Abstract
Background
The functional polymorphism -260 C>T in the LPS sensing TLR4 co-receptor CD14 gene enhances the transcriptional activity and results in a higher CD14 receptor density. Individuals carrying the T/T genotype also have significantly higher serum levels of soluble CD14. The T allele of this polymorphism has recently been linked to Chlamydia pneumoniae infection. We investigated the role of the CD14 -260 C>T polymorphism in the susceptibility to and severity (defined as subfertility and/or tubal pathology) of C. trachomatis infection in Dutch Caucasian women.
Methods
The different CD14 -260 C>T genotypes were assessed by PCR-based RFLP analysis in three cohorts: 1) A cohort (n = 576) of women attending a STD clinic, 2) a cohort (n = 253) of women with subfertility, and 3) an ethnically matched control cohort (n = 170). The following variables were used in the analysis: In cohort 1 the CT-DNA status, CT IgG serology status, self-reported symptoms and in cohort 2, the CT IgG serology status and the tubal status at laparoscopy.
Results
In the control cohort the CC, CT and TT genotype distribution was: 28.2%, 48.2%, and 23.5% respectively. No differences were found in the overall prevalence of CD14 -260 genotypes (28.1%, 50.7%, and 21.2%) in cohort 1 when compared to the control cohort. Also no differences were observed in women with or without CT-DNA, with or without serological CT responses, with or without symptoms, or in combinations of these three variables. In subfertile women with tubal pathology (cohort 2, n = 50) the genotype distribution was 28.0%, 48.0%, and 24.0% and in subfertile women without tubal pathology (n = 203), 27.6%, 49.3% and 23.2%. The genotype distribution was unchanged when CT IgG status was introduced in the analyses.
Conclusion
The CD14 -260 C>T genotype distributions were identical in all three cohorts, showing that this polymorphism is not involved in the susceptibility to or severity of sequelae of C. trachomatis infection.
Background
Chlamydia species are related to a broad clinical spectrum of human disease including Chlamydia pneumoniae in lung and cardiovascular disease, C. psittaci in pulmonary emphysema and psittacosis, and C. trachomatis in ocular and urogenital infections [1-3].
C. trachomatis is the most prevalent sexually transmitted disease in Europe and the USA. Due to the mostly asymptomatic course of infection, these women will most likely not be treated resulting in an enhanced risk for the development of late complications, which include pelvic inflammatory disease (PID), ectopic pregnancy and tubal infertility.
The female reproductive tract is a very complex system where many factors, including hormones, vaginal flora and immune mediators, combine to provide protection on the one hand, while on the other hand maintaining an environment suitable for conception [4]. Clear differences in the clinical course of infection have been described and are due to an interaction between environmental (e.g. co-infection), bacterial (e.g. virulence factors) and host factors (genetic differences between individuals). In previous studies no clear associations have been demonstrated between C. trachomatis serotype, C. trachomatis genotype, and the course of C. trachomatis infection[5,6], although differences in cytotoxicity for different serovars have been described[7] and an association between C. trachomatis serovar G and cervical squamous cell carcinoma has been suggested [8]. In addition, virulence gene expression studies, and genomic comparisons of strains, isolated from clearly symptomatic or asymptomatic infected persons, revealed no strong role for the CT bacterium in relation to the course of infection[9,10].
A limited number of studies have recently demonstrated the influence of host genetic factors on the susceptibility to and the severity of C. trachomatis infection. Host factors including HLA-DQ and interleukin 10 (IL-10) have been associated with Chlamydia infection[11].
The Toll Like Receptor (TLR) family is a group of pattern recognition receptors, which recognise several microbial products, including bacterial cell wall components and DNA[12]. Poltorak et al. associated TLR4 with lipopolysaccharide (LPS) recognition in mice[13]. Further studies in mice corroborated these data [14,15], while studies in human demonstrated associations between TLR4 mutations and LPS hyporesponsiveness[16]. We did not observe an association between the TLR4 Asp299Gly polymorphism in patients with tubal pathology although the study population was relatively small[17]. The lack of association can be explained by recent publications showing that heterozygous carriage of the TLR4 Asp299Gly mutation does not affect LPS responsiveness and that only the rare homozygous carriers are less responsive to LPS[18].
CD14 acts as a co-receptor for TLR4 and confers responsiveness to LPS, a component of the cell wall of most Gram-negative bacteria. CD14 forms a complex with LPS and the LPS-binding protein (LBP) (figure 1) [19]. Combined with TLR4 this complex induces NF-κB associated immune responses including the release of a broad spectrum of cytokines that include tumour necrosis factor alpha (TNF-α), IL-1, IL-6, and IL-8 to initiate immune response[20].
Figure 1.
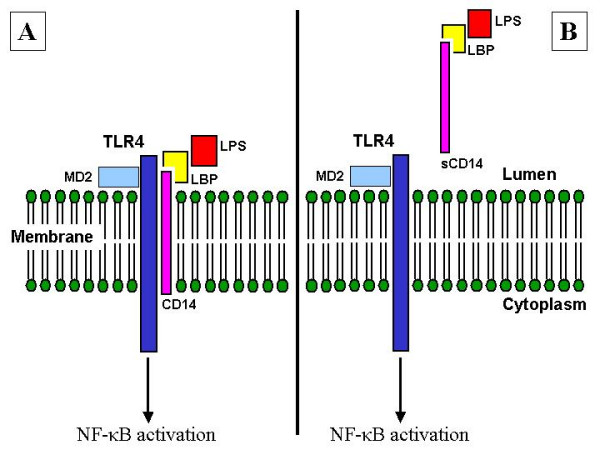
CD14 localisation. Panel A: Membrane-bound CD14 (mCD14) complexed with TLR4 and the LBP – LPS complex. Panel B: Soluble CD14 (sCD14). Abbreviations: TLR: Toll-Like Receptor; LBP: LPS Binding Protein; LPS: Lipopolysaccharide; NF-κB: Nuclear Factor κB.
The promotor region of the CD14 gene contains a single nucleotide polymorphism (SNP) at position -260. The -260 C>T genetic variation affects the binding of transcription factors[21] and has been associated with levels of sCD14 and inversely associated with serum IgE levels[20]. This SNP has been associated with myocardial infarction [22], Crohn's disease[23] and an increased susceptibility to develop chronic spondyloarthropathy in women[24].
Eng et al. demonstrated that carriers of the T allele of this promotor polymorphism have a higher expression of both mCD14 and sCD14 and that TNFα production is increased in the homozygous CD14 -260 T carriers when stimulated with either C. pneumoniae or C. trachomatis [25]. In a recent article, Rupp and colleagues described an association between the mutant allele and an increased susceptibility to chronic C. pneumoniae infection in coronary artery disease patients[26]. Since the CD14 -260 C>T is functional[25] and is associated with C. pneumoniae infection[26], one could hypothesize that in Chlamydia trachomatis infection this polymorphism could influence the susceptibility to and severity of this most prevalent sexually transmitted bacterium which is associated with female infertility.
Therefore, we investigated the role of the CD14 -260 C>T polymorphism in the susceptibility to and severity (defined as subfertility and/or tubal pathology) of C. trachomatis infection in Dutch Caucasian women. A cohort of women attending a STD clinic was used to assess the susceptibility to C. trachomatis infection, taking into account both C. trachomatis DNA and C. trachomatis IgG detection, symptoms and coinfections. A cohort of subfertile women with or without clinically well-defined tubal pathology was used to assess the role of CD14 in the severity of sequelae of C. trachomatis infection.
Methods
Patient populations
STD cohort
Women of Dutch Caucasian (DC) origin (n = 576), under the age of 33 (range 14 to 33 years; median 22 years) and visiting the STD outpatient clinic in Amsterdam, The Netherlands, were included in this study (collection period: July 2001 – December 2004) (Table 1). All 576 women were consecutively included as the first part of a large prospective study. For every CT-DNA positive woman two consecutive CT-DNA negative controls were included in the study. The women were asked to sign an informed consent and to fill out a questionnaire, regarding their complaints at that moment, varying from increased discharge, having bloody discharge during and/or after coitus, recent abdominal pain (not gastrointestinal or menses related) and/or dysuria. A cervical swab was taken for the detection of C. trachomatis DNA (CT-DNA) by PCR (COBAS AMPLICOR; Hoffman – La Roche, Basel, Switzerland)[27].
Table 1.
Patient characteristics in the STD and subfertility cohorts.
| STD cohort | Subfertility cohort | |||
| n | 576 | 253 | ||
| CT DNA (LCx) | + | 184 | ||
| - | 392 | |||
| CT IgG | + | 217 | ||
| - | 359 | |||
| CT IgG (MIF) | + (> = 32) | 41 | ||
| - (<32) | 212 | |||
| Coinfections | - | 401 | ||
| + | 175 | |||
| C. albicans | 160 | |||
| N. gonorrhoea | 7 | |||
| T. vaginalis | 6 | |||
| H. simplex virus 1 | 2 | |||
| H. simplex virus 2 | 5 | |||
| Symptoms | - | 335 | ||
| + | 221 | |||
| Vulvovaginal discharge | 157 | |||
| Abdominal pain | 81 | |||
| Dysuria | 58 | |||
| Bleeding during/after coitus | 25 | |||
| Age | Average | 23.6 y | 30 y | |
| Range | 15 – 41 y | 19 – 39 y | ||
| Median | 23 y | 31 y | ||
| Tubal Pathology | + | 50 | ||
| - | 203 |
Abbreviations: CT: C. trachomatis; STD: sexually transmitted disease; TP: tubal pathology
Peripheral venous blood was collected for the analysis of IgG antibodies against C. trachomatis (CT) (Medac Diagnostika mbH, Hamburg, Germany). A titre of ≥ 1:50 was considered positive. Samples with grey zone values, e.g. cut off ± 10%, were repeated and considered positive when the result was positive or again within the grey zone. Infections with the microorganisms: Candida albicans, Neisseria gonorrhoea, Trichomonas vaginalis, Herpes simplex virus 1/2, may result in symptoms similar to CT infection. Infection status for these microorganisms was recorded. HSV 1/2 was detected according the methods described by Bruisten et al[28]. N. gonorrhoea was detected according methods described by Spaargaren et al [29]. T. vaginalis was cultured on Trichosel medium according standard procedures [30] and detection of T. vaginalis was according the methods described by van der Schee et al [31]. C. albicans was cultured on Chrom agar and detection of C. albicans was performed according standard procedures [30].
Subfertility cohort
The study was performed in 253 consecutive Dutch Caucasian women who visited the department of Obstetrics and Gynaecology of the Academisch Ziekenhuis Maastricht, The Netherlands, between December 1990 and November 2000 because of subfertility [32]. In these women a laparoscopy with tubal testing had been performed as part of their fertility work-up. Preoperatively blood was drawn from all patients for Chlamydia IgG antibody testing (CAT), and spare sera were cryopreserved.
Two independent investigators, who were unaware of the CAT results, scored the laparoscopy reports to assess the grade of tubal pathology. Tubal pathology was defined as extensive peri-adnexal adhesions and/or distal occlusion of at least one tube at laparoscopy [33]. Subfertile women who had no peri-adnexal adhesions and had patent tubes at laparoscopy served as negative controls. Based on these criteria, 50 women had tubal pathology and 203 women served as controls.
IgG antibodies to C. trachomatis were detected with a species-specific MIF test (AniLabSystems, Finland), as described previously [32], with comparable sensitivity and specificity as compared to the IgG ELISA from Medac used for the STD cohort [34]. A positive C. trachomatis IgG MIF test was defined as a titre ≥1:32. Findings at laparoscopy were correlated with the MIF test results. Based on the MIF test, 41 women were found to be CT IgG positive, while 212 were CT IgG negative. Of the CT IgG positive women 28 (68.8%) had tubal pathology, while 22 women (10.4%) of the CT IgG negative women had tubal pathology.
Healthy controls
A healthy Dutch Caucasian control group (n = 170) was included to assess the general frequency of the CD14 -260 genotypes in the Dutch Caucasian population.
Immunogenetic analyses
DNA Extraction
STD cohort
Eukaryotic DNA from PBMC was isolated using the isopropanol isolation method. In short: 100 μl PBMC in PBS were added to 600 μl L6 (Nuclisens Lysisbuffer, Organon Teknika, Boxtel, The Netherlands) and 1 μl glycogen (Roche Molecular Diagnostics, Almere, The Netherlands). The samples were incubated for 30 minutes at 65°C and left to cool at RT. An equal volume of cold (-20°C) isopropanol was added to the samples. The samples were then centrifuged (20 min at 20.000 G). The supernatant was discarded and the pellets were washed twice in 75% EtOH. The pellets were dissolved in T10 overnight (O/N) at 4°C and then stored at -20°C until further analysis.
Subfertility cohort
Genomic DNA was extracted out of the cryopreserved sera using High Pure PCR Template Preparation Kit (HPPTP kit) according to the manufacturer's instructions (Roche Molecular Biochemicals, Mannheim, Germany).
Healthy controls
Blood was collected in EDTA-tubes and stored at room temperature until the genomic DNA was extracted from peripheral blood leukocytes (PBMC) according to an in-house DNAzol (Invitrogen, The Netherlands) isolation procedure.
CD14 -260 C>T gene polymorphism
The C>T substitution in the proximal CD14 promoter GC box at position -260 from the translation start site (NCBI SNP CLUSTER ID:rs2569190) results in a HaeIII restriction site. We developed a PCR assay using the primers, 5' TCA CCT CCC CAC CTC TCT T 3' (sense) and 5' CCT GCA GAA TCC TTC CTG TT 3' (antisense) (Invitrogen Life Technologies, Breda, The Netherlands), flanking this restriction site. Amplification was performed using a thermal cycler Perkin-Elmer 9700 (Applied Biosystems, Forter City, CA, USA). The parameters were an initial denaturation at 95°C for 5 min, followed by 35 cycles: denaturation at 95°C for 30 s, annealing at 59°C for 30 s, and elongation at 72°C for 1 min. The final elongation was at 72°C for 7 min followed for a cooling to 4°C. The 107-bp fragments were digested overnight at 37°C with HaeIII (Invitrogen, The Netherlands) resulting in fragments that either were cut in two fragments of 83-bp and 24-bp (allele C) or were not restricted (T allele). These fragments were analyzed by electrophoresis on 4% low melting agarose gels (Tebu-Bio, The Netherlands) stained with ethidium bromide.
Statistical analyses
All groups were tested for Hardy-Weinberg equilibrium to check for Mendelian inheritance. Statistical analyses were performed using Instat Graphpad and SPSS version 11 (SPSS Inc., Chicago, IL, USA). Fisher exact and χ2 tests were used to test for differences in CD14 allele/genotype/carrier frequencies between the (sub)groups and p-values < 0.05 were considered statistically significant.
Results
All genotype distributions assessed were in Hardy-Weinberg Equilibrium. The CD14 -260 C>T SNP was assessed in the STD, subfertility and control cohorts.
CD14 -260 in the susceptibility to C. trachomatis infection
To determine the effects of CD14 -260 C>T on the susceptibility to C. trachomatis infection, the prevalence of CD14 -260 C>T genotypes were assessed in the STD cohort (table 2). The overall genotype distribution was 28.1% CC, 50.7% CT, 21.2% TT. This distribution was comparable to the healthy controls (figure 2). The distribution was 28.8% CC, 50.0% CT, 21.2% TT in CT DNA positive women, while in CT DNA negative women the distribution was 27.8% CC, 51.0% CT, 21.2% TT. In women with or without serological CT responses the distribution was 30.4% CC, 49.3% CT, 20.3% TT and 26.7% CC, 51.5% CT, 21.7% TT, respectively. No differences could be observed in women with or without symptoms. Coinfection with other microorganisms or combinations of these four variables (CT DNA, CT serology, symptoms and microorganisms) did not introduce statistically significant differences or trends in CD14 genotype distributions.
Table 2.
CD14 genotype distribution in the Dutch Caucasian STD cohort.
| CD14 -260 C>T | ||||||||
| 1.1 (CC) | 1.2 (CT) | 2.2 (TT) | ||||||
| Total | n | % | n | % | n | % | ||
| Total | 217 | 66 | 30,4% | 107 | 49,3% | 44 | 20,3% | |
| CT IgG+ | LCx+ (CT DNA+) | 135 | 38 | 28,1% | 69 | 51,1% | 28 | 20,7% |
| MO+ | 42 | 12 | 28,6% | 24 | 57,1% | 6 | 14,3% | |
| Symp | 56 | 14 | 25,0% | 31 | 55,4% | 11 | 19,6% | |
| LAP+ | 17 | 4 | 23,5% | 10 | 58,8% | 3 | 17,6% | |
| LCx- (CT DNA-) | 82 | 28 | 34,1% | 38 | 46,3% | 16 | 19,5% | |
| MO+ | 29 | 12 | 41,4% | 11 | 37,9% | 6 | 20,7% | |
| Symp | 43 | 16 | 37,2% | 14 | 32,6% | 13 | 30,2% | |
| LAP+ | 17 | 5 | 29,4% | 8 | 47,1% | 4 | 23,5% | |
| Total | 359 | 96 | 26,7% | 185 | 51,5% | 78 | 21,7% | |
| CT IgG- | LCx+ (CT DNA+) | 49 | 15 | 30,6% | 23 | 46,9% | 11 | 22,4% |
| MO+ | 16 | 3 | 18,8% | 10 | 62,5% | 3 | 18,8% | |
| Symp | 19 | 5 | 26,3% | 11 | 57,9% | 3 | 15,8% | |
| LAP+ | 10 | 3 | 30,0% | 6 | 60,0% | 1 | 10,0% | |
| LCx- (CT DNA-) | 310 | 81 | 26,1% | 162 | 52,3% | 67 | 21,6% | |
| MO+ | 88 | 20 | 22,7% | 53 | 60,2% | 15 | 17,0% | |
| Symp | 103 | 26 | 25,2% | 51 | 49,5% | 26 | 25,2% | |
| LAP+ | 37 | 6 | 16,2% | 20 | 54,1% | 11 | 29,7% | |
| Healthy Controls | 170 | 48 | 28,2% | 82 | 48,2% | 40 | 23,5% | |
C. trachomatis IgG positive and negative patients, divided in CT DNA (LCx) positive and negative and subdivided in coinfection with other microorganisms (N. gonorrhoea, T. vaginalis, C. albicans, H. simplex virus 1 & 2), symptoms (vulvovaginal discharge, abdominal pain, dysuria, bleeding during/after coitus) and lower abdominal pain. Abbreviations: CT: C. trachomatis; MO+: microorganism positive; LAP+: lower abdominal pain positive; Symp: symptoms positive
Figure 2.
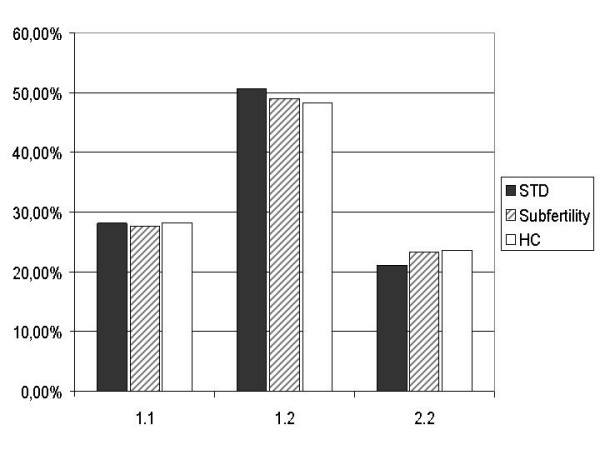
CD14 genotype distribution in the STD, subfertility and control cohorts. Abbreviations: STD: sexually transmitted disease; HC: healthy controls
CD14 -260 in the severity of sequelae of C. trachomatis infection
The effect of CD14 -260 C>T on the severity of sequelae of C. trachomatis infection was assessed in a cohort of subfertile women with clinically well-defined tubal pathology. The overall genotype distribution in the cohort was 27.7% CC, 49.0% CT and 23.3% TT (figure 2). The genotype distribution in women with tubal pathology was similar to the distribution in women without tubal pathology (28.0% CC, 48.0% CT, 24.0% TT and 27.6% CC, 49.3% CT, 23.2% TT respectively) and to the distribution in the healthy controls (table 3). Introduction of CT IgG serology, with special attention to C. trachomatis positive women who did develop tubal pathology as compared to those who did not develop tubal pathology, did not alter the observed genotype distribution.
Table 3.
CD14 genotype distribution in the Dutch Caucasian subfertility cohort.
| CD14 -260 C>T | |||||||
| 1.1 (CC) | 1.2 (CT) | 2.2 (TT) | |||||
| Total | n | % | n | % | n | % | |
| Total | 253 | 70 | 27,7% | 124 | 49,0% | 59 | 23,3% |
| TP+ | 50 | 14 | 28,0% | 24 | 48,0% | 12 | 24,0% |
| TP- | 203 | 56 | 27,6% | 100 | 49,3% | 47 | 23,2% |
| CT IgG+ TP+ | 28 | 9 | 32,1% | 15 | 53,6% | 4 | 14,3% |
| CT IgG+ TP- | 13 | 4 | 30,8% | 6 | 46,2% | 3 | 23,1% |
| Healthy Controls | 170 | 48 | 28,2% | 82 | 48,2% | 40 | 23,5% |
Distribution in the total cohort and subdivided in women with or without tubal pathology, and C. trachomatis IgG positive women with or without tubal pathology. C. trachomatis positivity defined as a titre ≥ 1:32 (MIF). Abbreviations: TP: tubal pathology; CT: Chlamydia trachomatis.
Discussion
We did not find an association between the functional upregulating CD14 -260 C>T polymorphism and the susceptibility to or subsequent severity of sequelae of C. trachomatis infection, as assessed in the STD and subfertility populations (figure 2). However, these results do not exclude that a still unknown CD14 expression decreasing SNP may influence the course of C. trachomatis infection.
Recent studies have shown that Chlamydia LPS is capable of inducing an inflammatory response through CD14[35,36], although the potency to induce an inflammatory response was 100 – 1000 times less when compared to the responses induced by S. minnesota, N. gonorrhoea [35] and the enterobacteria S. enterica and E. coli[36]. Heine et al. demonstrated that the CD14 associated inflammatory response was TLR4 but not TLR2 mediated[36]. These results are corroborated by studies showing the role of the CD14-TLR4-MD2 complex in intracellular signalling by LPS[13,37] and studies showing the dependency on CD14 of phagocytosis of Gram negative bacteria [38].
The absence of an association between CD14 and susceptibility to C. trachomatis infection might be explained by the compartmentalisation of TLR4. The differential expression of TLR4 has been described in immortalised cell-lines derived from the female urogenital tract [39] and recently demonstrated in cells isolated from patients by Pioli[40] and Fazeli[41]. TLRs 1 – 6 were found to be expressed in the epithelia of the female urogenital tract. TLR2 and TLR4 were the only Toll like receptors with a clear differential expression. Low expression in the lower urogenital tract and high expression in the upper genital tract[40,41]. The expression remained similar in all subjects irrespective of age or status of the reproductive cycle[41]. It is hypothesized that through this expression pattern TLR4 modulates immunological tolerance in the lower genital tract and induces host defence against ascending infection in the upper genital tract[41]. In the upper genital tract, Fazeli and colleagues found TLR4 positive vacuole like structures that seemed to be secreted from endocervical glands[42]. A secretory form of TLR4 has been described in mice, where the soluble TLR4 appears to inhibit LPS mediated signals, while at the same time sTLR4 mRNA is upregulated by LPS[43]. This may represent a feedback mechanism to prevent excessive responses to LPS in the endocervix, which can be seen as a boundary between the lower and upper genital tract. Further evidence for the regulation of immune responses to LPS by TLR4 is provided by the study of Harju et al., who demonstrated the intrauterine expression of TLR4 and endotoxin responsiveness in mice in the perinatal period [44]. mCD14 is expressed on human endometrial stromal cells but not on endometrial epithelial gland cells. The epithelial cells are dependent on sCD14 for LPS recognition [45]. Soluble CD14 is present in the cervical mucosa and may be present in the endometrium[46].
Combining the aforementioned studies with the knowledge that CD14 can signal through TLR4, it might be hypothesized that the absence of an association between the CD14 -260 SNP and the susceptibility to C. trachomatis infection might be due to the low expression or absence of TLR4 in the lower urogenital tract. In the upper genital tract, strict regulation of immune responses to LPS by TLR4 may inhibit CD14 signalling through TLR4 [43,44], thus limiting the influence of CD14 on the development of tubal pathology.
However, this hypothesis does not take into account the ability of CD14 to signal through TLR2[47], nor does it take into account that the study of Netea et al. which demonstrated that non-LPS components of Chlamydia pneumoniae can stimulate cytokine production through TLR2 dependent, CD14 independent pathways[48] and that a similar mechanism may exist and stimulate C. trachomatis induced cytokine production in urogenital infections.
Since TLR2 is involved in Chlamydia-induced TGF-beta, an anti-inflammatory cytokine with an important role in fibrosis, and thus very likely in post-infection tubal pathology, it might explain why CD14 polymorphisms may not severely impact the development of tubal pathology[49].
Darville et al. have demonstrated that TLR2 is an important mediator of innate immune responses in C. trachomatis infection in mice and plays an important role in early production of immune mediators and development of tubal pathology[50,51]. In a recent publication by Pitz et al. it was shown that C. pneumoniae is capable of activating endothelial cells by TLR2 as initial extracellular C. pneumoniae receptor, whereas NOD1 was shown to be a potent intracellular immune receptor for C. pneumoniae in endothelial cells. Further research may extend these results to C. trachomatis infections. Overall, the recognition of bacterial LPS involves a complex system of multiple receptors and a complex orchestration of protein-protein interactions [52].
Conclusion
Our study showed that the functional up-regulating CD14 -260 C>T SNP did neither influence the susceptibility to nor the severity of late sequelae of Chlamydia trachomatis infection. However, this does not exclude a prominent role for CD14 in the course of an active C. trachomatis infection and not yet described CD14 expression decreasing SNPs may affect the course of C. trachomatis infection profoundly. Further studies on the immunogenetics of C. trachomatis infection will provide more insight in the clear differences in the clinical course that this microorganism induces in individuals and lead to potential vaccine candidates.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
SO: Data acquisition, data/statistical analyses, drafting the manuscript
JS: Sample collection, drafting the manuscript, critically revising for medical content
JAL and JEDH: Sample collection, critically revising for medical content
JSAF: Sample collection, critically revising for medical content
JP: Data acquisition, data analyses
ASP: Study design and conception, critically revising for immunogenetic content
SAM: Study design, conception and coordination, critically revising for immunogenetic content
All authors contributed to writing of the final manuscript.
All authors read and approved the final manuscript
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
Sander Ouburg is an AstraZeneca Nederland BV fellow.
Servaas A. Morré is supported by the Department of Internal Medicine of the VU University Medical Centre, the Netherlands.
The authors are indebted to Prof. Cathrien Bruggeman, head of the department of Medical Microbiology, Academisch Ziekenhuis Maastricht, Maastricht, The Netherlands, for the serological testing of the subfertility cohort.
The ICTI consortium (Integrated approach to Chlamydia trachomatis Infections [53]) provides a broad specialized network for the multidisciplinary studies described.
Contributor Information
Sander Ouburg, Email: s.ouburg@vumc.nl.
Joke Spaargaren, Email: jspaargaren@ggd.amsterdam.nl.
Janneke E den Hartog, Email: Jlan@sgyn.azm.nl.
Jolande A Land, Email: Jedh@sgyn.azm.nl.
Johan SA Fennema, Email: hfennema@ggd.amsterdam.nl.
Jolein Pleijster, Email: j.pleijster@vumc.nl.
A Salvador Peña, Email: aspena@planet.nl.
Servaas A Morré, Email: samorretravel@yahoo.co.uk.
the ICTI consortium, Email: samorretravel@yahoo.co.uk.
References
- Numazaki K, Asanuma H, Niida Y. Chlamydia trachomatis infection in early neonatal period. BMC Infect Dis. 2003;3:2. doi: 10.1186/1471-2334-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smieja M, Mahony J, Petrich A, Boman J, Chernesky M. Association of circulating Chlamydia pneumoniae DNA with cardiovascular disease: a systematic review. BMC Infect Dis. 2002;2:21. doi: 10.1186/1471-2334-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smieja M, Leigh R, Petrich A, Chong S, Kamada D, Hargreave FE, Goldsmith CH, Chernesky M, Mahony JB. Smoking, season, and detection of Chlamydia pneumoniae DNA in clinically stable COPD patients. BMC Infect Dis. 2002;2:12. doi: 10.1186/1471-2334-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunological Reviews. 2005 doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Workowski KA, Stevens CE, Suchland RJ, Holmes KK, Eschenbach DA, Pettinger MB, Stamm WE. Clinical manifestations of genital infection due to Chlamydia trachomatis in women: differences related to serovar. Clin Infect Dis. 1994;19:756–760. doi: 10.1093/clinids/19.4.756. [DOI] [PubMed] [Google Scholar]
- Lyons JM, Ito Jr JI, Morré SA. Journal of Clinical Pathology. Vol. 57. England; 2004. Chlamydia trachomatis serovar E isolates from patients with different clinical manifestations have similar courses of infection in a murine model: host factors as major determinants of C trachomatis mediated pathogenesis; pp. 657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JM, Ito Jr JI, Peña AS, Morré SA. Journal of Clinical Pathology. Vol. 58. England; 2005. Differences in growth characteristics and elementary body associated cytotoxicity between Chlamydia trachomatis oculogenital serovars D and H and Chlamydia muridarum; pp. 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila T, Saikku P, Koskela P, Bloigu A, Dillner J, Ikaheimo I, Jellum E, Lehtinen M, Lenner P, Hakulinen T, Närvänen A, Pukkala E, Thoresen S, Youngman L, Paavonen J. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA. 2001;285:47–51. doi: 10.1001/jama.285.1.47. [DOI] [PubMed] [Google Scholar]
- Morré SA, Ossewaarde JM, Savelkoul PHM, Stoof J, Meijer CJLM, van den Brule AJC. Analysis of genetic heterogeneity in Chlamydia trachomatis clinical isolates of serovars D, E and F by Amplified Fragment length Polymorphism. J Clin Microbiol. 2000;38:3463–3466. doi: 10.1128/jcm.38.9.3463-3466.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek Y, Spaargaren J, Langerak AAJ, Merks J, Morré SA, van der Ende A. Interrelationship between polymorphisms of incA, fusogenic properties of Chlamydia trachomatis strains, and clinical manifestations in patients in The Netherlands. Journal of Clinical Microbiology. 2005;43:2441–2443. doi: 10.1128/JCM.43.5.2441-2443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen AH, Surcel HM, Lehtinen M, Karhukorpi J, Tiitinen A, Halttunen M, Bloigu A, Morrison RP, Karttunen R, Paavonen J. HLA DQ alleles and interleukin-10 polymorphism associated with Chlamydia trachomatis-related tubal factor infertility: a case-control study. Human Reproduction. 2002;17:2073–2078. doi: 10.1093/humrep/17.8.2073. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Annual Review of Immunology. Vol. 21. United States; 2003. Toll-like receptors; pp. 335–376. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Current Opinion in Immunology. 2000;12:20–26. doi: 10.1016/S0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- Netea MG, Van Der Graaf CAA, Vonk AG, Verschueren I, van der Meer JWM, Kullberg BJ. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis. 2002;185:1483–1489. doi: 10.1086/340511. [DOI] [PubMed] [Google Scholar]
- Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- Morré SA, Murillo LS, Bruggeman CA, Peña AS. The role that the functional Asp299Gly polymorphism in the toll-like receptor-4 gene plays in susceptibility to Chlamydia trachomatis-associated tubal infertility. J Infect Dis. 2003;187:341–342. doi: 10.1086/346044. [DOI] [PubMed] [Google Scholar]
- Erridge C, Stewart J, Poxton IR. Monocytes Heterozygous for the Asp299Gly and Thr399Ile Mutations in the Toll-like Receptor 4 Gene Show No Deficit in Lipopolysaccharide Signalling. J Exp Med. 2003;197:1787–1791. doi: 10.1084/jem.20022078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD. A Polymorphism* in the 5' flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol. 1999;20:976–983. doi: 10.1165/ajrcmb.20.5.3494. [DOI] [PubMed] [Google Scholar]
- Zhang DE, Hetherington CJ, Tan S, Dziennis SE, Gonzalez DA, Chen HM, Tenen DG. Sp1 is a critical factor for the monocytic specific expression of human CD14. J Biol Chem. 1994;269:11425–11434. [PubMed] [Google Scholar]
- Hubacek JA, Rothe G, Pit'ha J, Škodová Z, Stanek V, Poledne R, Schmitz G. C(-260)-->T polymorphism in the promoter of the CD14 monocyte receptor gene as a risk factor for myocardial infarction. Circulation. 1999;99:3218–3220. doi: 10.1161/01.cir.99.25.3218. [DOI] [PubMed] [Google Scholar]
- Klein W, Tromm A, Griga T, Folwaczny C, Hocke M, Eitner K, Marx M, Duerig N, Epplen JT. Interaction of polymorphisms in the CARD15 and CD14 genes in patients with Crohn disease. Scandinavian Journal of Gastroenterology. 2003;38:834–836. doi: 10.1080/00365520310003147. [DOI] [PubMed] [Google Scholar]
- Repo H, Anttonen K, Kilpinen SK, Palotie A, Salven P, Orpana A, Leirisalo-Repo M. CD14 and TNfa promoter polymorphisms in patients with acute arthritis. Special reference to development of chronic spondyloarthropathy. Scand J Rheumatol. 2002;31:355–361. doi: 10.1080/030097402320817086. [DOI] [PubMed] [Google Scholar]
- Eng HL, Wang CH, Chen CH, Chou MH, Cheng CT, Lin TM. A CD14 promoter polymorphism is associated with CD14 expression and Chlamydia-stimulated TNFalpha production. Genes & Immunity. 2004. [DOI] [PubMed]
- Rupp J, Goepel W, Kramme E, Jahn J, Solbach W, Maass M. CD14 promoter polymorphism -159C>T is associated with susceptibility to chronic Chlamydia pneumoniae infection in peripheral blood monocytes. Genes & Immunity. 2004. [DOI] [PubMed]
- van Doornum GJJ, Schouls LM, Pijl AS, Cairo I, Buimer M, Bruisten SM. Comparison between the LCx probe system and the COBAS AMPLICOR system for detection of Chlamydia trachomatis and Neisseria gonorrhoeae infections in patients attending a clinic for treatment of sexually transmitted diseases in Amsterdam, The Netherlands. Journal of Clinical Microbiology. 2001;39:829–835. doi: 10.1128/JCM.39.3.829-835.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruisten SM, Cairo I, Fennema H, Pijl A, Buimer M, Peerbooms PGH, van Dyck E, Meijer A, Ossewaarde JM, van Doornum GJJ. Diagnosing genital ulcer disease in a clinic for sexually transmitted diseases in Amsterdam, The Netherlands. J Clin Microbiol. 2001;39:601–605. doi: 10.1128/JCM.39.2.601-605.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaargaren J, Stoof J, Fennema H, Coutinho RA, Savelkoul PH. Amplified fragment length polymorphism fingerprinting for identification of a core group of Neisseria gonorrhoeae transmitters in the population attending a clinic for treatment of sexually transmitted diseases in Amsterdam, The Netherlands. J Clin Microbiol. 2001;39:2335–2337. doi: 10.1128/JCM.39.6.2335-2337.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PR, Baron EJ, Pfaller MA, Tenover FC and Yolken RH, editor. Manual of Clinical Microbiology. 6. Washington D.C., American Society for Microbiology Press, Washington D.C., USA; 1995. [Google Scholar]
- van der Schee C, Sluiters HJF, van der Meijden WI, van Beek P, Peerbooms PGH, Verbrugh H, van Belkum A. Host and pathogen interaction during vaginal infection by Trichomonas vaginalis and Mycoplasma hominis or Ureaplasma urealyticum. Journal of Microbiological Methods. 2001;45:61–67. doi: 10.1016/S0167-7012(01)00224-X. [DOI] [PubMed] [Google Scholar]
- Land JA, Gijsen AP, Kessels AGH, Slobbe MEP, Bruggeman CA. Performance of five serological chlamydia antibody tests in subfertile women. Human Reproduction. 2003;18:2621–2627. doi: 10.1093/humrep/deg479. [DOI] [PubMed] [Google Scholar]
- Land JA, Evers JLH, Goossens VJ. How to use Chlamydia antibody testing in subfertility patients. Human Reproduction. 1998;13:1094–1098. doi: 10.1093/humrep/13.4.1094. [DOI] [PubMed] [Google Scholar]
- Morré SA, Munk C, Persson K, Krüger-Kjaer S, van Dijk R, Meijer CJLM, van den Brule AJC. Comparison of three commercially available peptide-based immunoglobulin G (IgG) and IgA assays to microimmunofluorescence assay for detection of Chlamydia trachomatis antibodies. Journal of Clinical Microbiology. 2002;40:584–587. doi: 10.1128/JCM.40.2.584-587.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalls RR, Rice PA, Qureshi N, Takayama K, Lin JS, Golenbock DT. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infection and Immunity. 1995;63:3125–3130. doi: 10.1128/iai.63.8.3125-3130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine H, Müller-Loennies S, Brade L, Lindner B, Brade H. Endotoxic activity and chemical structure of lipopolysaccharides from Chlamydia trachomatis serotypes E and L2 and Chlamydophila psittaci 6BC. European Journal of Biochemistry. 2003;270:440–450. doi: 10.1046/j.1432-1033.2003.03392.x. [DOI] [PubMed] [Google Scholar]
- Akashi S, Saitoh S, Wakabayashi Y, Kikuchi T, Takamura N, Nagai Y, Kusumoto Y, Fukase K, Kusumoto S, Adachi Y, Kosugi A, Miyake K. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. Journal of Experimental Medicine. 2003;198:1035–1042. doi: 10.1084/jem.20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald U, Fan X, Jack RS, Workalemahu G, Kallies A, Stelter F, Schütt C. J Immunol. Vol. 157. UNITED STATES; 1996. Monocytes can phagocytose Gram-negative bacteria by a CD14-dependent mechanism; pp. 4119–4125. [PubMed] [Google Scholar]
- Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. J Immunol. Vol. 168. United States; 2002. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling; pp. 2424–2432. [DOI] [PubMed] [Google Scholar]
- Pioli PA, Amiel E, Schaefer TM, Connolly JE, Wira CR, Guyre PM. Infection and Immunity. Vol. 72. United States; 2004. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract; pp. 5799–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli A, Bruce C, Anumba DO. Human Reproduction. Vol. 20. England; 2005. Characterization of Toll-like receptors in the female reproductive tract in humans; pp. 1372–1378. [DOI] [PubMed] [Google Scholar]
- Bauer S, Kirschning CJ, Häcker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Proc Natl Acad Sci U S A. Vol. 98. United States; 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition; pp. 9237–9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwami KI, Matsuguchi T, Masuda A, Kikuchi T, Musikacharoen T, Yoshikai Y. J Immunol. Vol. 165. UNITED STATES; 2000. Cutting edge: naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling; pp. 6682–6686. [DOI] [PubMed] [Google Scholar]
- Harju K, Ojaniemi M, Rounioja S, Glumoff V, Paananen R, Vuolteenaho R, Hallman M. Pediatric Research. Vol. 57. United States; 2005. Expression of toll-like receptor 4 and endotoxin responsiveness in mice during perinatal period; pp. 644–648. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Meijer L, Normark S, Richter-Dahlfors A. Cell Microbiol. Vol. 4. England; 2002. TLR4-dependent recognition of lipopolysaccharide by epithelial cells requires sCD14; pp. 493–501. [DOI] [PubMed] [Google Scholar]
- Lawn SD, Subbarao S, Wright Jr TC, Evans-Strickfaden T, Ellerbrock TV, Lennox JL, Butera ST, Hart CE. J Infect Dis. Vol. 181. UNITED STATES; 2000. Correlation between human immunodeficiency virus type 1 RNA levels in the female genital tract and immune activation associated with ulceration of the cervix; pp. 1950–1956. [DOI] [PubMed] [Google Scholar]
- Manukyan M, Triantafilou K, Triantafilou M, Mackie A, Nilsen N, Espevik T, Wiesmüller KH, Ulmer AJ, Heine H. Eur J Immunol. Vol. 35. Germany; 2005. Binding of lipopeptide to CD14 induces physical proximity of CD14, TLR2 and TLR1; pp. 911–921. [DOI] [PubMed] [Google Scholar]
- Netea MG, Kullberg BJ, Galama JMD, Stalenhoef AFH, Dinarello CA, van der Meer JWM. Eur J Immunol. Vol. 32. Germany; 2002. Non-LPS components of Chlamydia pneumoniae stimulate cytokine production through Toll-like receptor 2-dependent pathways; pp. 1188–1195. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Morimoto Y, Iwagaki H, Itoh H, Saito S, Kobayashi N, Yagi T, Tanaka N. Journal of International Medical Research. Vol. 29. England; 2001. Bacterial lipopolysaccharide induces transforming growth factor beta and hepatocyte growth factor through toll-like receptor 2 in cultured human colon cancer cells; pp. 409–420. [DOI] [PubMed] [Google Scholar]
- Darville T, O'Neill JM, Andrews J, Nagarajan UM, Stahl L, Ojcius DM. J Immunol. Vol. 171. United States; 2003. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection; pp. 6187–6197. [DOI] [PubMed] [Google Scholar]
- Erridge C, Pridmore A, Eley A, Stewart J, Poxton IR. Journal of Medical Microbiology. Vol. 53. England; 2004. Lipopolysaccharides of Bacteroides fragilis, Chlamydia trachomatis and Pseudomonas aeruginosa signal via Toll-like receptor 2; pp. 735–740. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Triantafilou K. Trends Immunol. Vol. 23. England; 2002. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster; pp. 301–304. [DOI] [PubMed] [Google Scholar]
- Morré SA, Spaargaren J, Ossewaarde JM, Land JA, Bax CJ, Dörr PJ, Oostvogel PM, Vanrompay D, Savelkoul PHM, Pannekoek Y, van Bergen JEAM, Fennema HSA, de Vries HJC, Crusius JBA, Peña AS, Ito Jr JI, Lyons JM. Description of the ICTI consortium: an integrated approach to the study of chlamydia trachomatis infection. Drugs of Today. 2006. [PubMed]


