of living cells expressing eGFP in the combination treatment compared to virus alone (24.3% Vs. 7.8% and 44.4% Vs. 40.7 at 24 and 96 hours after treatment - Figure 6).
Figure 5. Viral plaque assay KU19-19 treated with NV1066 and mitomycin-C.
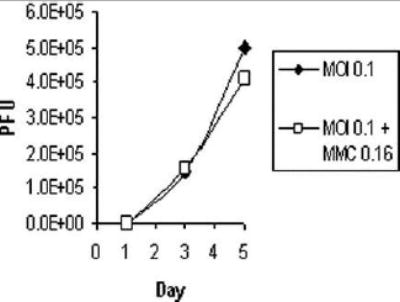
Ku19-19 cells, plated at 2 * 104 cells per well, with (MOI 0.1+MMC 0.16) or without (MOI 0.1) 2 hour exposure to 0.16 μGr/cc mitomycin-C (MMC). Cells were all subsequently infected with NV1066 at multiplicity of infection (MOI) of 0.1, viral plaques were grown and counted on confluent Vero cells in a standard viral plaque assay, results are expressed as number of plaque forming units (pfu).
Figure 6. GFP expression in live KU19-19 cells at 24 and 96 hours after infection.
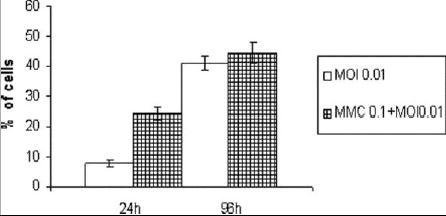
Ku19-19 Cells plated at 2 * 104 cells per well, with (MMC 0.1+MOI 0.01) or without (MOI 0.01) 2 hours exposure to 0.16 μGr/cc mitomycin-C (MMC) and subsequently infected with NV1066 at multiplicity of infection (MOI) of 0.01. The green fluorescent protein (GFP)-positive live cells at 24 and 96 hours after treatment were expressed as percentage of all cells in the sample. 7-amino-actinomycin (7-AAD) was added as an exclusion dye for cell viability.
Discussion
Current guidelines call for the treatment of all high grade, T1 or Cis superficial bladder cancer patients with adjuvant intravesical treatment 16. Mitomycin-C is a natural anti-tumor antibiotic, used clinically as a chemotherapy agent. The bioreductive activation of MMC allows for alkalization of guanine residues leading to DNA inter and intra-strand cross-linking 17. Two randomized prospective trials have demonstrated that immediate MMC instillation after TURT lowers the recurrence rate and increases recurrence free time for treated patients 1, 5. However, unlike BCG there is no evidence that MMC can reduce the overall disease progression 16.
Several approaches have been reported to increase the anti-tumor activity of MMC, among them combined treatment with BCG , hyperthermia 18 and 5-aminolevolaevulinic based photodynamic therapy 19.
We have previously demonstrated the efficacy of oncolytic HSV based therapy for both in vitro and in vivo bladder cancer models 11. In this study, we seek to determine the synergistic effect between MMC and oncolytic HSV in the treatment of human bladder cancer cell lines. Such synergism was demonstrated in lung cancer cell line by Toyoizumi et al12.
The strain of HSV used (NV1066), has the transgene of eGFP under the control of a CMV promoter, which acts as a reporter gene. When combining MMC and NV1066 in nine different combinations, a significantly improved cell kill for the combination treatment was demonstrated in KU19-19 cell line, (P<0.001). In order to assess for synergy, we used the combination index and isobologram methods of Chou and Talalay. This type of analysis determines synergy based on extrapolated equation and is an acceptable method for assessing efficacy for chemotherapy combination therapies as well as combination therapy of chemotherapy and viral therapy 12, 15, 20. Based on the nine different combination ratios tested, we determined that a ratio of 1:10 NV1066 to mitomycin-C conveyed the strongest synergistic effect, as the combination index achieved for this ratio was the lowest. We studied the effect of combination treatment on two cell lines, KU19-19 and SKUB using a 1:10 ratio. Based on these data, a significant dose-reduction-index (DRI) was demonstrated (Figure 4, Table-2). DRI is an important parameter in determining the clinical use of combination therapy because it shows the potential benefit of dose reduction and subsequent lower toxicity without sacrificing any therapeutic effect. The synergistic interaction between NV1066 and MMC may provide an opportunity to eradicate bladder cancer microscopic deposits that each agent alone at acceptable dosage cannot reach. However it is difficult to assess in an in vitro study whether this synergistic effect can translate not only to dose reduction but more importantly in the case of TCC, a lower rate of recurrence and progression.
The transgene of eGFP under the control of a CMV inserted into NV1066 can enable the identification of small tumor deposits as demonstrated by Stiles et al 9 for esophageal cancer. Therefore, NV1066 might allow both treatment and diagnosis of residual disease that could be identified and resected in a follow up TURBT.
We assessed for eGFP expression by both florescent microscopy, in which more cells exposed to combination treatment expressed eGFP per high magnification filed (data not shown) and by the Vector spread assay evaluating the expression of eGFP in living cells with a FACS analysis (Figure 6). At 24 hours combination therapy yielded a higher percent of eGFP expression. However, due to the nature of replication competent viruses, at a later time point this advantage tended to disappear, penetrance of the rapidly replicating virus was high in both conditions eliminating any measurable difference. Viral growth demonstrated that MMC does not interfere with viral replication and proliferation (Figure 5).
Conclusions
HSV therapy synergistically enhanced the cytotoxicity of MMC in the treatment of two bladder cancer cell lines (KU19-19 and SKUB). This synergistic effect allowed considerable dose reduction for both agents in both cell lines. The data provide the cellular basis for the clinical investigation of combined use of mitomycin-C with oncolytic HSV therapy in the treatment of superficial bladder cancer. Both agents can be instilled intravesically following trans-urethral resection of bladder tumor, to achieve synergistic efficacy while minimizing dosage and toxicity.
Footnotes
Supported by: AACR-AstraZeneca Cancer Research and Prevention grant (P.S.A), grants R01 CA 76416 and R01 CA/DK80982 (Y.F) from the National Institutes of Health, grant MBC-99366 (Y.F) from the American Cancer Society, grant BC024118 from the US Army (Y.F), grant IMG0402501 from the Susan Komen Foundation (Y.F) and grant 032047 from Flight Attendant Medical Research Institute (Y.F and P.S.A).
References
- 1.Solsona E, Iborra I, Ricos JV, Monros JL, Casanova J, Dumont R. Effectiveness of a single immediate mitomycin C instillation in patients with low risk superficial bladder cancer: short and long-term followup. J Urol. 1999;161:1120. [PubMed] [Google Scholar]
- 2.Sylvester RJ, van der MA, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Facts and Figures 2003American Cancer Society, vol. 2003
- 4.Malkowicz S: Managment of Superficial BladderCancer, 8 ed: Saunders, p. 2785, 2002
- 5.Tolley DA, Parmar MK, Grigor KM, Lallemand G, Benyon LL, Fellows J, et al. The effect of intravesical mitomycin C on recurrence of newly diagnosed superficial bladder cancer: a further report with 7 years of follow up. J Urol. 1996;155:1233. [PubMed] [Google Scholar]
- 6.Galanis E, Vile R, Russell SJ. Delivery systems intended for in vivo gene therapy of cancer: targeting and replication competent viral vectors. Crit Rev Oncol Hematol. 2001;38:177. doi: 10.1016/s1040-8428(01)00103-2. [DOI] [PubMed] [Google Scholar]
- 7.Logg CR, Logg A, Matusik RJ, Bochner BH, Kasahara N. Tissue-specific transcriptional targeting of a replication-competent retroviral vector. J Virol. 2002;76:12783. doi: 10.1128/JVI.76.24.12783-12791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pebody RG, Andrews N, Brown D, Gopal R, De Melker H, Francois G, et al. The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sex Transm Infect. 2004;80:185. doi: 10.1136/sti.2003.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stiles BM, Bhargava A, Adusumilli PS, Stanziale SF, Kim TH, Rusch VW, et al. The replication-competent oncolytic herpes simplex mutant virus NV1066 is effective in the treatment of esophageal cancer. Surgery. 2003;134:357. doi: 10.1067/msy.2003.244. [DOI] [PubMed] [Google Scholar]
- 10.Cozzi PJ, Burke PB, Bhargav A, Heston WD, Huryk B, Scardino PT, et al. Oncolytic viral gene therapy for prostate cancer using two attenuated, replication-competent, genetically engineered herpes simplex viruses. Prostate. 2002;53:95. doi: 10.1002/pros.10138. [DOI] [PubMed] [Google Scholar]
- 11.Cozzi PJ, Malhotra S, McAuliffe P, Kooby DA, Federoff HJ, Huryk B, et al. Intravesical oncolytic viral therapy using attenuated, replication-competent herpes simplex viruses G207 and Nv1020 is effective in the treatment of bladder cancer in an orthotopic syngeneic model. Faseb J. 2001;15:1306. doi: 10.1096/fj.00-0533fje. [DOI] [PubMed] [Google Scholar]
- 12.Toyoizumi T, Mick R, Abbas AE, Kang EH, Kaiser LR, Molnar-Kimber KL. Combined therapy with chemotherapeutic agents and herpes simplex virus type 1 ICP34.5 mutant (HSV-1716) in human non-small cell lung cancer. Hum Gene Ther. 1999;10:3013. doi: 10.1089/10430349950016410. [DOI] [PubMed] [Google Scholar]
- 13.Steube KG, Meyer C, Tachibana M, Murai M, Drexler HG. Bladder carcinoma cell line KU-19-19-derived cytokines support proliferation of growth factor-dependent hematopoietic cell lines: modulation by phorbol ester, interferon-gamma and interleukin-1 beta. Biochem Biophys Res Commun. 1998;242:497. doi: 10.1006/bbrc.1997.8002. [DOI] [PubMed] [Google Scholar]
- 14.Wong RJ, Joe JK, Kim SH, Shah JP, Horsburgh B, Fong Y. Oncolytic herpesvirus effectively treats murine squamous cell carcinoma and spreads by natural lymphatics to treat sites of lymphatic metastases. Hum Gene Ther. 2002;13:1213. doi: 10.1089/104303402320138998. [DOI] [PubMed] [Google Scholar]
- 15.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 16.Smith JA, Jr, Labasky RF, Cockett AT, Fracchia JA, Montie JE, Rowland RG. Bladder cancer clinical guidelines panel summary report on the management of nonmuscle invasive bladder cancer (stages Ta, T1 and TIS) The American Urological Association J Urol. 1999;162:1697. [PubMed] [Google Scholar]
- 17.Paz MM, Das TA, Tomasz M. Mitomycin C linked to DNA minor groove binding agents: synthesis, reductive activation, DNA binding and cross-linking properties and in vitro antitumor activity. Bioorg Med Chem. 1999;7:2713. doi: 10.1016/s0968-0896(99)00223-0. [DOI] [PubMed] [Google Scholar]
- 18.Gofrit ON, Shapiro A, Pode D, Sidi A, Nativ O, Leib Z, et al. Combined local bladder hyperthermia and intravesical chemotherapy for the treatment of high-grade superficial bladder cancer. Urology. 2004;63:466. doi: 10.1016/j.urology.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 19.French AJ, Datta SN, Allman R, Matthews PN. Investigation of sequential mitomycin C and photodynamic therapy in a mitomycin-resistant bladder cancer cell-line model. BJU Int. 2004;93:156. doi: 10.1111/j.1464-410x.2004.04576.x. [DOI] [PubMed] [Google Scholar]
- 20.Barret JM, Etievant C, Hill BT. In vitro synergistic effects of vinflunine, a novel fluorinated vinca alkaloid, in combination with other anticancer drugs. Cancer Chemother Pharmacol. 2000;45:471. doi: 10.1007/s002800051021. [DOI] [PubMed] [Google Scholar]


