Abstract
Background
The genome sequence of the pufferfish Takifugu rubripes is an enormously useful tool in the molecular physiology of fish. Euryhaline fish that can survive both in freshwater (FW) and seawater (SW) are also very useful for studying fish physiology, especially osmoregulation. Recently we learned that there is a pufferfish, Takifugu obscurus, common name "mefugu" that migrates into FW to spawn. If T. obscurus is indeed a euryhaline fish and shares a high sequence homology with T. rubripes, it will become a superior animal model for studying the mechanism of osmoregulation. We have therefore determined its euryhalinity and phylogenetic relationship to the members of the Takifugu family.
Results
The following six Takifugu species were used for the analyses: T. obscurus, T. rubripes, T. niphobles, T. pardalis, T. poecilonotus, and T. porphyreus. When transferred to FW, only T. obscurus could survive while the others could not survive more than ten days in FW. During this course of FW adaptation, serum Na+ concentration of T. obscurus decreased only slightly, but a rapid and large decrease occurred even in the case of T. niphobles, a peripheral fresh water species that is often seen in brackish river mouths. Phylogenetic analysis using nucleotide sequences of the mitochondrial 16S ribosomal RNA gene of each species indicated that the six Takifugu species are very closely related with each other.
Conclusion
T. obscurus is capable of adapting to both FW and SW. Its genomic sequence shares a very high homology with those of the other Takifugu species such that the existing Takifugu genomic information resources can be utilized. These properties make "mefugu", which has drawn little attention from animal physiologists until this study, a useful model animal for studying the molecular mechanism of maintaining body fluid homeostasis.
Background
Maintenance of a stable internal environment is important for vertebrate animals to survive in a variety of habitats. Even small changes in ionic balance, osmolarity, and pH of body fluid seriously affect the survival of the animals. Strategies for maintaining body fluid homeostasis are different depending on animals and their habitats. Freshwater (FW) teleosts (modern bony fish) maintain the osmolarity of extracellular fluid around 300 mOsM, while the osmolarity of the environmental freshwater is generally less than 10 mOsM. In order to balance passive loss of salts and gain of water, they take up salts from FW through the gills and excrete a lot of dilute urine from which most of the salts have been reabsorbed by the kidney [1]. Marine teleosts also maintain the osmolarity of extracellular fluid to a level similar to that of freshwater fish, despite that the osmolarity of seawater (SW) is approx. 1000 mOsM. In order to balance passive loss of water and gain of salts, they drink seawater, absorb salts and water both in the intestine, and excrete salts through the gills [1]. The systems used by teleosts to adapt to FW and SW differ not only in the direction of ion and water movements but also in their molecular components. Euryhaline fish adapts to both FW and SW by switching these systems.
To identify the molecular components involved in body fluid homeostasis, the change of expression of each gene during adaptation of euryhaline fishes to different salinities is a potential useful marker since the genes involved are expected to be drastically up- or down-regulated during the adaptation. In fact, several genes have been identified by this strategy using euryhaline fishes such as tilapia [2], salmon [3-5], killifish [6], and eel [7-10]. However, this systematic approach is very laborious because genome sequences are not available for the euryhaline fishes that are currently being used for molecular physiological studies.
Takifugu is a genus of puffer fish and belongs to the family Tetradontidae of teleost fish. It consists of approx. 20 species living in the Northwest Pacific Ocean around China, Korea, and Japan [11,12]. Takifugu species are famous for their puffing behavior, powerful toxins in the internal organs, and edible muscle. Two species are farmed on a commercial scale: T. rubripes is farmed in Japan and T. obscurus, in Korea and China. The Takifugu species have an advantage as animal model, in that they have a short genome (~400 Mb) compared to those of other vertebrates including Homo sapiens (human, 3000 Mb), Mus musculus (mouse, 3000 Mb), Gallus gallus (chicken, 1200 Mb), Xenopus laevis (African clawed frog, 3100 Mb), Danio rerio (zebrafish, 1700 Mb), and Oryzias latipes (medaka, 1100 Mb) [13]. In 2002, the genome project of T. rubripes was completed and the sequence information is now available for free on the websites [14].
Within the genus Takifugu, two species are known to be anadromous, namely, T. obscurus [15-19] and T. ocellatus [16]. T. obscurus (Figure 1, Table 1) is found in the East China Sea, the South China Sea, and inland waters in China and the Korean Peninsula. It lives in the bottom layer of inshore and inland waters, and grows 20–40 cm in length. Most of the growth takes place in the sea but they spawn in brackish and fresh water. During the spawning season, which is from late spring to early summer, the sexually mature fish run into river estuaries and spawn in inland waters including rivers, lakes, and ponds. The fingerlings grow in the inland water and either return to the sea the next spring or they there for a few months before returning to the sea. In the sea they grow to sexually mature fish over several years, and then return to the inland water again to spawn. T. ocellatus is also found in an area similar to that of T. obscurus. T. ocellatus is a small species and grows to around 15 cm in length. The life cycle of T. ocellatus has not been well described but is expected to be similar to that of T. obscurus.
Figure 1.
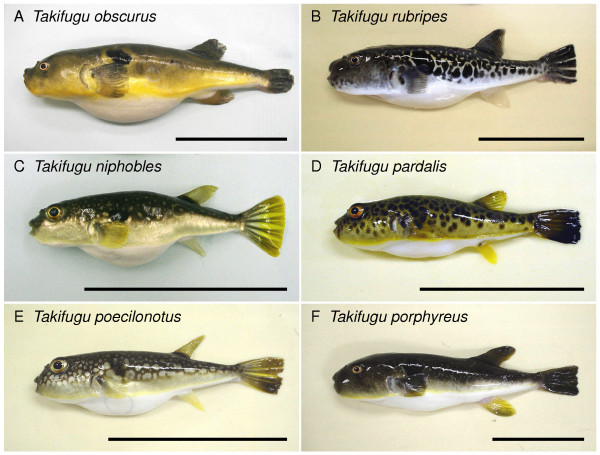
Six Takifugu species used in this study. A. T. obscurus. B. T. rubripes. C. T. niphobles. D. T. pardalis. E. T. poecilonotus. F. T. porphyreus. Scale bars represent 50 mm.
Table 1.
List of Takifugu species used in this study.
| Adaptability | Species (Common name) | Environment (Total length) | Spawning | Region | Ref. |
| Seawater fish, Anadromous freshwater fish | Takifugu obscurus (Obscure puffer, Mefugu) | Sea River 20–40 cm |
River | East Asia | [12,15-17,46] |
| Seawater fish, Peripheral freshwater fish | Takifugu niphobles (Grass puffer, Kusafugu) | Seacoast, River mouth* 15 cm |
Sea | East Asia | [12,15,47] |
| Seawater fish | Takifugu pardalis (Panther puffer, Higanfugu) | Sea, River mouth** 20–38 cm |
Sea | East Asia | [12,22] |
| Takifugu poecilonotus (Fine-patterned puffer, Komonfugu) | Sea, River mouth*** 20 cm |
Sea | East Asia | [12,15] | |
| Takifugu rubripes (Ocellate puffer, Tiger puffer, Torafugu) | Sea, River mouth*** 35–80 cm |
Sea | East Asia | [12,15,22] | |
| Takifugu porphyreus (Genuin puffer, Purple puffer, Mafugu) | Sea 20–50 cm |
Sea | East Asia | [12,22] |
* Adult fish are often seen in BW river mouths and sometimes seen in FW rivers.
** Adult fish are sometimes seen in BW river mouths [48].
*** Fingerlings are often seen in brackish river mouths [22].
In this study, we focus on the suitability of T. obscurus as a novel animal model for studying the molecular mechanism of body fluid homeostasis. First we compared the adaptability of T. obscurus to FW with those of other Takifugu species, and showed that only T. obscurus is fully adaptable to both SW and FW. Next we demonstrated that changes in blood Na+ concentration of T. obscurus during FW adaptation are kept within the physiological range while those of T. niphobles decline beyond the range. Finally we isolated and sequenced 16S ribosomal genes from six Takifugu species including T. obscurus, T. niphobles, and T. rubripes, and demonstrated that those sequences are 99% identical within the genus Takifugu. With the euryhalinity and applicability of the currently available fugu genome sequence, we conclude that T. obscurus is a useful animal model for studying the mechanism of osmoregulation.
Results
Survival of Takifugu species in FW
A summary on six Takifugu species used in this study is shown in Figure 1 and Table 1. The survival rate of each species after transfering from SW to FW is shown in Figure 2A. The results show the mean values of several experiments. The mean survival in FW was: 1.2 ± 0.2 days, 3.6 ± 0.2 days, 5.5 ± 0.4 days, 7.0 ± 0.3, 7.5 ± 0.8 days, and more than 10 days for T. porphyreus, T. poecilonotus, T. rubripes, T. niphobles, T. pardalis, and T. obscurus, respectively. In a separate experiment, we confirmed that T. obscurus could survive for at least 3 weeks in FW without any apparent difficulties (data not shown). These data suggest that only T. obscurus is fully adaptable to both FW and SW among the six Takifugu species tested. Of the five species that could not survive in FW, four (T. niphobles, T. rubripes, T. pardalis and T. poecilonotus) were able to adapt to BW (14% SW) (Figure 2B).
Figure 2.
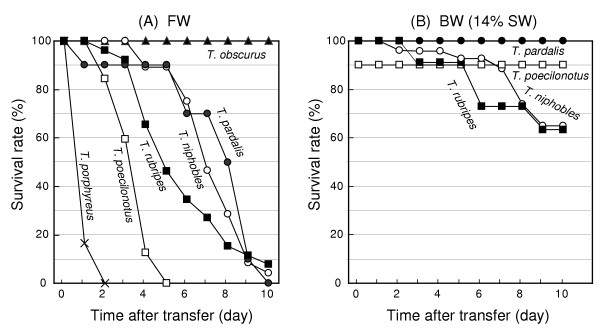
Survival rates of the Takifugu species after a direct transfer from seawater (SW) to freshwater (FW) or brackish water (BW). Fourteen-percent SW was used as BW. Numbers of fishes used for the analyses were: T. obscurus, n = 18 for FW; T. niphobles, n = 35 for FW and n = 36 for BW; T. pardalis, n = 10 for FW and n = 6 for BW; T. poecilonotus, n = 32 for FW and n = 10 for BW; T. rubripes, n = 26 for FW and n = 11 for BW; and T. porphyreus, n = 6 for FW.
The fishes that survived for 10 days in BW were transferred to FW and survival was monitored (time course data not shown). Mean survival in FW were: 3.1 ± 0.6 days, 4.6 ± 0.6 days, and 5.5 ± 0.7 days for T. niphobles (n = 7), T. poecilonotus (n = 8), and T. pardalis (n = 6), respectively. Mean survival in FW following the transfer from BW did not differ significantly between T. poecilonotus and T. pardalis, and was short for T. niphobles (P < 0.001) when compared to the survival of those that were transferred from SW to FW. These results indicate that 10 days' adaptation to BW does not improve the adaptability of T. poecilonotus, T. pardalis, and T. niphobles to FW.
Changes in serum osmolarity and concentrations of ions and urea during adaptation
To gain insights into the way that the Takifugu species adapt to different salinities, we sampled the blood from two species, T. obscurus and T. niphobles, and determined serum osmolarity and concentrations of ions and urea (Table 2). In SW, serum osmolarity and ion concentration of T. obscurus and T. niphobles were similar to those reported for other teleost fish [1]. When transferred to FW, however, significant changes were observed in serum osmolarity and concentrations of Na+ and Cl- for T. niphobles, whereas the changes were small for T. obscurus. The reductions in osmolarity during FW adaptation of T. obscurus and T. niphobles were -17 and -148 mOsM, respectively. The decrements of serum concentrations of Na+ and Cl- during FW adaptation were -13 and -16 mM in T. obscurus, and -69 and -52 mM in T. niphobles, respectively. These results suggest that T. obscurus has a much stronger ability to maintain body fluid homeostasis against salinity fluctuations and can survive in FW.
Table 2.
Serum osmolarity (mOsM) and concentration (mM) of ions and urea
| Species | Condition | mOsM | Na+ | K+ | Ca2+ | Mg2+ | Cl- | Urea |
| T. obscurus | SW | 363 ± 9.4 | 179 ± 3.9 | 2.8 ± 0.4 | 3.1 ± 0.2 | 1.8 ± 0.1 | 144 ± 3.3 | 2.6 ± 0.4 |
| FW | 346 ± 5.7 | 166 ± 1.2 | 3.0 ± 0.7 | 3.5 ± 0.3 | 1.3 ± 0.1 | 128 ± 2.1* | 3.7 ± 0.4 | |
| T. niphobles | SW | 392 ± 5.6 | 197 ± 3.8 | N.D. | 2.8 ± 0.1 | 1.6 ± 0.1 | 126 ± 3.6 | 1.0 ± 0.4 |
| FW | 244 ± 9.5** | 128 ± 5.7** | N.D. | 2.2 ± 0.2 | 0.9 ± 0.2 | 74 ± 2** | 0.4 ± 0.1 | |
| BW | 355 ± 33 | 181 ± 15 | N.D. | 3.0 ± 0.3 | 2.0 ± 0.2 | 104 ± 12 | 0.6 ± 0.1 |
Values are means ± SE; n = 4. *P < 0.01, **P < 0.001.
Figure 3 shows the time course of changes in serum Na+ concentration following exposure of T. obscurus and T. niphobles to low salinities. In the case of T. obscurus, a slightly decreased level that was observed on day 1, remained throughout the course, but in the case of T. niphobles, a relatively large decrease occurred continuously until death. In BW where T. niphobles exhibited 64% survival rate (Figure 2), a significant recovery of the decreased serum Na+ levels was observed on day 9 (Figure 3). The standard errors of serum Na+ concentration of T. niphobles (7.6–32 mM) were much larger than those of T. obscurus (2.3–7.9 mM), suggesting that the individual differences of adaptability to FW and BW are large in T. niphobles. In T. niphobles the decrease in serum Cl- was more extensive than that in serum Na+. In T. obscurus serum Cl- decreased while Na+ and osmolarity remained unchanged.
Figure 3.
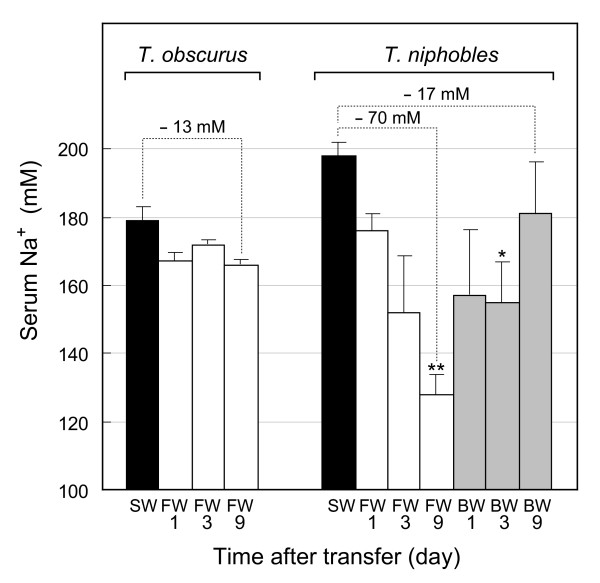
Changes in serum Na+ concentration during transfer from seawater (SW) to freshwater (FW) or to brackish water (BW). Results of T. obscurus and T. niphobles are shown on the left and right, respectively. Serum Na+ concentrations of the fish that adapted to SW, FW, and BW are shown as the black, white, and gray bars, respectively (n = 3–4). 14% SW was used as BW. *P < 0.01, **P < 0.001.
Comparison of nephron structure of Takifugu species
Kidney sections of the six Takifugu species were analyzed to compare their nephron structures. Under light microscope, a number of glomeruli were observed within all sections stained with hematoxylin-eosin, demonstrating that all six Takifugu species have glomerular nephrons (Figure 4A–C). The glomeruli of FW-acclimated T. obscurus appeared to be loose compared to those of the SW-acclimated fish (Figure 4D–E). There was no clear difference between those species rich in glomeruli at the histological level.
Figure 4.
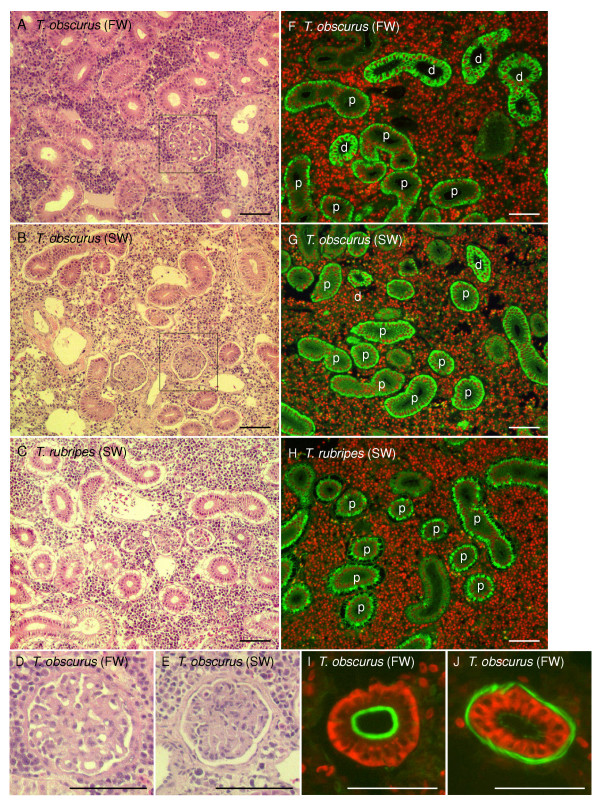
Renal structure of Takfiugu kidneys. A–E. Paraffin-embedded sections of the kidneys of indicated Takifugu species were stained with hematoxylin and eosin and examined for abundance of glomeruli. All the other species, T. niphobles, T. pardalis, T. poecilonotus, and T. porphyreus, also have glomerulous nephron (data not shown). D. Higher magnification view of the glomeruli of FW-acclimated T. obscurus indicated by a box in A. E. Higher magnification views of the glomeruli of SW-acclimated T. obscurus indicated by boxes in B. F–H. Paraffin-embedded sections of the kidneys of indicated Takifugu species were stained with anti-Na+-K+-ATPase (NKA) antibody (green) and Hoechst 33342 (red). NKA antibody strongly stained basolateral surface of proximal segment (p) and entire cell of distal segment (d). T. niphobles, T. pardalis, and T. poecilonotus showed similar result to T. rubripes (data not shown). I–J. Frozen sections of the kidneys of T. obscurus were stained with anti-NKA antibody (red) and Alaxa Fluor 488-labeled phalloidin (green). Phalloidin binds to actin filaments, and strongly stains a well-developed apical brush border of proximal segments. I. Proximal segment of the nephron of T. obscurus. J. Distal segment of the nephron of T. obscurus. All scale bars represent 50 μm.
To characterize the segments of the renal tubules, kidney sections of T. obscurus, T. rubripes, T. niphobles, T. pardalis, and T. poecilonotus were stained with anti-Na+-K+-ATPase (NKA) antibody, and observed under a fluorescence microscope. NKA is the most important molecule that provides a driving force for many transporting systems in the renal tubules, and the patterns of NKA localization are different among the segments of renal tubule (Figure 4I–J) [20]. In both FW- and SW-acclimated T. obscurus, proximal and distal segments were clearly observed (Figure 4F–G). In contrast, the distal segment is not found in T. rubripes, T. niphobles, T. pardalis, and T. poecilonotus (Figure 4H).
Phylogeny of Takifugu species
To know the phylogenetic relationship of the Takifugu species, we isolated the mitochondrial 16S rRNA gene from each species and determined the sequence. Resulting data were compared with the sequences of the 16S rRNA genes of other species in databases, and a phylogenetic tree was constructed (Figure 5). Surprisingly, the Takifugu species were very closely related each other. The identities of 16S rRNA within the Takifugu species are 99% whereas those between Takifugu and Tetraodon nigroviridi, Oryzias latipes, or Homo sapiens were 86%, 77%, and 63%, respectively. Our preliminary results of the nucleotide sequences of several cDNA clones for ion transporters (Na+/H+ exchangers; accession numbers AB200326–AB200333) and hormone receptors (members of the adrenomedullin receptor family: accession numbers AB219765–AB219771, AB219835–AB219840) [21] of T. obscurus were 99% identical to those of T. rubripes including the non-coding sequences (data not shown). These results suggest that the Takifugu species diversified very recently and the genome resources of T. rubripes can be used for studying the T. obscurus genes and their products.
Figure 5.
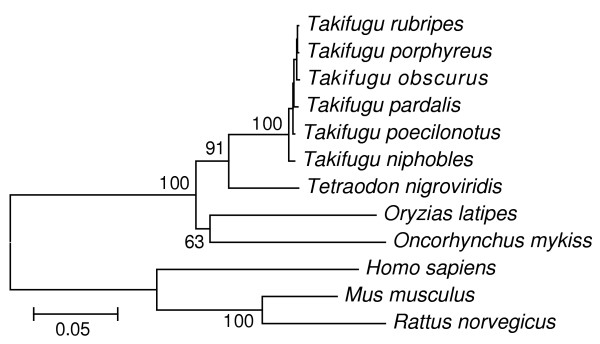
Phylogenetic relationship between Takifugu and other species. 1.1-kb nucleotide sequences of the mitochondrial 16S ribosomal RNA gene of each species were used for the analyses. Bootstrap values from 2,000 times replications are indicated at major nodes. Bars indicate 5% replacement of a nucleotide per site. Accession numbers were as follows: T. niphobles, AB199318; T. poecilonotus, AB199319; T. pardalis, AB199320; T. rubripes, AB199321; T. obscurus, AB199322; T. porphyreus, AB199323; T. nigroviridis, CR688806; O. latipes, NC_004387; O. mykiss, NC_001717; H. sapiens, J01415; M. musculus, J01420; and R. norvegicus, X14848.
Discussion
Through analyses of the ability of the Takifugu species to adapt to FW, we have demonstrated that only T. obscurus exhibits a high adaptability to both FW and SW. This observation is consistent with their natural anadromous habitats (Table 1). In our analyses, we used sexually immature fish (~10 g) and large fish (~350 g) with well developed testis or ovary. All the T. obscurus survived in both FW and SW for more than 10 days and they looked healthy, suggesting that size and sexual maturation do not affect their adaptability. Recently, Yan et al. reported the effect of salinity on food intake, growth, and survival of T. obscurus (~45 g) [19]. They cultured T. obscurus in FW, BW, and SW for 54 days and compare their level of food-intake and growth rates. The fish survived and grew under all conditions tested, and the growth rates in low-salinity BW (23% SW) were better than those in FW, SW and high-salinity BW (51% SW). Their observation demonstrated that T. obscurus grows under a wide range of salinities and low-salinity BW is the best condition for young T. obscurus to grow.
Our analyses also demonstrated that many other Takifugu species exhibit a relatively high ability to cope with salinity changes. T. niphobles, T. rubripes, T. pardalis, and T. poecilonotus can survive in FW for several days and in BW for more than 10 days, suggesting that the Takifugu species are potentially euryhaline. These results are consistent with their natural brackish/marine habitats; they are sometimes found in brackish river mouths (Table 1). It is known that T. rubripes spawn in the entrance of bays. The fingerlings grow in shallow and river mouths of bays for one year, and then go to the broad ocean [22]. Han et al. demonstrated that the best growing salinity of T. rubripes weighting ~0.02, ~1.2 and ~25 g were 73–91%, 29%, and 43% SW, respectively [23]. Thus change of the environmental salinity is important for the growth of the fingerlings of T. rubripes.
During the acclimation to FW, serum Cl- of T. obscurus decreased although Na+ and osmolarity remained unchanged. In T. niphobles the decrease in serum Cl- was more extensive than that in serum Na+. These results suggest that the mechanisms whereby Cl- and Na+ are regulated differ. The decrease in serum Cl- during FW acclimation has also been observed in Japanese eel (Anguilla japonica) [24] and spotted green pufferfish (Tetraodon nigroviridis) [20]. In Tetraodon and Takifugu species, the other electrolytes that compensate for Cl- were not determined. In the case of Japanese eel, serum SO42- concentration increases from ~1 to ~19 mM during acclimation from SW to FW [24]. The expressions of kidney sulfate transporters are drastically induced during FW acclimation, suggesting that the serum SO42- reabsorbed by the kidney compensates for Cl- and helps improve the survival of eel in FW [24].
Some reports have categorized pufferfish as aglomerular [25,26]. However, glomerular nephrons was observed in the species of from four genuses of the Tetraodontidae family, namely, Canthigaster rivulatus [27], Tetraodon nigroviridis [20], Sphoeroides testudineus [28], two Takifugu species reported by Ogawa [27], and six Takifugu species in this study (Figure 4). We think that many of the Tetraodontidae species are glomerular. The increase in size of the glomerulus after transferring to FW (Figure 4D–E) was also found in the threespine stickleback (Gasterosteus aculeatus L.) [29]. In general, the largest difference between FW fish and glomerular SW fish regarding structure of the renal tubules is the presence or absence of a distal segment, which acts as a urine-diluting segment in FW fish [30]. Most of the euryhaline fish have a FW-fish type of nephron such as the European eel (Anguilla vulgaris), Pacific pink salmon (Oncorhynchus gorbuscha), rainbow trout (Oncorhynchus mykiss), southern flounder (Paralichthys lethostigma), armored sculpin (Leptocottus armatus), medaka (Oryzias latipes), and spotted green pufferfish [20,30]. In the Takifugu species, we demonstrated that only mefugu (T. obscurus) has the FW-fish type of nephron with a distal segment, and the other species have a SW-fish type of nephron lacking a distal segment (Figure 4F–J). These results are completely consistent with the ability of those species to adapt to FW, thus the presence of a distal segment is one of the most important factors that allow T. obscurus to be highly adaptalbe to a wide range of salinities.
Tetraodon nigroviridis (spotted green pufferfish) is a small pufferfish less than 10 cm in length that lives in brackish river and estuaries of Southeast Asia. T. nigroviridis also has a compact genome like the Takifugu species, and the whole genome was sequenced in 2004 [31]. Recently, Lin et al. have demonstrated the strong adaptability of T. nigroviridis to FW, BW, and SW and its use in studies on osmoregulation [20]. We think that both T. nigroviridis and T. obscurus are good models for studying osmoregulation. The advantage with T. nigroviridis is that it is readily available. The advantage with T. obscurus is that it can be used in a wide range of size (2–20 cm) and compare the functions of the gill and kidney with those of other Takifugu species that can not adapt to FW.
Many molecules have been identified as components of the chloride cells (or mitochondria-rich cells), the major site of ion regulation in the gill: transporters, channels, and pumps for Na+, K+, Cl-, HCO3-, H+, Ca2+, water, and urea; carbonic anhydrase; and hormone receptors [32]. However, the complete physiological function of the chloride cells cannot be explaned by those components alone, and identification of further players is necessary. Furthermore, little is known of the molecular biology of osmoregulation by the kidney and intestine of teleost fish: NKA [20], sulphate transporters [24], urea transporter [33], chloride channel [34], Ca2+-sensing receptor [35], V-type H+-ATPases [36] in the kidney; Na-Pi cotransporter [37] and aquaporin water channels in both the kidney and intestine [38-40]; and Na+/K+/2Cl- cotransporter in the stomach and intestine [41]. By determining the differences in gene expression patterns in the gill, intestine, and kidney of FW- and SW-acclimated mefugu (T. obscurus), we would be able to identify the genes that are important for osmoregulatory adaptation.
Conclusion
• Mefugu (T. obscurus) is an anadromous fish of the genus Takifugu that has a strong ability to maintain body fluid homeostasis during adaptation to low and high environmental salinities and is fully adaptable to both FW and SW.
• Members of the genus Takifugu are very closely related and share ~99% sequence identities in their genomes as shown by a phylogenetic analysis using the mitochondrial DNA sequence for the 16S ribosomal RNA gene.
• The nephrons of FW- or SW-acclimated T. obscurus exhibit a structure that is typical of FW fish. On the other hand, T. rubripes, T. niphobles, T. pardalis, T. poecilonotus, and T. porphyreus have nephrons of that are typical of SW glomerular fish.
• T. obscurus can be used as an animal model for studying the molecular mechanism of osmoregulation by exploiting the Takifugu genome resources.
Methods
Animals and transfer experiment
The animal protocols and procedures were approved by the Institutional Animal Care and Use Committee of Tokyo Institute of Technology and conformed to the American Physiological Society's Guiding Principles in the Care and Use of Laboratory Animals. T. obscurus (10–350 g) were cultured in a brackish river in Korea and China. The fish cultured in BW (14% SW) were transported to The Shimonoseki Marine Science Museum in Japan and kept in 150–2000-l tanks containing BW. The fish were then acclimated to SW for 7–14 days. None of the fish died during the acclimation to SW. To determe FW adaptability, the SW in the tank was gradually replaced with FW by pouring FW at a speed that allowed a complete replacement after 1–2 h. Some fish were transferred to FW directly. Survival was then monitored every 12 h for 10 days.
Other species were caught or cultured in seawater. T. rubripes (30–4200 g) were cultured and sampled at the Japan Sea. T. niphobles (18–128 g), T. pardalis (29–175 g), T. poecilonotus (18–43 g), and T. porphyreus (521–1000 g) were sampled at the Japan Sea. They were transported to the Aquarium and kept in 200–5700-l tanks containing SW. Their adaptability to FW and BW were determined as described above.
All fish used in the analyses were adult fish. The normal size of each species is shown in Table I. Most of the T. niphobles, T. pardalis, T. poecilonotus, and T. porphyreus were sexually mature adult fish. T. obscurus and T. rubripes were mixtures of mature and immature fish. The distinction between the species was performed according to Nakabo [11].
Blood analyses
T. obscurus and T. niphobles were maintained in SW and transferred to FW or BW (14% SW). Bloods were collected from the fish in SW and those in FW or BW after 1, 3, and 9 days of the transfer. Healthy fish that had adapted to in various conditions were anaesthetized by immersion in 0.1% ethyl m-aminobenzoate methanesulfonate, and blood was collected from the hepatic vein or heart. Serum from T. obscurus and T. niphobles were diluted in water at the ratio of 1:2 and 1:8, respectively, and used for the analyses. Serum osmolarity was measured by a cryoscopic method. Concentrations of Na+, K+, and Cl- were measured by the established electrode methods. Ca2+ and Mg2+ concentrations were determined by the o-cresolphthalein complexone method and xylysine blue method, respectively. Urea nitrogen concentration in the serum was measured by standard urease assay. The dilution of serum in water did not affect the results (data not shown). These measurements were conducted by SRL Laboratories (Tokyo, Japan).
Histochemistry
Kidneys of pufferfish were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, embedded in paraffin, sectioned, and stained with hematoxylin and eosin according to standard procedures.
For the immunohisotchemical analyses, the kidneys were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, at 4°C for 2 h, and rinsed in phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 6.5 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.4) containing 10% (w/v) sucrose. The fixed tissues were cryoprotected through a range of increasing sucrose concentrations up to 20%, quick frozen in an optimum cutting temperature compound (Tissue Tek).
The frozen sections (6 μm) and paraffin-embedded sections (6 μm) were prepared, permeabilized with 0.1% Triton X-100 in PBS at 20°C for 10 min, incubated with 5% fetal bovine serum (FBS) in PBS at 20°C for 1 h, and incubated with anti-Na+-K+-ATPase rabbit antiserum [8] (1:1,000) in PBS containing 5% FBS at 20°C for 8 h. After washing with PBS, the sections were incubated with a mixture of Alexa Fluor-488- or Alexa Fluor-546-labeled secondary antibody (Molecular Probes; 1:2,000 dilution), Alexa Fluor-488-labeled phalloidin (Molecular Probes; 0.15 μM), and Hoechst 33342 (Molecular Probes; 100 ng/ml) in PBS containing 5% FBS at 20°C for 1 h. The sections were mounted on antifade glycerol (90% glycerol, 10% 10 × PBS, and 0.1% 1,4-phenylenediamine, pH 7.4). Fluorescence was detected using a fluorescence microscope (Carl Zeiss). The images were obtained with a high-resolution digital charge-coupled device (CCD) camera (AxioCam HRm, Carl Zeiss) and processed with an AxioVision 4.1 software (Carl Zeiss).
The following pufferfish were used for the analyses: T. obscurus acclimated to FW for 9 days; T. obscurus acclimated to SW for 9 days; T. rubripes, T. niphobles, T. pardalis, T. poecilonotus, and T. porphyreus maintained in SW.
Phylogenetic analyses
Mitochondrial DNA from T. obscurus, T. rubripes, T. niphobles, T. pardalis, T. poecilonotus, and T. porphyreus was extracted from the fin, and used for isolation of genes for 16S rRNA by PCR as described elsewhere [42]. Two sets of primers were used: L1854 (5'-AAACCTCGTACCTTTTGCAT-3') and H2582 (5'-ATTGCGCTACCTTTGCACGGT-3') for amplification of the anterior half of the 16S rRNA genes, and L2503 (5'-CACAAGCCTCGCCTGTTTACCA-3') and H3058 (5'-TCCGGTCTGAACTCAGATCACGTA-3') for the amplification of the posterior half. Products of PCR were purified and directly sequenced by the dideoxy chain termination method with an automated DNA sequencer (Model 310; Applied Biosystems, Foster City, CA). The GenBank accession number for the sequence of each gene is as follows: T. niphobles, AB199318; T. poecilonotus, AB199319; T. pardalis, AB199320; T. rubripes, AB199321; T. obscurus, AB199322; and T. porphyreus, AB199323.
For the evolutionary analyses, the nucleotide sequences were aligned using Clustal W software [43], and then a phylogenetic tree was constructed by the neighbor-joining method [44] using MEGA software [45] based on Jukes-Cantor evolutionary distances [44]. Statistical analysis was performed by bootstrap methods [44].
List of abbreviations
SW – seawater, FW – freshwater, BW – brackish water
Authors' contributions
AK and SH planned of and designed the study, and wrote the manuscript. HD planned the sections of the study, and performed the operations relating to the supply, transfer, and maintenance of the fish. HD and AK performed the salinity transfer analyses. HS cloned and sequenced genes for 16S rRNA, and collected information on ecobiology of the Takifugu species. AK performed blood assays and construction of the phylogenetic tree. TN performed the histochemical analyses. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Toshiaki Ishibashi, Kenji Mizushima, and Masashi Wada for their assistance with use of the facilities in the aquarium, Tomo Akita, Kumi Kimura, and Rieko Akashi for maintaining the fish, Koji Ishigaki for transferring the T. obscurus from South Korea, Chunshen Li and Osame Tabeta for translating reference for 17 in Chinese language, Yukihiro Kurita for the discussion, and Setsuko Sato for the secretarial assistance. This work was supported by Grants-in-Aid for Scientific Research (14104002, 16710145) from the Ministry of Education, Culture, Sport, Science and Technology of Japan (MEXT) and the 21st Century COE Program of MEXT.
Contributor Information
Akira Kato, Email: akirkato@bio.titech.ac.jp.
Hiroyuki Doi, Email: doi@kaikyokan.com.
Tsutomu Nakada, Email: tnakada@bio.titech.ac.jp.
Harumi Sakai, Email: sakaih@fish-u.ac.jp.
Shigehisa Hirose, Email: shirose@bio.titech.ac.jp.
References
- Karnaky KJ. Osmotic and ionic regulation. In: Evans DH, editor. The Physiology of Fishes. Boca Raton (Florida): CRC Press; 1998. pp. 157–176. [Google Scholar]
- Fiol DF, Kultz D. Rapid hyperosmotic coinduction of two tilapia (Oreochromis mossambicus) transcription factors in gill cells. Proc Natl Acad Sci U S A. 2005;102:927–932. doi: 10.1073/pnas.0408956102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Zarate J, Bradley TM. A homolog of the E3 ubiquitin ligase Rbx1 is induced during hyperosmotic stress of salmon. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1643–R1653. doi: 10.1152/ajpregu.00571.2001. [DOI] [PubMed] [Google Scholar]
- Pan F, Zarate J, Choudhury A, Rupprecht R, Bradley TM. Osmotic stress of salmon stimulates upregulation of a cold inducible RNA binding protein (CIRP) similar to that of mammals and amphibians. Biochimie. 2004;86:451–461. doi: 10.1016/j.biochi.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Smith TR, Tremblay GC, Bradley TM. Hsp70 and a 54 kDa protein (Osp54) are induced in salmon (Salmo salar) in response to hyperosmotic stress. J Exp Zool. 1999;284:286–298. doi: 10.1002/(SICI)1097-010X(19990801)284:3<286::AID-JEZ6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Scott GR, Richards JG, Forbush B, Isenring P, Schulte PM. Changes in gene expression in gills of the euryhaline killifish Fundulus heteroclitus after abrupt salinity transfer. Am J Physiol Cell Physiol. 2004;287:C300–C309. doi: 10.1152/ajpcell.00054.2004. [DOI] [PubMed] [Google Scholar]
- Mistry AC, Kato A, Tran YH, Honda S, Tsukada T, Takei Y, Hirose S. FHL5, a novel actin-binding protein, is highly expressed in eel gill pillar cells and responds to wall tension. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1141–R1154. doi: 10.1152/ajpregu.00108.2004. [DOI] [PubMed] [Google Scholar]
- Mistry AC, Honda S, Hirata T, Kato A, Hirose S. Eel urea transporter is localized to chloride cells and is salinity dependent. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1594–R1604. doi: 10.1152/ajpregu.2001.281.5.R1594. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Itakura M, Kashiwagi M, Nakamura N, Matsuki T, Sakuta H, Naito N, Takano K, Fujita T, Hirose S. Identification by differential display of a hypertonicity-inducible inward rectifier potassium channel highly expressed in chloride cells. J Biol Chem. 1999;274:11376–11382. doi: 10.1074/jbc.274.16.11376. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Nakamura N, Kashiwagi M, Honda S, Kato A, Hasegawa S, Takei Y, Hirose S. RING finger, B-box, and coiled-coil (RBCC) protein expression in branchial epithelial cells of Japanese eel, Anguilla japonica. Eur J Biochem. 2002;269:6152–6161. doi: 10.1046/j.1432-1033.2002.03332.x. [DOI] [PubMed] [Google Scholar]
- Nakabo T. Fishes of Japan – with pictorial keys to the species. English. Tokyo: Tokai University Press; 2002. [Google Scholar]
- Masuda H, Amaoka K, Araga C, Uyeno T, Yoshino T. The Fishes of the Japanese Archipelago. Tokyo: Tokai University Press; 1984. [Google Scholar]
- Venkatesh B, Gilligan P, Brenner S. Fugu: a compact vertebrate reference genome. FEBS Lett. 2000;476:3–7. doi: 10.1016/S0014-5793(00)01659-8. [DOI] [PubMed] [Google Scholar]
- Aparicio S, Chapman J, Stupka E, Putnam N, Chia JM, Dehal P, Christoffels A, Rash S, Hoon S, Smit A, Gelpke MD, Roach J, Oh T, Ho IY, Wong M, Detter C, Verhoef F, Predki P, Tay A, Lucas S, Richardson P, Smith SF, Clark MS, Edwards YJ, Doggett N, Zharkikh A, Tavtigian SV, Pruss D, Barnstead M, Evans C, Baden H, Powell J, Glusman G, Rowen L, Hood L, Tan YH, Elgar G, Hawkins T, Venkatesh B, Rokhsar D, Brenner S. Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science. 2002;297:1301–1310. doi: 10.1126/science.1072104. [DOI] [PubMed] [Google Scholar]
- Kim IS. Freshwater Fishes. Seoul: Samhwa Publishing; 1997. [Ministry of Education (Series Editor): Illustrated Encyclopedia of Fauna & Flora of Korea, vol 37.] [Google Scholar]
- Wu HL, Jin XB, Ni Y. Toxic and Pharmacological Fish in China. Shanghai: Shanghai Scientific & Technical Publishers; 1978. [Google Scholar]
- Xu C. Takifugu obscurus (Abe) In: East China Sea Fisheries Institute, Chinese Academy of Fisheries Science, editor. The Fishes of Shanghai Area. Shanghai: Shanghai Scientific & Technical Publishers; 1990. pp. 377–378. [Google Scholar]
- Yang Z, Chen YF. Induced ovulation in obscure puffer Takifugu obscurus by injections of LHRH-a. Aquacul Int. 2004;12:215–223. doi: 10.1023/B:AQUI.0000032082.17825.f2. [DOI] [Google Scholar]
- Yan M, Li Z, Xiong B, Zhu J. Effects of salinity on food intake, growth, and survival of pufferfish (Fugu obscurus) J Appl Ichthyol. 2004;20:146–149. doi: 10.1046/j.1439-0426.2003.00512.x. [DOI] [Google Scholar]
- Lin CH, Tsai RS, Lee TH. Expression and distribution of Na, K-ATPase in gill and kidney of the spotted green pufferfish, Tetraodon nigroviridis, in response to salinity challenge. Comp Biochem Physiol A Mol Integr Physiol. 2004;138:287–295. doi: 10.1016/j.cbpb.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Nag K, Kato A, Nakada T, Hoshijima K, Mistry AC, Takei Y, Hirose S. Molecular and functional characterization of adrenomedullin receptors in pufferfish. Am J Physiol Regul Integr Comp Physiol. 2006;290:R467–R478. doi: 10.1152/ajpregu.00507.2005. [DOI] [PubMed] [Google Scholar]
- Fujita S. Studies on life history and aquaculture of Japanese puffer fishes. Rep Nagasaki Pref Inst Fish. 1962;2:1–121. [Google Scholar]
- Han K, Chuang H, Matsui S, Furuichi M, Kitajima C. Effect of ambient salinity on growth, survival rate, and feed efficiency in the early stage of puffer fish Takifugu rubripes. Nippon Suisan Gakkaishi. 1995;61:21–26. [Google Scholar]
- Nakada T, Zandi-Nejad K, Kurita Y, Kudo H, Broumand V, Kwon CY, Mercado A, Mount DB, Hirose S. Roles of Slc13a1 and Slc26a1 sulfate transporters of eel kidney in sulfate homeostasis and osmoregulation in freshwater. Am J Physiol Regul Integr Comp Physiol. 2005;289:R575–R585. doi: 10.1152/ajpregu.00725.2004. [DOI] [PubMed] [Google Scholar]
- Vize PD. A Homeric view of kidney evolution: A reprint of H.W. Smith's classic essay with a new introduction. Anat Rec A Discov Mol Cell Evol Biol. 2004;277:344–354. doi: 10.1002/ar.a.20017. [DOI] [PubMed] [Google Scholar]
- Edwards JG. Studies on aglomerular and glomerular kidneys. Am J Anat. 1928;42:75–107. doi: 10.1002/aja.1000420104. [DOI] [Google Scholar]
- Ogawa M. Comparative study on the internal structure of the teleostean kidney. Sci Rept Saitama Univ. 1962;B4:107–129. [Google Scholar]
- Prodocimo V, Freire CA. Glomeruli and renal tubules are restricted to the cranial kidney of the adult estuarine Sphoeroides testudineus. J Fish Biol. 2003;63:1258–1265. doi: 10.1046/j.1095-8649.2003.00242.x. [DOI] [Google Scholar]
- Ogawa M. Seasonal difference of glomerular change of marine form of the stickleback, Gasterosteus aculeatus L. after transferred into fresh water. Sci Rept Saitama Univ. 1968;B5:117–123. [Google Scholar]
- Hickman CP, Jr, Trump BF. The Kidney. In: Hoar WS, Randall DJ, editor. Fish Physiology. New York: Academic Press; 1969. pp. 91–239. [Google Scholar]
- Jaillon O, Aury JM, Brunet F, Petit JL, Stange-Thomann N, Mauceli E, Bouneau L, Fischer C, Ozouf-Costaz C, Bernot A, Nicaud S, Jaffe D, Fisher S, Lutfalla G, Dossat C, Segurens B, Dasilva C, Salanoubat M, Levy M, Boudet N, Castellano S, Anthouard V, Jubin C, Castelli V, Katinka M, Vacherie B, Biemont C, Skalli Z, Cattolico L, Poulain J, De BV, Cruaud C, Duprat S, Brottier P, Coutanceau JP, Gouzy J, Parra G, Lardier G, Chapple C, McKernan KJ, McEwan P, Bosak S, Kellis M, Volff JN, Guigo R, Zody MC, Mesirov J, Lindblad-Toh K, Birren B, Nusbaum C, Kahn D, Robinson-Rechavi M, Laudet V, Schachter V, Quetier F, Saurin W, Scarpelli C, Wincker P, Lander ES, Weissenbach J, Roest CH. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature. 2004;431:946–957. doi: 10.1038/nature03025. [DOI] [PubMed] [Google Scholar]
- Hirose S, Kaneko T, Naito N, Takei Y. Molecular biology of major components of chloride cells. Comp Biochem Physiol B Biochem Mol Biol. 2003;136:593–620. doi: 10.1016/S1096-4959(03)00287-2. [DOI] [PubMed] [Google Scholar]
- Mistry AC, Chen G, Kato A, Nag K, Sands JM, Hirose S. A novel type of urea transporter, UT-C, is highly expressed in proximal tubule of seawater eel kidney. Am J Physiol Renal Physiol. 2005;288:F455–F465. doi: 10.1152/ajprenal.00296.2004. [DOI] [PubMed] [Google Scholar]
- Miyazaki H, Kaneko T, Uchida S, Sasaki S, Takei Y. Kidney-specific chloride channel, OmClC-K, predominantly expressed in the diluting segment of freshwater-adapted tilapia kidney. Proc Natl Acad Sci U S A. 2002;99:15782–15787. doi: 10.1073/pnas.242611099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loretz CA, Pollina C, Hyodo S, Takei Y, Chang W, Shoback D. cDNA cloning and functional expression of a Ca2+-sensing receptor with truncated C-terminal tail from the Mozambique tilapia (Oreochromis mossambicus) J Biol Chem. 2004;279:53288–53297. doi: 10.1074/jbc.M410098200. [DOI] [PubMed] [Google Scholar]
- Perry SF, Furimsky M, Bayaa M, Georgalis T, Shahsavarani A, Nickerson JG, Moon TW. Integrated responses of Na+/HCO3- cotransporters and V-type H+-ATPases in the fish gill and kidney during respiratory acidosis. Biochim Biophys Acta. 2003;1618:175–184. doi: 10.1016/j.bbamem.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Kohl B, Herter P, Hulseweh B, Elger M, Hentschel H, Kinne RK, Werner A. Na-Pi cotransport in flounder: same transport system in kidney and intestine. Am J Physiol. 1996;270:F937–F944. doi: 10.1152/ajprenal.1996.270.6.F937. [DOI] [PubMed] [Google Scholar]
- Aoki M, Kaneko T, Katoh F, Hasegawa S, Tsutsui N, Aida K. Intestinal water absorption through aquaporin 1 expressed in the apical membrane of mucosal epithelial cells in seawater-adapted Japanese eel. J Exp Biol. 2003;206:3495–3505. doi: 10.1242/jeb.00579. [DOI] [PubMed] [Google Scholar]
- Martinez AS, Cutler CP, Wilson GD, Phillips C, Hazon N, Cramb G. Regulation of expression of two aquaporin homologs in the intestine of the European eel: effects of seawater acclimation and cortisol treatment. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1733–R1743. doi: 10.1152/ajpregu.00747.2004. [DOI] [PubMed] [Google Scholar]
- Martinez AS, Cutler CP, Wilson GD, Phillips C, Hazon N, Cramb G. Cloning and expression of three aquaporin homologues from the European eel (Anguilla anguilla): effects of seawater acclimation and cortisol treatment on renal expression. Biol Cell. 2005;97:615–627. doi: 10.1042/BC20040111. [DOI] [PubMed] [Google Scholar]
- Cutler CP, Cramb G. Two isoforms of the Na+/K+/2Cl- cotransporter are expressed in the European eel (Anguilla anguilla) Biochim Biophys Acta. 2002;1566:92–103. doi: 10.1016/s0005-2736(02)00596-5. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Iguchi K, Hosoya K, Nishida M. Phylogenetic relationships of the Japanese minnows, Pseudorasbora (Cyprinidae), as inferred from mitochondrial 16S rRNA gene sequences. Ichthyol Res. 2000;47:43–50. [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Kumar S. Molecular Evolution and Phylogenetics. New York: Oxford University Press Inc; 2000. [Google Scholar]
- Kumar S, Tamura K, Jakobsen IB, Nei M. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics. 2001;17:1244–1245. doi: 10.1093/bioinformatics/17.12.1244. [DOI] [PubMed] [Google Scholar]
- Abe T. Taxonomic studies on the puffers (Tetraodontidae, Teleostei) from Japan and adjacent regions – V. Synopsis of the puffers from Japan and adjacent regions. Bull Biogeogr Soc Jpn. 1949. pp. 89–140.
- Miyadi D, Kawanabe H, Mizuno N. Colored Illustrations of the Freshwater Fishes of Japan. New. Osaka: Hoikusha Publishing; 1976. [Google Scholar]
- Society for nature conservation research of Fukui prefecture . Animals of inland waters of Fukui prefecture. Fukui: Fukui prefecture; 1998. [Google Scholar]


