Abstract
Transcriptional regulation of the ferric citrate transport genes of Escherichia coli is initiated by the binding of ferric citrate to the outer membrane protein FecA. This binding elicits a signal that is transmitted by FecR across the cytoplasmic membrane into the cytoplasm, where the sigma factor FecI directs the RNA polymerase to the promoter upstream of the fecABCDE genes. An in vivo deletion analysis using a bacterial two-hybrid system assigned the interaction of the FecR and FecI proteins to the cytoplasmic portion of the FecR transmembrane protein and region 4 of FecI. Missense mutations randomly generated by PCR were localized to region 4 of FecI, and the mutants were impaired with regard to the interaction of FecR with FecI and fecB-lacZ transcription. The cloned region 4 of FecI interfered with fecB-lacZ transcription. Interaction of N-proximal regions of predicted FecR homologs with region 4 of predicted FecI homologs of Pseudomonas aeruginosa was demonstrated. The interaction was specific in that only cognate protein pairs interacted with each other; no interactions occurred between heterologous combinations of the P. aeruginosa proteins and between a P. aeruginosa FecI homolog and E. coli FecR. The results demonstrate that region 4 of FecI specifically binds FecR and that this binding is necessary for FecI to function as a sigma factor.
The ferric citrate transport system transports iron into the cytoplasm of Escherichia coli. Fe3+ is bound to citrate in the culture medium and probably forms an Fe3+-dicitrate complex (30, 37). Fe3+-dicitrate is bound to the outer membrane protein FecA, which plays a dual role (3, 12, 16). It transports Fe3+-dicitrate across the outer membrane and, independently of transport, induces transcription of the fecABCDE transport genes in the presence of Fe3+-dicitrate. Transport-negative fecA mutants fully retain the inducing activity. The signal transmitted by FecA loaded with Fe3+-dicitrate across the outer membrane is then transmitted across the cytoplasmic membrane by FecR, which is oriented such that residues 1 to 85 are in the cytoplasm, residues 86 to 100 form a transmembrane segment, and residues 101 to 317 are in the periplasm (44). The signal activates the sigma factor FecI, which directs the RNA polymerase to the promoter upstream of the fecA gene (1, 7). The fecI and fecR genes are located further upstream of fecA and are regulated by the intracellular iron concentration through the Fur protein, which when loaded with Fe2+ represses fecIR transcription (2). Fe2+-Fur also directly represses fec transport gene transcription by binding to the promoter upstream of fecA (2). Lack of intracellular iron results in the synthesis of FecIR; in the presence of extracellular Fe3+-dicitrate, this results in the synthesis of the FecABCDE transport proteins, which in turn bring the required iron into the cells.
The model of a signaling cascade from the cell surface into the cytoplasm is supported by the finding that the N-proximal end of FecA interacts with the periplasmic portion of FecR and that the cytoplasmic portion of FecR interacts with FecI (8, 28). A FecA derivative lacking the N-terminal portion no longer has the inducing activity but fully retains the transport activity (16). FecR1-85 (which consists of the 85 N-proximal residues of FecR) causes a constitutive transcription of the fec transport genes (27, 44), and single amino acid replacements in FecR1-85 abolish induction and interaction with FecI (38).
FecI belongs to the extracytoplasmic function (ECF) sigma factors (1, 7), which respond to extracellular signals (21). The Fec system is the only system in which the signal and the signaling cascade have been completely determined (3). Regulatory systems homologous to FecIR have been identified in Shigella flexneri (FecIR, encoded on a novel pathogenicity island) (22), Pseudomonas putida (PupIR) (18), Bordetella avium (RhuIR) (17), Bordetella pertussis (HurIR) (42), and Bordetella bronchiseptica (BupIR) (31) and additional homologs have been deduced from the genome sequences of these organisms and from those of Pseudomonas aeruginosa and Caulobacter crescentus (27, 39).
The regulatory proteins of the FecIR type form a subgroup among the ECF sigma factors. ECF sigma factors are usually regulated by anti-sigma factors (10, 13, 14, 26, 45). This is apparently not the case for FecIR, since FecR or FecR1-85 has to be present in vivo for transcription of the fec transport genes to occur. At present, it is not clear whether the inducing signal changes the conformation of FecR and whether the altered FecR activates FecI by altering the FecI conformation similar to the way allosteric enzymes are regulated. Alternatively, and closer to the anti-sigma factor concept, FecR could bind FecI and prevent FecI from being inactivated by precipitation or proteolytic degradation. When the inducing signal arrives at FecR, FecI might dissociate from FecR and immediately interact with the RNA polymerase. Biochemical studies to decide between these alternatives are hampered by the presence of very low amounts of FecR and FecI [below 1% of σ70 (23)], the insolubility of overexpressed FecI, and degradation of FecR (1, 45, 46).
In this report, we further studied the interaction between FecR and FecI and identified the region of FecI that binds to FecR. As found for the soluble anti-sigma factors Rsd and AsiA that bind to σ70 (6, 15, 41), the transmembrane FecR interacted with region 4 of FecI.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The E. coli strains and plasmids used in this study are listed in Table 1. Cells were grown in tryptone-yeast extract (TY) or nutrient broth (NB) as previously described by Miller (24). Antibiotics were added to the media to the following concentrations: ampicillin, 50 μg/ml; chloramphenicol, 40 μg/ml; tetracycline, 12 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | endA1 hsdR17 (rK− mK+) supE44 thi1 recA1 gyrA relA1 Δ(lacZYA-argF)U169 φ80ΔlacZM15 | 11 |
| ZI418 | araD139 ΔlacU169 rpsL 150 relA1 flbbB5301 deoC1 ptsF25 rbsR thi aroB fecB::Mud1 (Ap, lac) | 43 |
| MO704 | fecB::Mud1 (Ap, lac) fecl::KanraroB araD139 ΔlacU169 rpsL 150 relA1 flbB5301 deoC1 ptsF25 rbsR thi | 43 |
| SU202 | lexA71::Tn5 sulA211 sulA(op408/op+)::lacZ Δ(lacIPOZYA) 169/F′ lacIqlacZΔM15::Tn9 | 5 |
| P. aeruginosa | ||
| PAO1 | Wild type | Lab stock |
| Plasmids | ||
| pHSG576 | pSC101 derivative, Cmr | 40 |
| pSV66 | pHSG576 fecI fecR fecA | 12 |
| pMMO202 | pHSG576 NdeI/BglI (cleavage sites removed) | 38 |
| pMMO203 | pMMO202 fecI fecR | 38 |
| pSM39 | pMMO202 fecIΔ9-32 fecR | This study |
| pSM40 | pMMO202 fecIΔ32-55 fecR | This study |
| pSM41 | pMMO202 fecIΔ52-67 fecR | This study |
| pSM42 | pMMO202 fecIΔ65-83 fecR | This study |
| pSM43 | pMMO202 fecIΔ79-114 fecR | This study |
| pSM44 | pMMO202 fecIΔ114-134 fecR | This study |
| pSM53 | pMMO202 fecI(T1570C)(L113P) fecR | This study |
| pSM54 | pMMO202 fecI(T1642C)(L137P) fecR | This study |
| pSM55 | pMMO202 fecI(T1642G)(L137R) fecR | This study |
| pSM56 | pMMO202 fecI(T1669C/C1670G)(L146P) fecR | This study |
| pSM57 | pMMO202 fecI(T1669G/C1670G)(L146R) fecR | This study |
| pSM59 | pMMO202 fecI(T1729C)(L166P) fecR | This study |
| pSM60 | pMMO202 fecI(C1728A/T1729G)(L166R) fecR | This study |
| pMS604 | oriColE1 TetrlexA1-87-fos zipper | 5 |
| pSM173 | pMS604 lexA1-87-fecI | 8 |
| pSM20 | pMS604 lexA1-87-fecI Δ9-32 | This study |
| pSM21 | pMS604 lexA1-87-fecI Δ32-55 | This study |
| pSM22 | pMS604 lexA1-87-fecI Δ52-67 | This study |
| pSM23 | pMS604 lexA1-87-fecI Δ65-83 | This study |
| pSM24 | pMS604 lexA1-87-fecI Δ79-114 | This study |
| pSM25 | pMS604 lexA1-87-fecI Δ114-134 | This study |
| pSM26 | pMS604 lexA1-87-fecI Δ133-173 | This study |
| pSM76 | pMS604 lexA1-87-fecI64-173 | This study |
| pSM45 | pMS604 lexA1-87-fecI64-173(T1570C)(L113P) | This study |
| pSM49 | pMS604 lexA1-87-fecI64-173(T1642C)(L137P) | This study |
| pSM46 | pMS604 lexA1-87-fecI64-173 (T1669C)(L146P) | This study |
| pSM52 | pMS604 lexA1-87-fecI64-173 (A1613G/T1669G/T1702C)(E127E/L146R/V161A) | This study |
| pSM47 | pMS604 lexA1-87-fecI64-173 (T1729C)(L166P) | This study |
| pSM51 | pMS604 lexA1-87-fecI64-173 (T1729C/T1749G)(L166P/L173V) | This study |
| pSM61 | pMS604 lexA1-87-fecI109-173 | This study |
| pSM74 | pSM604 lexA1-87-fecI Δ132-148 | This study |
| pSM75 | pSM604 lexA1-87-fecI1-153 | This study |
| pDP804 | ori p15A AmprlexA1-87408-jun zipper | 5 |
| pSM85 | pDP804 lexA1-87408-fecR1-85 | 8 |
| pSM84 | pDP804 lexA1-87408-fecR | This study |
| pSM65 | pMS604 lexA1-87-pa24681-172 | This study |
| pSM66 | pMS604 lexA1-87-pa2468110-172 | This study |
| pSM68 | pMS604 lexA1-87-pa38991-170 | This study |
| pSM69 | pMS604 lexA1-87-pa3899105-170 | This study |
| pSM67 | pDP804 lexA1-87408-pa24671-90 | This study |
| pSM70 | pDP804 lexA1-87408-pa39001-85 | This study |
Construction of plasmids.
Plasmids carrying fecI with various deletions were constructed by insertion of a SacI restriction site and by outward PCR amplification. Plasmid pMMO203 was used as the DNA template for plasmids pSM39, pSM40, pSM41, pSM42, pSM43, and pSM44. For construction of plasmids pSM20, pSM21, pSM22, pSM23, pSM24, and pSM25, plasmid pSM173 was used as the DNA template. Plasmids pSM39 and pSM20 were obtained with primers FecI2.1SACRP (5′-CTCGAACGTTAAGAGCTCCGCGGTAGTGGC-3′) and FecI2.1PSAC (5′-GCTGGCTGACGGCGGAGCTCCAGTCTGC-3′), respectively. Plasmids pSM40 and pSM21 were constructed using primers FecI2.2NRPSAC (5′-GCATCAAAAGCAGACTGGAGCTCGCGCGTCAG-3′) and FecI2.2NPSAC (5′-GCGGGTAATGGTCAGCGAAGAGCTCTCGACGATCC-3′), respectively. Plasmids pSM41 and pSM22 were created with primers FecI2.3RPSAC (5′-CGAGAGCGTGAGCTCCGCCATTACCCG-3′) and FecI2.3PSAC (5′-CGCTCCTTCCTCGCGGAGCTCGCCAAACGC-3′), respectively. Plasmids pSM42 and pSM23 were constructed using primers FecI2.4RPSAC (5′-GGCGATAGTGCAGAGCTCCGCGCGAGGATCGCG-3′) and FecIP2.4SAC (5′-CGCCGAAACGCGGAGCTCAAAGCGTATCTGG −3′), respectively. Plasmids pSM43 and pSM24 were created with primers FecI3RPSAC (5′-CCAGATACGCTTTGAGCTCCGCGTTTCGGCG-3′) and FecI3PSAC (5′-CGAGACCCTAGCGGAGCTCGACAGCATGCTGGACGG-3′), respectively. Plasmid pSM44 was obtained using primers FecI4.2PPSAC (5′-CAGACCATCGAGCTCCGCAAGCAGAAACGC-3′) and FecI4.2PSAC (5′-CGTCTGGAGTATGCGGAGCTCATCCTTTGTTAACCG-3′). Plasmid pSM25 was created using primers FecI4.1NRPSAC (5′-CCGTCCAGCATGCTGTCGAGGAGCTCTAGGGTCTC-3′) and FecI4.1NPSAC (5′-GCGTTTCTGCTTTCGGAGCTCGATGGTCTG-3′). Plasmid pSM26 was constructed using primers FecIBstEII (5′-GATGCAGGTGACCATGTCTGACCGCGCC-3′) and FecIPstI (5′-GGTTAACACTGCAGCGAAAGCAGAAACGC-3′). The resulting fecI fragment was digested with BstEII/PstI and cloned into BstEII/PstI-restricted pSM604.
fecI was randomly mutagenized by PCR using primers 2.4BstEIIPr (5′-CCGCGATCCGGTCACCTTCCTCTGCACTATCGCC-3′) and FecIPstI (5′-GGTTAACACTGCAGCGAAAGCAGAAACGC-3′), and plasmid pSV66 was used as the DNA template. Each of the mutated fecI fragments was cloned into pMS604 restricted with BstEII and PstI, resulting in plasmids pSM45, pSM46, pSM47, pSM49, pSM51, and pSM52.
The fecI mutations described above were introduced by site-directed mutagenesis into plasmid pMMO203. The mutated fecI fragments were amplified by PCR with primers FecIL114P (5′-GCCAACTCGAGACCCTACAACCCCTCGACAGC-3′), L137P_rev (5′-CTCGCTGTATGTCGGACCATCCAGTTGCG-3′), L137R_rev (5′-CTCGCTGTATGTGCGACCATCCAGTTGCG-3′), TAQL146P (5′-GCGCACAAACCGGGTGTTTCCATCA-3′), TAQL146R (5′-GCGCACAAACGGGGTGTTTCCATCA-3′), FecI_L166P_rev (5′-GGATTCATATGCCATACTCCAGACGGAACGGCAGGC-5′), FecI_L166R_rev (5′-GGATTCATATGCCATACTCCAGACGGAACCTCAGGC-3′), FecIXhoI_for (5′-GCCAACTCGAGACCCTACAACTCC-3′) and FecINdeI_rev (5′-GGATTCATATGCCATACTCCAGACGG-3′), and plasmid pSV66 was used as the DNA template. The PCR fragments were digested with XhoI and NdeI and cloned into plasmid pMMO203 cleaved with XhoI/NdeI, resulting in plasmids pSM53, pSM54, pSM55, pSM56, pSM57, pSM59, and pSM60.
Truncated fragments of fecI were synthesized by PCR. For construction of plasmid pSM61, the fecI fragment was amplified by PCR with primers FecIReg4BstEII (5′-GCCAACTGGTGACCCTACAACTCCTCG-3′) and FecIPstI (5′-GGTTAACACTGCAGCGAAAGCAGAAACGC-3′), and plasmid pSV66 was used as the DNA template. The amplified fragment was cleaved with BstEII and PstI and ligated into BstEII/PstI-restricted pMS604. Plasmid pSM74 was constructed using primers FecI4.2RPSACI (5′-CAGACCATCGAGCTCCGCAAGCAGAAACGC-3′) and FecI_1.Helix (5′-GCGCAAACTCGAGCTCTCCATCAGCTCCG-3′), and plasmid pSM75 was created with FecI4.2PPSAC (5′-CAGACCATCGAGCTCCGCAAGCAGAAACGC-3′) and FecI4.2PSAC (5′-CGTCTGGAGTATGCGGAGCTCATCCTTTGTTAACCG-3′); in both cases, plasmid pSM173 was used as the DNA template.
Wild-type fecR was amplified by PCR with primers FecRXhoI (5′-GGAGTACTCGAGATGAATCCTTTGTTAA-3′) and LexFecR2 (5′-GGAAGATCTTCCACCTAGTTTACAGTGGTGAAATGTT-3′), with plasmid pSV66 as the DNA template. The resulting fecR fragment was cloned into XhoI/BglII-digested pDP804.
The wild-type pa2468 and pa3899 genes were amplified by PCR with primers Con48FecIBstEII_for (5′-GTGTCGCCCCGAGGTGACCATGTCCGCCCCGATCC-3′), and Con48FecIXhoI_rev (5′-CGTCCACGGCTCGAGTCATTCGCCGTAGCG-3′) and with Con52FecIBstEII_for (5′-CGTTAATCCTTGGTGACCGGGAATGTCCAGGTG-3′) and Con52FecIBstEII4_for (5′-GCGAAGAGACGGTGACCATCGTCCTGGAGACC-3′), respectively. The truncated pa2468 and pa3899 genes were amplified by PCR with primers Con48FecI4BstEII_for (5′-CCGAGGCGCGGGAACTGGTGACCGAACTGCTG-3′) and Con48FecIXhoI_rev (5′-CGTCCACGGCTCGAGTCATTCGCCGTAGCG-3′) and with Con52FecIBstEII4_for (5′-GCGAAGAGACGGTGACCATCGTCCTGGAGACC-3′) and Con52FecIXhoI_rev (5′-CTTGGGTGCTCGAGGCAGGCTCATGGCAGCTCGG-5′), respectively. The PCR fragments were digested with BstEII and XhoI and ligated into BstEII/XhoI-restricted pMS604, resulting in plasmids pSM65, pSM66, pSM68, and pSM69.
To obtain plasmids pSM67 and pSM70, the truncated pa2467 and pa3900 gene fragments were amplified by PCR using primers Con48FecRXhoI (5′-CCTGCTGCGCTCGAGCGAATGAGCGGAGCCGTG-3′) and Con48FecRBglII (5′-CAGCAGCGAAGACTCTTACAGCGCCTGGCGCCGCTG-3′) and using Con52FecRXhoI (5′-CTTCGCCGACTCGAGATGAGCCTGCCCGCCGCACC-3′) and Con52FecRBglII (5′-GGACCAGCAGAGATCTCTACAGCGCGCGCCGGCG-3′), respectively. The XhoI/BglII-restricted PCR fragments were cloned into XhoI/BglII-cleaved pDP804. For construction of plasmids pSM65, pSM66, pSM67, pSM68, pSM69, and pSM70, the chromosomal DNA of strain PAO1 was used as the DNA template.
All plasmids constructs were confirmed by DNA sequencing.
Recombinant DNA techniques.
Standard techniques (33) or the protocols of the suppliers were used for isolation of plasmid DNA, digestion with restriction endonucleases, ligation, transformation, and agarose gel electrophoresis. DNA was sequenced by the dideoxy chain-termination method (34) using the AutoRead sequencing kit (Pharmacia Biotech, Freiburg, Germany). The reaction products were sequenced on an A.L.F. DNA sequencer (Pharmacia Biotech). PCR amplification was carried out using Taq polymerase (Qiagen, Hilden, Germany) and standard conditions. DNA was initially denatured by heating to 94°C for 3 min. This was followed by 30 cycles consisting of denaturing at 94°C for 1 min, annealing at 54°C for 2 min, and extension at 72°C for 3 min. Random mutagenesis by PCR followed the method previously described (20). Site-directed mutagenesis was carried out according to the methods of Landt et al. (19).
Western blotting.
To estimate the amounts of wild-type FecI and mutated FecI obtained, a Western blot analysis was employed using anti-LexA antibodies (Invitrogen, Karlsruhe, Germany). In brief, transformants of E. coli SU202 carrying the fecI genes were grown in TY medium supplemented with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to a density of 8 × 108 cells per ml. Cell lysates were treated with sample buffer (50 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate, 10% glycerol) and separated on 15% polyacrylamide gels. Proteins were electroblotted onto nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). Blots were blocked overnight in 3% bovine serum albumin in TNT buffer (20 mM Tris-HCl, 500 mM NaCl, 0.05% Tween 20), probed with the anti-LexA antibodies, washed with TNT buffer, and incubated with anti-rabbit immunoglobulin G conjugated with alkaline phosphatase (Sigma). The blots were developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP; Serva, Heidelberg, Germany).
Determination of β-galactosidase activity.
β-galactosidase activity was determined according to the methods of Miller (24) and Giacomini et al. (9). To determine the induction level, cells were grown in NB medium with no additions or supplemented with 50 μM 2,2′-dipyridyl or 1 mM citrate. For the LexA-based repression system, cells were grown in TY medium supplemented with 1 mM IPTG.
Sequence alignments and prediction of secondary structure.
Sequences of Pseudomonas aeruginosa were obtained from the Pseudomonas Genome Project (http://www.pseudomonas.com/current_annotation.asp). The sequence of E. coli FecI was translated from the GenBank entries given in parentheses. Amino acid sequences were aligned using ClustalW multiple sequence alignment. The helix-turn-helix motif of region 4 of E. coli FecI was predicted using the PredictProtein program (http://www.embl-heidelberg.de/predictprotein).
RESULTS
Binding sites of FecR on FecI.
The bacterial two-hybrid LexA system was used to identify sites of interaction of FecR with FecI in vivo. The dimer of LexA represses sulA transcription. The dimerizing C-proximal end can be replaced by heterologous protein fragments that are thought to dimerize. The promoter of sulA has been mutagenized such that one half binds the wild-type N-proximal end of LexA (LexA1-87) and the other half binds a mutated N-proximal end of LexA (LexA1-87408) (5). Complete FecR and FecR1-85 (residues 1 to 85 of FecR) were fused to LexA1-87408, and FecI and deletion derivatives of FecI were fused to LexA1-87. When FecR1-85 interacts with FecI, the fec transport genes are constitutively transcribed, whereas when complete FecR interacts with FecI, transcription initiation of the fec transport gene requires ferric citrate and FecA (8, 27, 38, 44).
E. coli SU202, which contains lacZ under the control of the mutated sulA promoter, was transformed with plasmids carrying the truncated lexA-fecR and lexA-fecI fusion genes. LexA1-87-FecI109-173, which contained only region 4 of FecI combined with FecR and FecR1-85, repressed lacZ transcription (Table 2). Deletions were introduced into regions 2, 3, and 4 of FecI but not into region 1, since region 1.1 of FecI is lacking and deletion of region 1.2 does not affect FecI activity (38). FecI with deletions covering regions 2 and 3 formed dimers with FecR and FecR1-85, as evidenced by the background activity of β-galactosidase, in contrast to FecI with deletions in region 4, which showed high β-galactosidase activity (Table 2). Cells expressing FecIΔ79-114 and wild-type FecR had a 2.5-fold- higher β-galactosidase activity than fully repressed cells. These results are consistent with the proposal that region 4 of FecI binds FecR and that the cytoplasmic N-terminal end of FecR is sufficient for binding. Regions 2 and 3 of FecI did not contribute directly to binding or influence binding of region 4 to FecR.
TABLE 2.
Interaction of FecI deletion derivatives with complete FecR and FecR1-85
| Plasmid | Protein | Region deleted in FecI | β-Galactosidase activity (Miller units)a
|
|
|---|---|---|---|---|
| FecR1-85 | Wild-type FecR | |||
| None | No FecI | 241 | 216 | |
| pSM173 | Wild-type FecI | 25 | 36 | |
| pSM20 | FecI Δ9-32 | 2.1 | 29 | 27 |
| pSM21 | FecI Δ32-55 | 2.2 | 28 | 29 |
| pSM22 | FecI Δ52-67 | 2.3 | 23 | 28 |
| pSM23 | FecI Δ65-83 | 2.4 | 25 | 32 |
| pSM24 | FecI Δ79-114 | 3 | 29 | 71 |
| pSM25 | FecI Δ114-134 | 4.1 | 225 | 209 |
| pSM26 | FecI Δ133-173 | 4.2 | 213 | 216 |
| pSM61 | FecI109-173 | 1-3 | 32 | 31 |
Determined using the bacterial two-hybrid LexA-based system in E. coli SU202 sulA-lacZ.
Transcription activity of the FecI deletion derivatives.
To determine transcription levels of the fec transport genes in the fecI deletion mutants, wild-type fecI on plasmid pMMO203 fecI fecR was replaced by mutated fecI. Transcription levels were measured in E. coli MO704, which carried on the chromosome a kanamycin resistance gene in fecI and a fecB-lacZ fusion. E. coli MO704(pMMO203), grown in NB supplemented with 50 μM dipyridyl to limit the available iron, displayed approximately 8% (8 to 12 Miller units) of the β-galactosidase activity of cells grown in the presence of 1 mM citrate (291 Miller units). E. coli MO704 transformed with the vector pHSG576 displayed only 1.5% of the β-galactosidase activity of cells grown in the presence of 1 mM citrate; this value was also determined for cells that synthesized the FecI deletion derivatives listed in Table 2. All regions in which the deletions were found—2.1, 2.2, 2.3, 2.4, 3, and 4.2 (4.1 was not determined)—were important for sigma factor activity.
If FecR interacts with region 4 of FecI, a FecI fragment comprising region 4 should inhibit interaction of FecR with wild-type FecI and hence transcription of fecB-lacZ. E. coli ZI418, which carries on the chromosome a complete fec transport operon with the exception of fecB, which is fused to lacZ, was transformed with plasmid pSM61, which encodes FecI109-173. Transcription of the fecI fragment gene was induced by adding IPTG. The β-galactosidase activity of the citrate-induced culture decreased from 177 units without IPTG induction to 51 units after induction of FecI109-173 synthesis. This result further supports the involvement of region 4 in the FecR-mediated FecI activity.
Point mutations in FecI that affect binding to FecR.
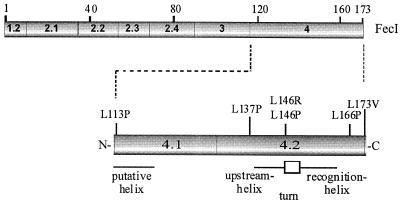
To further localize FecI regions that interact with FecR, point mutants were introduced into FecI. Inactive mutant proteins could then be used to correlate impaired interaction with FecR with the ability to induce transcription of fec genes. A fecI fragment comprising regions 2.4 to 4 was mutagenized via PCR to isolate FecI mutants that no longer interacted with FecR1-85. The formation of red colonies on MacConkey agar plates of E. coli SU202 transformed with the cloned mutagenized fecI fragments indicated impaired repression of sulA-lacZ. Of 3,000 colonies tested, only 6 were red. All mutations were located in region 4 (Fig. 1). Those FecI mutants with the highest lacZ transcription levels (Table 3) had a replacement of leucine by proline (L146P and L166P). The mutant with the L113P replacement, which is close to the border of region 3, displayed the least increase in β-galactosidase activity. The mutations are located in the predicted helix-turn-helix motif, and the L→P substitutions most likely affect the secondary structure.
FIG. 1.
Domain structure of FecI and location of the amino acid substitutions within the putative helix-turn-helix motif of FecI.
TABLE 3.
Binding of mutated FecI64-173 to wild-type FecR1-85
| Plasmid | fecI mutation(s) | Amino acid substitution(s) in FecI | β-Galactosidase activity (Miller units)a |
|---|---|---|---|
| pSM76 | None | None | 25 |
| pSM45 | T1570C | L113P | 58 |
| pSM49 | T1642C | L137P | 72 |
| pSM46 | T1669C | L146P | 180 |
| pSM52 | A1613G, T1669G, T1702C | E127E, L146R, V161A | 71 |
| pSM47 | T1729C | L166P | 178 |
| pSM51 | T1729C, T1749G | L166P, L173V | 205 |
Determined using the bacterial two-hybrid LexA-based system in E. coli SU202 sulA-lacZ.
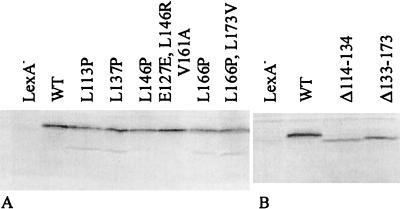
Inactivity of the mutated FecI derivatives linked to LexA could arise from instability of the hybrid proteins. Therefore, their amounts after IPTG induction were estimated by Western blot analysis using anti-LexA antibodies. All samples contained proteins which showed electrophoretic mobilities corresponding to expectations formed on the basis of their sizes (Fig. 2). The amounts of mutated FecI carrying point mutations and deletions were somewhat smaller than the amount of wild-type FecI, and the L→P mutants revealed a defined degradation product whose amount, however, was much less than those of the original products. The amounts of the mutated LexA-FecI hybrid proteins were still large and certainly sufficient to repress transcription of the chromosomal sulA-lacZ reporter gene.
FIG. 2.
Western blot analysis of E. coli SU202 cell extracts. LexA hybrid proteins of wild-type FecI, mutated FecI (A), and truncated FecI (B) were detected with anti-LexA antibodies.
Sigma factor activity of FecI point mutants.
To determine whether the L→P substitutions that impaired binding to FecR1-85 affected FecI sigma factor activity, fecB-lacZ transcription levels were determined in E. coli MO704 carrying the fecI mutant genes. In addition, these mutated leucine residues—L137, L146, and L166—were replaced by arginine via site-directed mutagenesis. All L→P mutants showed very low levels of fecB-lacZ transcription, including L113P and L137P (Table 4), which displayed residual interaction with FecR1-85 (Table 3). After induction with ferric citrate, the L→R mutants L137R and L166R showed levels of activity equal to that of wild-type FecI and L146R showed 22% of the wild-type FecI activity. These results demonstrate a correlation between FecR binding to FecI and the level of transcription and suggest that at least in the case of FecI(L137P) and FecI(L166P), secondary structure alterations disrupt interaction of FecI with FecR and hence sigma factor activity.
TABLE 4.
fecB-lacZ transcription by mutant FecI
| Plasmid | fecI mutation(s) | Amino acid substitution in FecI | β-Galactosidase activity (Miller units)a
|
|
|---|---|---|---|---|
| NB medium + 50 μM dipyridyl | NB medium + 1 mM citrate | |||
| pHSG576 | Vector | Vector | 5 | 6 |
| pMMO203 | Wild type | None | 23 | 376 |
| pSM53 | T1570C | L113P | 6 | 6 |
| pSM54 | T1642C | L137P | 12 | 13 |
| pSM55 | T1642G | L137R | 26 | 333 |
| pSM56 | T1669C, C1670G | L146P | 9 | 7 |
| pSM57 | T1669G, C1670G | L146R | 13 | 82 |
| pSM59 | T1729C | L166P | 5 | 6 |
| pSM60 | C1728A, T1729G | L166R | 41 | 393 |
Determined in E. coli MO704 fecI::Kan fecB-lacZ transformed with plasmids carrying mutated or wild-type fecI and wild-type fecR.
Interactions of proteins of P. aeruginosa homologous to FecI and FecR.
The genome of P. aeruginosa (39) encodes a number of proteins homologous to FecI and FecR of E. coli (38), among them PA2468 (172 residues) and PA3899 (170 residues), which are 46 and 47% identical to FecI (173 residues), respectively, and PA2467 (332 residues) and PA3900 (317 residues), which are 17 and 35% identical to FecR (317 residues), respectively. Genes pa2468 and pa2467 are seemingly controlled by the same promoter, as are genes pa3899 and pa3900. The region upstream of pa2467 contains a putative fur box, which points to iron-regulated transcription. The sequence similarities suggest that each of these sets of genes encodes an ECF sigma factor of the FecI type and a regulatory protein of the FecR type. To support this assumption and to obtain further data on the specificity of the interaction between FecI-type sigma factors and their regulatory proteins, the genes were fused to LexA1-87 and LexA1-87408 in various combinations and β-galactosidase activity resulting from sulA-lacZ transcription was determined. The fragments of the P. aeruginosa proteins used were homologous either to region 4 of FecI or to the cytoplasmic segment of FecR. The E. coli SU202 transformant that synthesized PA2468 and PA24671-90 and the transformant that synthesized PA3899 and PA39001-85 exhibited low β-galactosidase activity (Table 5), which indicated repression of sulA-lacZ transcription as a result of protein dimerization. When PA2468 was replaced by PA2468110-172 and PA3899 by PA3899105-170, both of which represent region 4 of the proteins, repression was even stronger (Table 5). However, the heterologous combinations, PA2468 with PA39001-85 and PA3899 with PA24671-90, did not repress sulA-lacZ transcription. The different amino acid sequences prevented interaction of the proteins (PA2468 is 48% identical to PA3899 and PA2467 is 42% identical to PA3900; see also Fig. 3). Furthermore, FecR1-85 combined with PA2468 did not repress sulA-lacZ transcription; this result in addition demonstrated that the identical turns (GVS) between the two helices in region 4 of PA2468 and of FecI do not determine the specificity of interaction between the sigma factors and the cognate regulatory proteins.
TABLE 5.
Interaction of the complete and truncated FecI homologs PA2468 and PA3988 of P. aeruginosa with the truncated FecR homologs PA2467 and PA3900 of P. aeruginosa and FecR1-85 of Escherichia coli
| Plasmids | Protein combination | β-galactosidase activity (Miller units)a |
|---|---|---|
| pMS604, pDP804 | Fos and Junb | 25 |
| pSM65, pSM67 | PA2468 and PA24671-90 | 28 |
| pSM66, pSM67 | PA2468110-172 and PA24671-90 | 14 |
| pSM68, pSM70 | PA3899 and PA39001-85 | 39 |
| pSM69, pSM70 | PA3899105-170 and PA39001-85 | 23 |
| pSM65, pSM70 | PA2468 and PA39001-85 | 397 |
| pSM68, pSM67 | PA3899 and PA24671-90 | 269 |
| pSM65, pSM85 | PA2468 and FecR1-85 | 181 |
Determined using the bacterial two-hybrid LexA-based system in E. coli SU202 sulA-lacZ.
Dimerization of Jun and Fos was used to develop the LexA-based dimerization assay (33).
FIG. 3.
Alignment of the conserved region 4 of E. coli (E.c.) FecI with P. aeruginosa (P.a.) FecI homologs. Shaded positions indicate three identical residues, and asterisks indicate two identical residues. The helix-turn-helix motif in Region 4.2 is indicated above the E. coli sequence.
DISCUSSION
The activity of those ECF sigma factors studied in some detail has been shown to be controlled by anti-sigma factors (13, 14). Exogenous signals are thought to activate the inactive sigma factors by causing dissociation of the anti-sigma factors from the sigma factors or by causing conformational changes in the sigma factors. In the absence of the anti-sigma factors, the ECF sigma factors initiate transcription without extracytoplasmic signals. This holds true for the PupIR regulatory system of P. putida, which shows sequence similarity to FecIR. lacZ fused to the promoter of the pupB gene, which encodes an outer membrane protein for the uptake of pseudobactin BN8, is strongly transcribed in pupI+ pupR− cells (18). In addition, the B. avium BhuR outer membrane protein, which is required for heme uptake, is synthesized in high amounts when rhuI, a fecI homolog, is overexpressed in the absence of heme as inducer (17).
In contrast, overexpression of FecI in the absence of FecR results in a very low level of transcription of chromosomal fecB-lacZ (29). Transcription of the fecABCDE transport genes requires FecR and ferric citrate for FecI to function as a sigma factor. Overexpression of plasmid-borne fecI in a chromosomal fecI+ fecR+ strain leads to high levels of fecB-lacZ transcription in the absence of ferric citrate; for example, in uninduced and in ferric-citrate-induced FecI+ FecR+ cells, the β-galactosidase activity is 185 and 190 units, respectively. Overexpression of plasmid-encoded fecR does not reduce uninduced or ferric-citrate-induced fecB-lacZ transcription in chromosomal fecI+ fecR+ cells (52 versus 51 units and 192 versus 199 units, respectively). C-terminally truncated FecR derivatives confer constitutive fecA-lacZ transcription in fecI+ fecR− cells (27, 38, 44). The electrophoretic mobility of a fecA promoter DNA fragment on a polyacrylamide gel is retarded when purified FecI and isolated RNA polymerase core enzyme are added together. When a crude cell extract of a FecIRA-producing cell is used, band shifting occurs at much lower levels of FecI than of purified FecI (1). However, since a detergent had to be used to solubilize FecI, it remains unclear whether a substantial fraction of FecI was denatured. The crude cell extract caused band shifting only when the cells expressed the fecIRA genes and were grown in the presence of ferric citrate. When FecR was omitted, no band shifting was observed (1). These data indicate the requirement of the presence of FecR for FecI activity.
The deviation of FecIR-mediated regulation from regulation by anti-sigma control of ECF sigma factors prompted this study on the sites of interaction between FecR and FecI. FecR and FecR1-85 interacted with complete FecI, as evidenced by repression of sulA-lacZ transcription through the FecI and FecR or FecR1-85 LexA fusions. Repression was retained when fragments of FecI were sequentially excised from the N terminus to the C terminus up to region 4.1. Deletion of residues 114 to 134 (region 4.1) and 133 to 173 (region 4.2) resulted in C-terminally truncated FecI derivatives that no longer combined with FecR or FecR1-85 to repress sulA-lacZ transcription. The finding that region 4 interacted with FecR was supported by results obtained with randomly generated fecI mutants, which in combination with FecR1-85 displayed lower repression of sulA-lacZ transcription and were exclusively mutated in region 4.1 or 4.2. Impaired dimerization correlated with lack of induction. None of the four FecI L→P mutants initiated transcription of fecB-lacZ. The finding that fragments covering regions 1.2 to 3 could be excised from FecI without affecting binding of region 4 to FecR supports the exclusive involvement of region 4 in FecR binding and indicates the structural and functional independence of region 4 with regard to FecR binding.
The FecI protein displays a helix-turn-helix motif from residues 139 to 158 (Fig. 1). Secondary structure analysis predicts an amphipathic helix for the first portion of the motif, of which Tyr139, Ile142, Ala143, and Leu146 form the hydrophobic side and Ser140, Glu141, His144, and Lys145 form the hydrophilic side (27, 43). The L→P mutations in region 4 probably changed the conformation of FecI, since leucine was replaced by proline in all of the mutations (proline inserted in an α-helix disrupts the secondary structure). This hypothesis was supported by the results obtained with mutants in which the leucine residues were replaced by arginine instead of proline. Despite the strong alteration in charge and steric requirements, two of the three examined mutants exhibited activities close to that of the wild type and only one, L146R, showed a lower activity (22% of wild-type FecI activity). These results clearly associate functional interactions of FecR with region 4 of FecI.
Regions 4.1 and 4.2 of FecI proved to be important for FecR binding, as shown by the abolishment of FecR binding caused by individual deletions in either region and the impairment of transcription of fecB-lacZ caused by missense mutations in either region. The involvement of regions 4.1 and 4.2 of FecI is similar to the situation with the AsiA anti-sigma factor of phage T4 and σ70; binding of AsiA to σ70 not only involves region 4.2 of σ70 (4, 25, 35, 36) but also region 4.1 (41). AsiA inhibits transcription of E. coli genes and early T4 genes and enhances transcription of middle T4 genes. In contrast to single alanine mutations in synthetic peptides of σ70, which alone do not strongly affect binding to AsiA (25), the FecI L→P substitutions reduced binding of FecR1-85 and abolished FecI activity. As with FecR1-85, an N-terminal fragment of AsiA of 20 residues was necessary and sufficient for binding of AsiA to σ70. In both FecR and AsiA, the sigma factor binding domain is located in the first quarter of the polypeptides [FecR contains 317 residues, and 68 residues are sufficient (8, 27); AsiA consists of 90 residues].
Region 4 of FecI participates in recognition of the −35 promoter sequence, which is rather well conserved in ECF-regulated promoters (21). The sequence of the −35 region of the promoter upstream of the fecABCDE transport genes is homologous to that of the ECF-regulated promoters, whereas the −10 region shows poor sequence similarity (7, 32). FecI mediates binding of the E. coli RNA polymerase core enzyme to a 75-bp fragment that encompasses the −10 and −35 fecA promoter regions (1). It does not seem that FecR binding to region 4 of FecI interferes with binding of FecI to the −35 region, as has been suggested for AsiA (4, 35), since overexpressed FecR does not affect FecI-mediated fecB-lacZ transcription (43). An additional binding site for the FecI-RNA polymerase apoenzyme has been revealed by randomly generated mutations in a fecA promoter DNA fragment. The nucleotide replacements that reduced binding are clustered around position +13 (1) relative to the fecA transcription initiation site (7). This unusual binding site is another indication that fec transcription regulation has certain properties distinct from those of other sigma regulatory mechanisms.
Binding of FecR to region 4 of FecI concurs with the binding of anti-sigma factors to binding sites on sigma factors. Anti-sigma-factor activity of FecR cannot be evaluated because FecI shows only very low levels of activity in the absence of FecR, and therefore FecI inhibition by FecR cannot be determined. If nascent FecI is rapidly inactivated by proteolysis or aggregation, binding to FecR could keep it in an active conformation. The signal exerted by ferric citrate bound to FecA could dissociate FecI from FecR, followed by immediate binding of FecI to RNA polymerase core enzyme and transcription initiation of the fecABCDE genes. In this model, FecR would act as a membrane-bound chaperone. This model is more appealing than a model that proposes binding of the FecI-RNA polymerase via FecR to the cytoplasmic membrane, because in the latter case a portion of the RNA polymerase would have to be sequestered in an inactive form as long as no induction by ferric citrate occurs—only when induction occurs would the FecI-RNA polymerase dissociate from the membrane and initiate transcription. In a third model, the signal would not dissociate FecR and FecI but rather would bind FecIR to the RNA polymerase core enzyme. Since FecR is inserted into the cytoplasmic membrane, this model would imply that transcription of the fec transport genes occurs while the RNA polymerase is bound to the membrane.
We have previously identified tryptophan residues 19, 39, and 50 of FecR as being important for interaction with FecI. In randomly generated mutants containing arginine in place of the tryptophan residues, binding of mutated FecR1-85 to FecI and fecB-lacZ transcription mediated by mutated FecR were abolished. Two mutations in FecI (S15A and H20E) that partially suppressed the FecR W→R mutations are clustered in region 2.1 (38). Since no allele specificity is observed and the mutated FecI suppressor proteins do not restore constitutive fecB-lacZ transcription of FecR1-85 W→R mutants, a direct interaction between FecR and region 2.1 of FecI is unlikely. Rather, the FecI suppressor mutants increase FecI activity, presumably by improving interaction with the RNA polymerase core enzyme.
Tryptophan residues 19 and 39 are strictly conserved in 22 FecR homologs found in GenBank, whereas tryptophan residue 50 is replaced by phenylalanine or tyrosine in a few proteins. Among these proteins are FecI and FecR homolog pair PA2468 and PA2467 and homolog pair PA3899 and PA3900 of P. aeruginosa. In this report, we showed that these pairs interact with each other but that heterologous pair PA2468 and PA3900 and heterologous pair PA3899 and PA2467 do not. Similar to the truncated FecI and FecR derivatives, the N-terminally truncated PA2468110-172 and the C-terminally truncated PA24671-90 interacted with each other. Since PA2468110-172 consists of predicted region 4 and binds even more strongly to PA24671-90 than complete PA2468, region 4 represents the binding site of the FecR homolog. These data strongly support the prediction that these proteins belong to the FecIR subgroup of ECF σ70 factors, and they underline the importance of region 4 for the interaction of the two regulatory proteins.
Acknowledgments
We thank Karen A. Brune for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (BR330-19/1) and the Fonds der Chemischen Industrie.
Footnotes
This paper is dedicated to Karlheinz Altendorf on the occasion of his 60th birthday.
REFERENCES
- 1.Angerer, A., S. Enz, M. Ochs, and V. Braun. 1995. Transcriptional regulation of ferric citrate transport in Escherichia coli K-12. FecI belongs to a new subfamily of σ70-type factors that respond to extracytoplasmic stimuli. Mol. Microbiol. 18:163-174. [DOI] [PubMed] [Google Scholar]
- 2.Angerer, A., and V. Braun. 1998. Iron regulates transcription of the Escherichia coli ferric citrate transport genes directly and through the transcription initiation proteins. Arch. Microbiol. 169:483-490. [DOI] [PubMed] [Google Scholar]
- 3.Braun, V. 1997. Surface signaling: novel transcription initiation mechanism starting from the cell surface. Arch. Microbiol. 167:325-331. [DOI] [PubMed] [Google Scholar]
- 4.Colland, F., G. Orsini, E. N. Brody, H. Buc, and A. Kolb. 1998. The bacteriophage T4 AsiA protein: a molecular switch for sigma 70-dependent promoters. Mol. Microbiol. 27:819-829. [DOI] [PubMed] [Google Scholar]
- 5.Dmitrova, M., G. Younes-Cauet, P. Oertel-Buchheit, D. Porte, M. Schnarr, and M. Granger-Schnarr. 1998. A new LexA-based genetic system for monitoring and analyzing protein heterodimerization in Escherichia coli. Mol. Gen. Genet. 257:205-212. [DOI] [PubMed] [Google Scholar]
- 6.Dove, S. L., and A. Hochschild. 2001. Bacterial two-hybrid analysis of interactions between region 4 of the σ70 subunit of RNA polymerase and the transcriptional regulators Rsd from Escherichia coli and AlgQ from Pseudomonas aeruginosa. J. Bacteriol. 183:6413-6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enz, S., V. Braun, and J. Crosa. 1995. Transcription of the region encoding the ferric dicitrate transport system in Escherichia coli: similarity between promoters for fecA and for extracytoplasmic function sigma factors. Gene 163:13-18. [DOI] [PubMed] [Google Scholar]
- 8.Enz, S., S. Mahren, U. H. Stroeher, and V. Braun. 2000. Surface signaling in ferric citrate transport gene induction: interaction of the FecA, FecR, and FecI regulatory proteins. J. Bacteriol. 182:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giacomini, A., B. Corich, F. J. Ollero, A. Squartini, and M. P. Nuti. 1992. Experimental conditions may affect reproducibility of the β-galactosidase assay. FEMS Microbiol. Lett. 100:87-90. [DOI] [PubMed] [Google Scholar]
- 10.Gross, C. A., C. Chan, A. Dombroski, T. Gruber, M. Sharp, J. Tupy, and B. Young. 1998. The functional and regulatory roles of sigma factors in transcription. Cold Spring Harbor Lab. Symp. Quant. Biol. 63: 141-155. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan, D. 1983. Studies in transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557.. [DOI] [PubMed] [Google Scholar]
- 12.Härle, C., K. Insook, A. Angerer, and V. Braun. 1995. Signal transfer through three compartments: transcription initiation of the Escherichia coli ferric citrate transport system from the cell surface. EMBO J. 14:1430-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helmann, J. D. 1999. Anti-sigma factors. Curr. Opin. Microbiol. 2:135-141. [DOI] [PubMed] [Google Scholar]
- 14.Hughes, K. T., and K. Mathee. 1998. The anti-sigma factors. Annu. Rev. Microbiol. 52:231-286. [DOI] [PubMed] [Google Scholar]
- 15.Jishage, M., D. Dasgputa, and A. Ishihama. 2001. Mapping of the Rsd contact site on the sigma 70 subunit of Escherichia coli RNA polymerase. J. Bacteriol. 183:2952-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, I., A. Stiefel, S. Plantör, A. Angerer, and V. Braun. 1997. Transcription induction of the ferric citrate transport genes via the N-terminus of the FecA outer membrane protein, the Ton system and the electrochemical potential of the cytoplasmic membrane. Mol. Microbiol. 23:333-344. [DOI] [PubMed] [Google Scholar]
- 17.Kirby, A. E., D. J. Metzger, E. R. Murphy, and T. D. Connell. 2001. Heme utilization in Bordetella avium is regulated by RhuI, a heme-responsive extracytoplasmic function sigma factor. Infect. Immun. 69:6951-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koster, M., W. van Klompenburg, W. Bitter, J. Leong, and P. Weisbeek. 1994. Role for the outer membrane ferric siderophore receptor PupB in signal transduction across the bacterial cell envelope. EMBO J. 13:2805-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landt, O., H. P. Grunert, and U. Hahn. 1990. A general method for rapid site-directed mutagenesis using the polymerase chain reaction. Gene 96:125-128. [DOI] [PubMed] [Google Scholar]
- 20.Leung, D. W., E. Chen, and D. V. Goeddel. 1989. A method for random mutagenesis of a defined DNA segment using a modified polymerase chain reaction. Technique 1:11-15. [Google Scholar]
- 21.Lonetto, M. A., K. L. Brown, K. E. Rudd, and M. J. Buttner. 1994. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase σ factors involved in the regulation of extracytoplasmic functions. Proc. Natl. Acad. Sci. USA 91:7573-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luck, S. N., S. A. Turner, K. Rajakumar, H. Sakellaris, and B. Adler. 2001. Ferric dicitrate transport system (Fec) of Shigella flexneri 2a YSH6000 is encoded on a novel pathogenicity island carrying multiple antibiotic resistance genes. Infect. Immun. 69:6012-6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda, H., N. Fujita, and A. Ishihama. 2000. Competition among seven Escherichia coli sigma subunits: relative binding affinities to the core RNA polymerase. Nucleic Acids Res. 28:3497-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, N.Y.
- 25.Minakhin, L., J. A. Camarero, M. Holford, C. Parker, T. W. Muir, and K. Severinov. 2001. Mapping the molecular interface between the sigma(70) subunit of E. coli RNA polymerase and T4 AsiA. J. Mol. Biol. 306:631-642. [DOI] [PubMed] [Google Scholar]
- 26.Missiakas, D., and S. Raina. 1998. The extracytoplasmic function sigma factors: role and regulation. Mol. Microbiol. 28:1059-1066. [DOI] [PubMed] [Google Scholar]
- 27.Nierman, W. C., T. V. Feldblyum, M. T. Laub, I. T. Paulsen, K. E. Nelson, J. Eisen, J. F. Heidelberg, M. R. Alley, N. Ohta, J. R. Maddock, I. Potocka, W. C. Nelson, A. Newton, C. Stephens, N. D. Phadke, B. Ely, R. T. DeBoy, R. J. Dodson, A. S. Durkin, M. L. Gwinn, D. H. Haft, J. F. Kolonay, J. Smit, M. B. Craven, H. Khouri, J. Shetty, K. Berry, T. Utterback, K. Tran, A. Wolf, J. Vamathevan, M. Ermolaeva, O. White, S. L. Salzberg, J. C. Venter, L. Shapiro, and C. M. Fraser. 2001. Complete genome sequence of Caulobacter crescentus. Proc. Natl. Acad. Sci. USA 98:4136-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ochs, M., S. Veitinger, I. Kim, D. Welz, A. Angerer, and V. Braun. 1995. Regulation of citrate-dependent iron transport of Escherichia coli: FecR is required for transcription activation by FecI. Mol. Microbiol. 15:119-132. [DOI] [PubMed] [Google Scholar]
- 29.Ochs, M., A. Angerer, S. Enz, and V. Braun. 1996. Surface signaling in transcriptional regulation of the ferric citrate transport system of Escherichia coli: mutational analysis of the alternative sigma factor FecI supports its essential role in fec transport gene transcription. Mol. Gen. Genet. 250: 455-465. [DOI] [PubMed] [Google Scholar]
- 30.Pierre, J. L., and I. Gautier-Luneau. 2000. Iron and citric acid: a fuzzy chemistry of ubiquitous biological relevance. BioMetals 13:9-96. [DOI] [PubMed] [Google Scholar]
- 31.Pradel, E., and C. Locht. 2000. Expression of the putative siderophore receptor gene bfrZ is controlled by the extracytoplasmic-function sigma factor BupI in Bordetella bronchiseptica. J. Bacteriol. 183:2910-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pressler, U., H. Staudenmaier, L. Zimmermann, and V. Braun. 1988. Genetics of the iron dicitrate transport system of Escherichia coli. J. Bacteriol. 170:2716-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, N.Y.
- 34.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Severinova, E., K. Severinova, and S. A. Darst. 1998. Inhibition of Escherichia coli RNA polymerase by bacteriophage T4 AsiA. J. Mol. Biol. 279:9-18. [DOI] [PubMed] [Google Scholar]
- 36.Sharma, U. K., S. Ravishankar, R. K. Shandil, P. V. Praveen, and T. S. Balganesh. 1999. Study of the interaction between bacteriophage T4 AsiA and Escherichia coli σ70, using the yeast two-hybrid system: neutralization of AsiA toxicity to E. coli cells by coexpression of a truncated σ70 fragment. J. Bacteriol. 181:5855-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiro, T. G., G. Bates, and P. Saltman. 1967. The hydrolytic polymerization of ferric citrate. II. The influence of excess citrate. J. Am. Chem. Soc. 89:5463-5467. [Google Scholar]
- 38.Stiefel, A., S. Mahren, M. Ochs, P. T. Schindler, S. Enz, and V. Braun. 2001. Control of the ferric citrate transport system of Escherichia coli: mutations in region 2.1 of the FecI extracytoplasmic-function sigma factor suppress mutations in the FecR transmembrane regulatory protein. J. Bacteriol. 183:162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulsen. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 40.Takeshita, S., M. Sato, M. Toba, W. Masahashi, and T. Hashimoto-Gotoh. 1987. High-copy-number and low-copy-number plasmid vectors for lacZ α-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63-74. [DOI] [PubMed] [Google Scholar]
- 41.Urbauer, J. L., K. Adelman, R. J. Urbauer, M. F. Simeonov, J. M. Gilmore, M. Zolkiewski, and E. N. Brody. 2001. Conserved regions 4.1 and 4.2 of σ70 constitute the recognition sites for the anti-σ factor AsiA, and AsiA is a dimer free in solution. J. Biol. Chem. 276:41128-41132. [DOI] [PubMed] [Google Scholar]
- 42.Vanderpool, C. K., and S. K. Armstrong. 2001. The Bordetella bhu locus is required for heme iron utilization. J. Bacteriol. 183:4278-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Hove, B., H. Staudenmaier, and V. Braun. 1990. Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coli K-12. J. Bacteriol. 172:6749-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welz, D., and V. Braun. 1998. Ferric citrate transport of Escherichia coli: functional regions of the FecR transmembrane regulatory protein. J. Bacteriol. 180:2387-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wosten, M. M. 1998. Eubacterial sigma-factors. FEMS Microbiol. Rev. 22:127-150. [DOI] [PubMed] [Google Scholar]
- 46.Wriedt, K., A. Angerer, and V. Braun. 1995. Transcriptional regulation from the cell surface: conformational changes in the transmembrane protein FecR lead to altered transcription of the ferric citrate transport genes in Escherichia coli. J. Bacteriol. 177:3320-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]