Abstract
OBJECTIVE
Pregnancy is associated with major changes in calcium metabolism because the neonatal skeleton contains approximately 30 grams of calcium, which are largely deposited in the third trimester. Osteoprotegerin (OPG) acts as a decoy receptor for the “Receptor Activator of Nuclear Factor-κ B Ligand” (RANKL), which is an essential factor for bone remodeling. This study was conducted to determine whether there were changes in maternal plasma OPG concentration during normal pregnancy.
STUDY DESIGN
A cross-sectional study was performed in 433 patients of reproductive age (40 non-pregnant and 393 pregnant). Pregnant patients were classified into 4 groups according to gestational age: group 1: 11–14 weeks (n=100); group 2: 15–18 weeks (n=99); group 3: 27–30 weeks (n=100); and group 4: 37–42 weeks (n=94). Plasma OPG concentrations were measured using a sensitive and specific immunoassay. Non-parametric statistics were used for analysis.
RESULTS
OPG was detected in the plasma of all women tested. The median OPG concentration was significantly higher in term patients than in those in early pregnancy [median: 6.63 pmol/L (range: 1.57–25.57) vs. median: 3.98 pmol/L (range: 0.41–13.71), p<0.001)]. There was no significant difference in plasma OPG concentrations between non-pregnant women and those in groups 1 or 2 [non-pregnant women median: 3.86 pmol/L (range: 1.64–15.29) vs. group 1 median: 3.98 pmol/L (range: 0.41–13.71) vs. group 2 median: 3.87 pmol/L (range: 1.14–69.83), p=0.75].
CONCLUSION
The median maternal plasma OPG concentration is higher in the third trimester than in the first trimester of pregnancy. OPG may be involved in the regulation of bone turnover during pregnancy.
Keywords: Osteoprotegerin, osteoporosis in pregnancy, maternal bone remodeling, fetal skeleton mineralization, calcium homeostasis
INTRODUCTION
Normal pregnancy is a “maternal calcium stress state” 1 because the development of the fetal skeleton at term requires approximately 20–30 g of calcium, 80% of which accumulates in the third trimester.2,3 Therefore, calcium homeostasis is of major physiologic and clinical importance and depends on the dynamic interaction between the large pool of calcium present in the skeleton, the small pool present in extracellular fluid, input from the diet, and regulation of excretion, mainly through the kidneys.3
Active transport of calcium across the placenta permits higher concentrations of calcium in fetal blood than in maternal blood.4 If maternal calcium intake were sufficient to meet fetal demands, then physiologic adaptations to conserve calcium within the maternal compartment would not be needed. However, a body of evidence suggests that there is significant bone remodeling during pregnancy, leading to a flux of calcium out of the maternal skeleton to meet the needs of the fetus. Most evidence suggests that maternal bone turnover increases5–7 and bone mineral density (BMD) decreases during pregnancy.7–11
Recently, the Osteoprotegerin/RANKL/RANK system was discovered.12,13 Osteoprotegerin (OPG), a soluble tumor necrosis factor (TNF)-like protein secreted by osteoblasts, inhibits osteoclast formation and bone resorption.14 Receptor Activator of Nuclear Factor-κ B Ligand (RANKL) is a cytokine that regulates the formation and activation of osteoclasts and bone resorption.15 To perform this biological function, RANKL binds to its transmembrane signaling receptor RANK (Receptor Activator of Nuclear Factor- B) on the osteoclast precursor.16 OPG is capable of blocking the binding between the RANKL and its receptor, RANK, and can therefore act as an endogenous decoy receptor for RANKL.13 At present, maternal plasma OPG concentrations during pregnancy are not known. The aim of this study was to describe normal plasma OPG concentration during pregnancy.
MATERIALS AND METHODS
Study design
This cross-sectional study was conducted using our perinatal database and bank of biological samples. The study included 433 individuals: non-pregnant patients (n=40) and normal pregnant patients (n=393). Pregnant patients were sub-classified into four groups according to gestational age: group 1: 11–14 weeks (n=100); group 2: 15–18 weeks (n=99); group 3: 27–30 weeks (n=100); and group 4= 37–42 weeks (n=94). Pregnant and non-pregnant patients were enrolled between January 1998 and December 2003 at both Sotero del Rio Hospital, Puente Alto, Chile, and Hutzel Hospital, Detroit, Michigan. The collection of samples for research was approved by the Institutional Review Boards of Sotero del Rio Hospital, Wayne State University, and the National Institute of Child Health and Human Development, NIH, DHHS. Written informed consent was obtained from all participants. The inclusion criteria for pregnant patients were: 1) singleton pregnancy; 2) no obstetrical, maternal or fetal complications during pregnancy; and 3) delivery of a healthy neonate at term with appropriate weight for gestational age (>10th and < 90th percentile). The inclusion criteria for non-pregnant women were: 1) the absence of medical complications; and 2) women were not taking oral contraceptives.
Plasma samples and osteoprotegerin immunoassay
Blood samples were collected in vials containing ethylenediaminetetra-acetic acid (EDTA). Plasma was collected after centrifugation of the sample for 10 minutes at 4°C, then stored at −70°C until analysis. Plasma OPG concentrations were measured with a commercially available enzyme-linked immunosorbent assay (Biomedica Gruppe, Vienna, Austria). The sensitivity of the test was 0.532pmol/L and inter-and intra-assay coefficients of variation were 6.81% and 4.64%, respectively.
Statistical analysis
Non-parametric tests (Kruskal-Wallis and Mann-Whitney U tests) were used to analyze differences between groups. Spearman’s correlation test was used to examine the relationship between maternal plasma OPG concentrations, maternal age and BMI. Proportions were compared with Fisher’s exact test. P values <0.05 were considered statistically significant. Statistical analysis was performed with SPSS v12.0 (SPSS Inc, Chicago, Illinois).
RESULTS
The clinical characteristics of the study population and maternal plasma OPG concentrations are displayed in Tables I and II. No differences were noted among pregnant groups in maternal age, the proportion of nulliparity, mean birthweight, newborn gender or maternal BMI.
Table 1.
Clinical characteristics of the study population
| Non-Pregnant (n=40) | 11–14 weeks (n=100) | 15–18 weeks (n=99) | 27–30 weeks (n=100) | 37–42 weeks (n=94) | |
|---|---|---|---|---|---|
| Gestational age at sample collection | * | 12.7 ± 0.9 | 16.5 ± 0.9 | 28.4 ± 0.9 | 39.9 ± 1.2 |
| Maternal age (mean ± SD, years) | 27.8 ± 6.0 | 26.8 ± 6.0 | 26.2 ± 6.0 | 26.9 ± 6.5 | 26.1 ± 6.4 |
| Nulliparity (%) | 60 | 33 | 28 | 34 | 42 |
| Birthweight (mean ± SD, g) | * | 3421 ± 261 | 3402 ± 324 | 3470 ± 290 | 3444 ± 307 |
| Neonatal gender, male (%) | * | 55 | 47 | 57 | 52 |
| BMI – before pregnancy (mean ± SD) | 24.3 ± 5.4 | 24.4 ± 3.9 | 23.6 ± 3.1 | 24.3 ± 4.4 | 24.6 ± 4.4 |
| BMI – at sampling(mean ± SD) | 24.3 ± 5.4 | 24.4 ± 3.9 | 25.3 ± 3.2 | 27.7 ± 4.2 | 30.1 ± 4.6 |
Not applicable
Table 2.
Percentiles for plasma OPG concentrations during normal pregnancy (pmol/L).
| 5th | 10th | 25th | 50th | 75th | 90th | 95th | |
|---|---|---|---|---|---|---|---|
| Non Pregnant (n=40) | 2.00 | 2.09 | 3.15 | 3.86 | 5.82 | 8.10 | 11.82 |
| 11–14 weeks (n=100) | 2.09 | 2.41 | 3.04 | 3.98 | 5.20 | 6.65 | 7.37 |
| 15–18 weeks (n=99) | 2.10 | 2.27 | 3.12 | 3.87 | 5.10 | 7.23 | 9.11 |
| 27–30 weeks (n=100) | 2.21 | 2.66 | 3.66 | 5.48 | 7.27 | 10.19 | 14.41 |
| 37–42 weeks (n=94) | 2.70 | 3.39 | 4.43 | 6.63 | 9.51 | 13.42 | 17.74 |
OPG was detected in the plasma of all pregnant and non-pregnant women (Fig 1). No significant differences in plasma OPG concentrations were observed between non-pregnant patients and those in groups 1 and 2 [non-pregnant patients median: 3.86 pmol/L (range: 1.64–15.29) vs. group 1 median: 3.98 pmol/L (range: 0.41–13.71) vs. group 2 median: 3.87 pmol/L (range: 1.14–69.83); p=0.75). In contrast, plasma OPG concentrations increased significantly with advancing gestational age after early pregnancy [15–18 weeks: median 3.87 pmol/L (range: 1.14–69.83) vs. 27–30 weeks: median 5.48 pmol/L (range 1.86–20.68) vs. 37–42 weeks: median 6.63 pmol/L (range: 1.57–25.57); Spearman’s rho=0.383; p<0.001] (Figures 1 and 2).
Figure 1a and 1b.
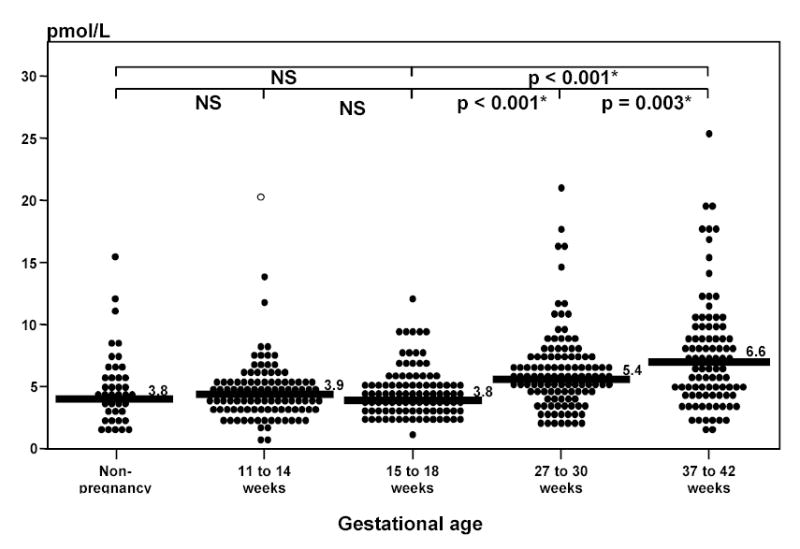
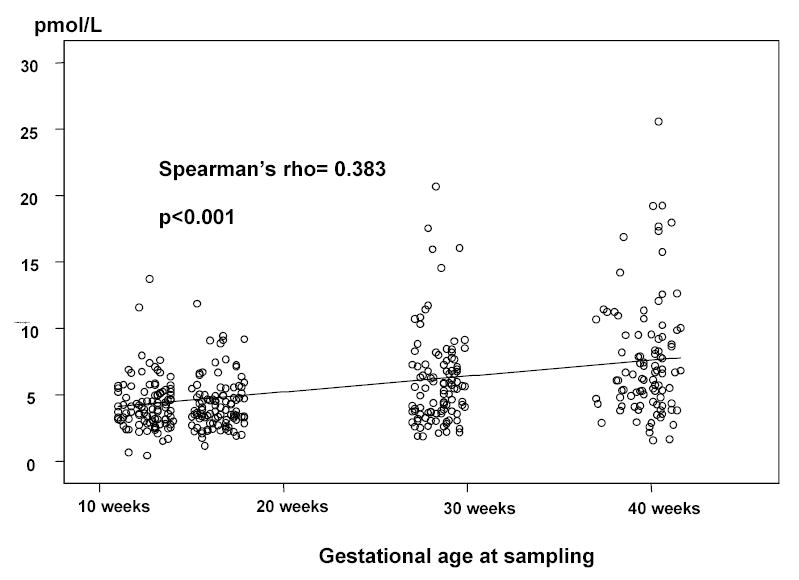
Maternal plasma osteoprotegerin concentrations. There was no significant difference in plasma OPG concentrations between non-pregnant and pregnant women in early (11–14 and 15–18 weeks) gestation [non-pregnant women: median 3.86 pmol/L (range: 1.64–15.29) vs. 11–14 weeks: median 3.98 pmol/L (range: 0.41–13.71) vs. 15–18 weeks: median 3.87 pmol/L (range: 1.14–69.83); p=0.75]. Plasma OPG concentrations increased significantly with advancing gestational age after early pregnancy [15–18 weeks: median 3.87 pmol/L (range: 1.14–69.83) vs. 27–30 weeks: median 5.48 pmol/L (range 1.86–20.68) vs. 37–42 weeks: median 6.63 pmol/L (range 1.57–25.57); Spearman’s rho=0.383; p<0.001].
No relationship was found between plasma OPG concentration and maternal age, maternal BMI prior to pregnancy or at the time of blood sampling, neonatal gender, or parity (data not shown).
DISCUSSION
Principal findings of the study
This study shows that the median maternal plasma OPG concentration increases with advancing gestational age and it is higher in the third trimester than in the first or second trimester of pregnancy. We propose that the increase in maternal plasma OPG in the third trimester may serve a homeostatic role and protect the maternal skeleton from excessive bone loss.
Clinical and biological significance of the findings
Pregnancy is associated with major changes in calcium homeostasis that are thought to represent maternal adaptive responses to ensure: 1) adequate development of the fetal skeleton; and 2) maintenance of normal calcium serum levels in the presence of a dramatic expansion of total extracellular volume.3 Calcium sources available for transfer to the fetus include maternal dietary intake, maternal extracellular sources, and the maternal skeleton.17
The maternal adaptive mechanisms involved to secure a calcium supply to the fetus include: 1) increased intestinal absorption of calcium; and 2) increased resorption of calcium from the maternal skeleton.17 Increased maternal serum free and total 1,25 hydroxivitamin D18 are thought to be responsible for the enhanced intestinal calcium absorption during pregnancy.5 However, most investigators consider that increased dietary intake and intestinal absorption are not sufficient to provide the calcium required by the fetus.8,10 The maternal skeleton is therefore believed to be a potential source of calcium for the fetus.
Longitudinal studies of bone mineralization during pregnancy have yielded conflicting reports. Most suggest that there is bone mineral density loss,8 while others do not.5,19 The most recent and largest longitudinal study of bone mineral density during pregnancy provided strong evidence for trabecular, but not cortical, bone loss.10 The clinical implications of this are severalfold. First, pregnancy may affect the future fracture risk if there is irreversible bone loss. Second, pregnancy among growing adolescents may pose conflicts between the calcium demands of the mother and those of the fetus. Indeed, pregnant adolescents have been reported to have greater bone loss than adult women.8 Third, multiple gestations in older women undergoing assisted reproductive technologies may affect maternal bone density to a magnitude which has not been recognized and predispose to osteoporosis. Fourth, there is now improved understanding of pregnancy-associated osteoporosis that may occur spontaneously or after the chronic administration of heparin,20 magnesium sulfate21 and steroids.22
The OPG/RANKL/RANK system plays a central role in bone homeostasis.12,13 In 1997, OPG was discovered to be a cytokine capable of protecting bone mass by inhibiting osteoclast differentiation and activation; hence, the term “osteoprotegerin.”14 Overexpression of OPG in transgenic mice results in osteopetrosis,14 while OPG-deficient mice (OPG −/−) have decreased total bone density and a high frequency of fractures.23 OPG exerts its inhibitory effects on osteoclastogenesis by binding to the OPG ligand (also known as OPGL or RANKL). When binding to RANKL, OPG prevents direct RANK-induced differentiation of osteoclast hematopoietic precursors, as well as the activation of mature osteoclast in vivo and in vitro.15
The biological importance of the OPG/RANKL/RANK system has been recently recognized. Alterations in the OPG/RANKL ratio have been implicated in the pathogenesis of bone diseases characterized by bone resorption, such as post-menopausal osteoporosis24 and gluco-corticoid-induced osteoporosis.22
A role for OPG during pregnancy
Our observations indicate that maternal plasma OPG concentrations increased in the third trimester of pregnancy, a time when the demand for calcium for fetal bone mineralization is at its highest. We interpret our findings as suggesting that OPG may protect the maternal skeleton from excessive catabolism. In addition, OPG has recently been found to be associated with coronary artery disease and confers a higher risk for the progression of atherosclerosis and cardiovascular mortality.25 Thus, a role for OPG in the pathophysiology of preeclampsia should also be considered. Our study has established a normal reference range for OPG in pregnancy. This is crucial for future studies of the role of this novel and important cytokine in obstetrics.
Strengths and weaknesses of the study
This report is based upon a cross-sectional study. Longitudinal studies are desirable to accurately characterize the changes in OPG concentrations in the same patient. The strengths of this study are the large sample size, the criteria for normal outcome, and the cross-sectional nature that allows establishment of a reference range to assess changes in OPG concentrations in a particular pregnancy.
Unanswered questions and proposed future research
The consequences of high and low concentrations of OPG in calcium metabolism during pregnancy and maternal bone mineral status remain to be determined and should be the subject of future research. An important question is whether OPG may play a role in the pathogenesis of preeclampsia, for this cytokine has been implicated in the pathophysiology of atherosclerosis.
Footnotes
Presented at the Twenty-Fifth Annual Meeting of the Society for Maternal-Fetal Medicine, Reno, NV, February 7-12, 2005.
Condensation: The plasma concentrations during normal pregnancy of osteoprotegerin, a cytokine involved in bone remodeling, are described.
References
- 1.Cundy T, Kanis JA. Calcium homoeostasis during pregnancy. Br Med J (Clin Res Ed) 1981;283:562–63. doi: 10.1136/bmj.283.6290.562-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Givens MH, Macy IC. The chemical composition of the human fetus. J Biol Chem. 1933;102:7–17. [Google Scholar]
- 3.Pitkin RM. Calcium metabolism in pregnancy and the perinatal period: a review. Am J Obstet Gynecol. 1985;151:99–109. doi: 10.1016/0002-9378(85)90434-x. [DOI] [PubMed] [Google Scholar]
- 4.Saxe A, Dean S, Gibson G, Pandian MR, Levy J. Parathyroid hormone and parathyroid hormone-related peptide in venous umbilical cord blood of healthy neonates. J Perinat Med. 1997;25:288–91. [PubMed] [Google Scholar]
- 5.Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr. 1995;61:514–23. doi: 10.1093/ajcn/61.3.514. [DOI] [PubMed] [Google Scholar]
- 6.Yamaga A, Taga M, Minaguchi H, Sato K. Changes in bone mass as determined by ultrasound and biochemical markers of bone turnover during pregnancy and puerperium: a longitudinal study. J Clin Endocrinol Metab. 1996;81:752–56. doi: 10.1210/jcem.81.2.8636299. [DOI] [PubMed] [Google Scholar]
- 7.More C, Bhattoa HP, Bettembuk P, Balogh A. The effects of pregnancy and lactation on hormonal status and biochemical markers of bone turnover. Eur J Obstet Gynecol Reprod Biol. 2003;106:209–13. doi: 10.1016/s0301-2115(02)00237-3. [DOI] [PubMed] [Google Scholar]
- 8.Sowers MF, Scholl T, Harris L, Jannausch M. Bone loss in adolescent and adult pregnant women. Obstet Gynecol. 2000;96:189–93. doi: 10.1016/s0029-7844(00)00903-0. [DOI] [PubMed] [Google Scholar]
- 9.Pearson D, Kaur M, San P, Lawson N, Baker P, Hosking D. Recovery of pregnancy mediated bone loss during lactation. Bone. 2004;34:570–78. doi: 10.1016/j.bone.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Promislow JH, Hertz-Picciotto I, Schramm M, Watt-Morse M, Anderson JJ. Bed rest and other determinants of bone loss during pregnancy. Am J Obstet Gynecol. 2004;191:1077–83. doi: 10.1016/j.ajog.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 11.Black AJ, Topping J, Durham B, Farquharson RG, Fraser WD. A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. J Bone Miner Res. 2000;15:557–63. doi: 10.1359/jbmr.2000.15.3.557. [DOI] [PubMed] [Google Scholar]
- 12.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–55. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 13.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 14.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 15.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 16.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–79. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 17.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18:832–72. doi: 10.1210/edrv.18.6.0319. [DOI] [PubMed] [Google Scholar]
- 18.Seki K, Makimura N, Mitsui C, Hirata J, Nagata I. Calcium-regulating hormones and osteocalcin levels during pregnancy: a longitudinal study. Am J Obstet Gynecol. 1991;164:1248–52. doi: 10.1016/0002-9378(91)90694-m. [DOI] [PubMed] [Google Scholar]
- 19.Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, et al. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. 1998;67:693–701. doi: 10.1093/ajcn/67.4.693. [DOI] [PubMed] [Google Scholar]
- 20.Barbour LA, Kick SD, Steiner JF, LoVerde ME, Heddleston LN, Lear JL, et al. A prospective study of heparin-induced osteoporosis in pregnancy using bone densitometry. Am J Obstet Gynecol. 1994;170:862–69. doi: 10.1016/s0002-9378(94)70299-3. [DOI] [PubMed] [Google Scholar]
- 21.Levav AL, Chan L, Wapner RJ. Long-term magnesium sulfate tocolysis and maternal osteoporosis in a triplet pregnancy: a case report. Am J Perinatol. 1998;15:43–46. doi: 10.1055/s-2007-993897. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki N, Kusano E, Ando Y, Yano K, Tsuda E, Asano Y. Glucocorticoid decreases circulating osteoprotegerin (OPG): possible mechanism for glucocorticoid induced osteoporosis. Nephrol Dial Transplant. 2001;16:479–82. doi: 10.1093/ndt/16.3.479. [DOI] [PubMed] [Google Scholar]
- 23.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–68. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–30. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, et al. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–80. doi: 10.1161/01.CIR.0000127957.43874.BB. [DOI] [PubMed] [Google Scholar]


