Abstract
OBJECTIVE
Genome-wide screening studies of the chorioamniotic membranes have unexpectedly identified an increase in the expression of bone morphogenetic protein 2 (BMP2) in spontaneous labor at term. The objective of this study was to determine whether BMP2 mRNA and protein expression are altered in the chorioamniotic membranes of patients with term labor, preterm labor, and preterm premature rupture of membranes (PPROM).
STUDY DESIGN
Chorioamniotic membranes were obtained from patients at term (with and without labor), with preterm labor (with and without histologic chorioamnionitis), and with PPROM (with and without histologic chorioamnionitis). The expression of BMP2 was studied by real-time quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR; n=88) and immunohistochemistry (IHC; n=124). Non-parametric statistics were used for analysis. Primary amnion cells obtained from women at term not in labor were treated with BMP2 to examine whether there was increased prostaglandin E2 expression.
RESULTS
1) The median BMP2 mRNA and protein expression were significantly higher in the membranes of patients with spontaneous labor at term than in those of patients not in labor at term (P < 0.001 for both). 2) BMP2 mRNA and protein expression were increased in patients with preterm labor with histologic chorioamnionitis than in those without histologic chorioamnionitis (P < 0.05 and P < 0.001, respectively). 3) There was no difference in BMP2 mRNA and protein expression in patients with PPROM, regardless of chorioamnionitis (P = 0.13 and P = 0.08). 4) There was a correlation between BMP2 and cyclooxygenase 2 protein expression in chorioamniotic membranes (R = 0.34; P < 0.001).
CONCLUSION
BMP2 mRNA and protein expression are increased in the chorioamniotic membranes of patients with spontaneous labor at term and patients with preterm labor associated with histologic chorioamnionitis. Its expression pattern and biologic effects strongly suggest that BMP2 is involved in human parturition.
Keywords: parturition, chorioamniotic membrane, immunohistochemistry, real-time quantitative reverse-transcriptase PCR, BMP-2, Preterm labor, Preterm premature rupture of membranes, PPROM
INTRODUCTION
Human parturition consists of an orchestrated series of physiological events that include myometrial contractility, cervical ripening and dilatation, and chorioamniotic membrane rupture.1–3 These processes are associated with changes in gene expression. 4–6 Yet, the understanding of the mechanisms responsible for parturition remains incomplete.3,7 To elucidate molecular events involved in human parturition, we performed a genome-wide screening of the transcriptome of the chorioamniotic membranes obtained from patients in labor at term.8
Unexpectedly, bone morphogenetic protein (BMP) 2 messenger RNA (mRNA) was among the genes that were dramatically up-regulated in the membranes obtained from patients in spontaneous labor at term than in those from patients not in labor. BMPs belong to the transforming growth factor-β (TGF-β) superfamily, which is a large group of extracellular growth factors involved in diverse and critical biological processes such as cell growth and differentiation. More than 30 BMPs have been identified to date.9 BMP2 was discovered as a distinct type of BMP in 1988, and knock-out mice had defects in the heart and chorioamnion.10,11 BMP2 promotes proliferation, differentiation, and apoptosis of rat uterine cells during the menstrual cycle, and several BMPs are expressed in the human placenta.12–14
This study was conducted to determine whether BMP2 mRNA and protein expression are altered in the chorioamniotic membranes of patients with term labor, preterm labor and preterm premature rupture of membranes (PPROM).
MATERIAL AND METHODS
Study design
A cross-sectional study was designed to describe the expression pattern of BMP2 in the chorioamniotic membranes of patients. All samples were taken from the files of the Pathology Section, Perinatology Research Branch, NICHD. All fetal membranes were obtained from placentas delivered at Hutzel Women’s Hospital between October, 1999 and October, 2002.
BMP2 mRNA and protein expression were analyzed by real-time quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR; n=88) and immunohistochemistry (IHC; n=124), respectively. The study consisted of patients in the following groups: 1) term not in labor and term in labor (qRT-PCR and IHC; n=44 and n=44, respectively); 2) preterm labor with and without histologic chorioamnionitis (qRT-PCR and IHC; n=18 and n=40, respectively); and 3) PPROM with and without histologic chorioamnionitis (qRT-PCR and IHC; n=26 and n=40, respectively). All 88 cases analyzed by qRT-PCR were among the 124 cases (70.9%) subjected to IHC.
Preterm labor was defined by the presence of regular uterine contractions that occurred at a frequency of at least 2 every 10 minutes, with cervical changes which led to delivery at <37 weeks of gestation. PPROM was diagnosed by sterile speculum examination with vaginal pooling, ferning, and nitrazine testing. Pregnancies were considered normal when there was no evidence of medical, obstetric or surgical complications, and resulted in a term delivery (≥37 gestational weeks). The criteria for the diagnosis of histologic chorioamnionitis have been previously described.15 All women provided written, informed consent prior to the collection of samples. The collection of samples and their use for research purposes were approved by the Institutional Review Boards of both Wayne State University and the National Institute of Child Health and Human Development.
Real-time quantitative reverse transcriptase-PCR (qRT-PCR)
The tissue samples were collected and kept in RNAlater (Ambion, Austin, TX) until total RNA extraction using the guanidinium isothiocyanate/cesium chloride method.16 Specific primers and probes for BMP2 were as follows: forward 5′-CTGTGATGCGGTGGACTGC-3′, reverse 5′-AAGTGGGCCACTTCCACCA-3′, and probe 5′-CAGGGACACGCCAACCATGGATTC-3′ (Applied Biosystems, Foster City, CA). qRT-PCR reactions were performed with TaqMan Universal PCR Master Mix Reagents (Applied Biosystems). The reaction was analyzed with a Sequence Detection System 7700 (Applied Biosystems). The ratios between BMP2 and 18S RNA were calculated and compared. Comparisons were done with the use of log-transformed data and Mann-Whitney U test (SPSS 12; SPSS Inc, Chicago, IL).
Immunohistochemistry
Five micron-thick paraffin sections were used for immunohistochemical staining. Murine monoclonal anti-hBMP2 and antibodies to cyclooxygenase-2 (PTGS2; COX-2 antibodies) and its blocking peptides were purchased from R&D Systems (cat# MAB355, Minneapolis, MN) and Santa Cruz (cat# sc-19999, Santa Cruz, CA), respectively. Immunoreactivity of amnion cells was semi-quantitatively analyzed using an immunostaining score. The immunostaining score was calculated by multiplying the intensity score (negative: 0, weak positive: 1, strong positive: 2) by the fraction (%) of immunopositive cells. At least five microscopic fields (X200) were analyzed using a microscope (Nikon Americas Inc, Melville, NY) with the SPOT advanced software (RT Slider, version 4.0.5, Diagnostic Instruments, Sterling Heights, Mich). A Mann-Whitney U test was used. Significance was assumed for a p value of < 0.05. Evaluation of the immunohistochemical results was done by two of the authors (GJK and YMK) who were blinded to the clinical information.
Amnion cell culture
Primary human amnion cells were obtained from three women at term not in labor. The cells were split in a 24-well plate (2 x 105 cells/well) in Ham’s F-12/DMEM media with 10% fetal bovine serum and antibiotics. The cells were incubated with 10 ng/mL of various cytokines (Interleukin (IL)-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, tumor necrosis factor [TNF]-α), and lipopolysaccharide (LPS) ((100 ng/mL) for 24 hours. The cells were also treated with different concentrations of recombinant human BMP2 (rhBMP2, 1 and 10 ng/mL) to analyze its effect on PGE2 expression by amnion cells. All cytokines and LPS were purchased from Sigma-Aldrich (Saint Louis, MO).
Immunofluorescence staining
Methanol-fixed amnion cells were immunostained with mouse anti-hBMP2 (1:50) and rabbit anti-PGE2 (Abcam Inc., Cambridge, MA) (1:50) antibodies for 2 hours at room temperature. After washing, the slides were incubated for one hour with FITC-conjugated anti-mouse IgG and FITC-conjugated anti-rabbit IgG for the detection of BMP2 and PGE2, respectively.
RESULTS
The characteristics of the study groups are summarized in Table I. The median BMP2 mRNA expression was significantly higher in both women with spontaneous labor at term and patients with preterm labor with histologic chorioamnionitis, than in those not in labor at term and without histologic chorioamnionitis, respectively (P < 0.001 and P < 0.05; Figure 1A and B). However, there was no significant difference in the median BMP2 mRNA expressions between the PPROM cases with histologic chorioamnionitis and the cases without (P = 0.13; Figure 1,C).
Table I.
Demographic and clinical characteristics of the study population.
| Characteristics | Term not in labor (n=22) | Term in labor (n=22) | PTL without chorioamnionitis (n=20) | PTL with chorioamnionitis (n=20) | PPROM without chorioamnionitis (n=20) | PPROM with chorioamnionitis (n=20) |
|---|---|---|---|---|---|---|
| Maternal age (years) | 26.6 ± 5.1 (19–39) | 23.0 ± 4.4 (17–36) | 22.0 ± 4.8 (16–37) | 23.9 ± 4.9 (16–30) | 27.4 ± 6.3 (16–42) | 27.2 ± 6.4 (18–38) |
| Gestational age at Delivery (weeks) | 39.0 ± 0.5 (38.0–40.3) | 39.6 ± 1.06 (37.4–42.0) | 29.2 ± 4.3 (21.0–34.0) | 29.3 ± 3.9 (22.1–34.1) | 29.4 ± 4.1 (20.1–34.0) | 28.9 ± 1.0 (20.6–33.3) |
| Gravidity | 3.5 ± 1.5 (1–7) | 3.5 ± 2.5 (1–9) | 2.7 ± 1.4 (1–6) | 3.8 ± 2.6 (1–10) | 3.9 ± 2.2 (1–8) | 4.6 ± 2.3 (1–10) |
| Parity | 1.7 ± 0.9 (0–4) | 1.1± 1.2 (0–4) | 1.0± 1.0 (0–3) | 1.6 ± 1.7 (0–6) | 1.7 ± 2.0 (0–7) | 2.4 ± 2.4 (0–9) |
The values are given as mean ± SD; the range is given in parentheses. PPROM: preterm premature rupture of membranes. BMP2 mRNA and protein expression values were expressed as mean ± standard deviation of log (BMP2 mRNA/18S RNA). The number in the parentheses represents the number of cases subjected to qRT-PCR or immunohistochemistry.
Figure 1.
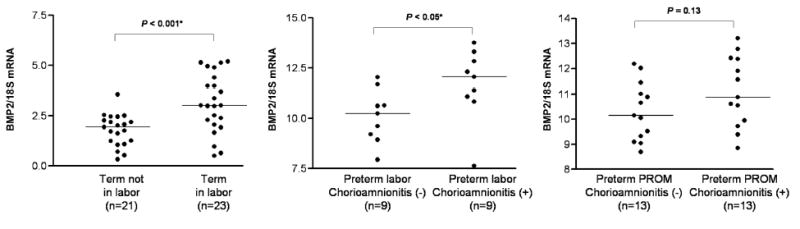
BMP2 mRNA expression in the chorioamniotic membranes in each study group.
BMP2 immunoreactivity was localized mainly to amniotic epithelial cells, although decidual and stromal cells, as well as intermediate trophoblasts of the choriodecidua, were also weakly positive (Figure 2, A and B). The BMP2 immunostaining score was significantly higher in the chorioamnion of women with spontaneous labor at term or in women with preterm labor with histological chorioamnionitis than in women without labor at term or without evidence of histological chorioamnionitis, (P < 0.001 and P < 0.001, respectively; Figure 2, D and E; Table II). However, as was the case in mRNA expression, there was no difference in BMP2 protein expression between PPROM patients with histologic chorioamnionitis and those without (P = 0.08; Figure 2, F).
Figure 2.
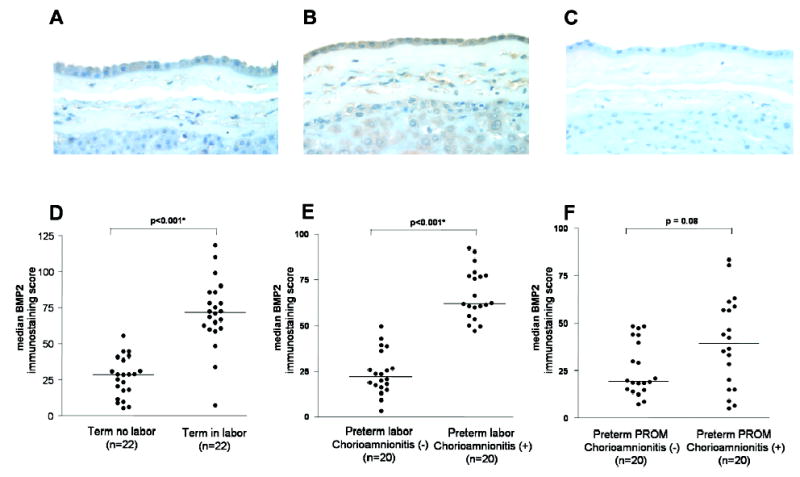
BMP2 protein expression in the chorioamniotic membranes. (A) Term in labor (B) Preterm labor with histologic chorioamnionitis (C) Negative control (X400) (D-F) Scatterplots of BMP2 immunostaining scores showing medians.
Table II.
BMP2 mRNA expression in amnion of patients with preterm labor (PTL), preterm premature rupture of membranes and normal pregnancy at term.
| Term not in labor | Term in labor | P a value | PTL without chorioamnionitis | PTL with chorioamnionitis | P b value | PPROM without chorioamnionitis | PPROM with chorioamnionitis | P c value | |
|---|---|---|---|---|---|---|---|---|---|
| mRNA (BMP2/18S mRNA) | 1.79 ± 0.79 (n=21) | 3.12 ± 1.45 (n=23) | < 0.001* | 10.11 ± 1.31 (n=9) | 11.69 ± 1.81 (n=9) | < 0.05* | 10.32 ± 1.15 (n=13) | 11.09 ± 1.39 (n=13) | 0.13 |
| Protein | 26.7 ± 13.8 (n=22) | 71.3 ± 23.8 (n=22) | < 0.001* | 24.1 ± 11.8 (n=20) | 67.2 ± 13.7 (n=20) | < 0.001* | 25.8 ± 14.2 (n=20) | 39.7 ± 23.6 (n=20) | 0.08 |
BMP2 mRNA and protein expression values were expressed as mean ± standard deviation of log (BMP2 mRNA/18S RNA).
n= The number of cases subjected to qRT-PCR or immunohistochemistry. PPROM, preterm, premature rupture of membranes.
: p <0.05
Comparison between pregnant women at term not in labor and in labor.
Comparison between pregnant women with PTL with and without chorioamnionitis.
Comparison between pregnant women with PPROM with and without chorioamnionitis.
Up-regulation of BMP2 mRNA and protein expression in the amnion cells associated with labor and histologic chorioamnionitis led us to look for its potential biological link to parturition. The immunohistochemical analysis of COX-2, a critical enzyme in PG synthesis, revealed strong immunoreactivity in amnion cells (Figure 3, A). COX-2 expression was significantly increased in the chorioamnion of women with spontaneous labor at term and with preterm labor with chorioamnionitis compared to cases of no labor at term and no chorioamnionitis, respectively (P < 0.001 and P < 0.001). Chorioamniotic membranes of patients with preterm labor and intact membranes with histologic chorioamnionitis also showed a greater expression of COX-2 than patients at term not in labor and preterm labor without chorioamnionitis (P < 0.001 for each). There was a significant correlation between COX-2 and BMP2 expression (Figure 3, B; R=0.34, P < 0.001).
Figure 3.
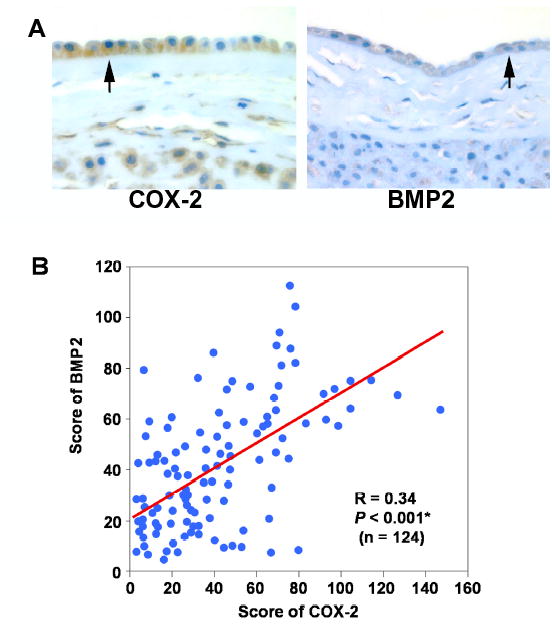
Correlation between BMP2 and COX-2 protein expressions in the chorioamniotic membranes. (A) Immunohistochemical staining for BMP2 and COX-2 in the chorioamnion in women at term. (B) Correlation between BMP2 and COX-2 (R = 0.34, P < 0.001).
We further tested whether BMP2 treatment of amnion cells can affect PGE2 synthesis. There was a prominent increase in PGE2 immunofluorescence in amnion cells treated with rhBMP2 (Figure 4). IL-1α, IL-1β, IL-6, IL-8, TNF-α and LPS treatment induced co-expression of BMP2 and PGE2 in amnion cells. The increase in BMP2 expression was most prominent after the treatment with IL-1β and TNF-α (Figure 5). Significant changes in BMP2 expression were not detected in IL-4 and IL-10 treated cells (data not shown).
Figure 4.
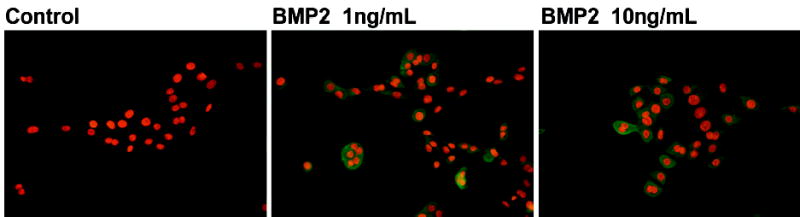
The effects of BMP2 on PGE2 expression. Increased cytoplasmic green immunofluorescence is evident in BMP2-treated amnion cells.
Figure 5.
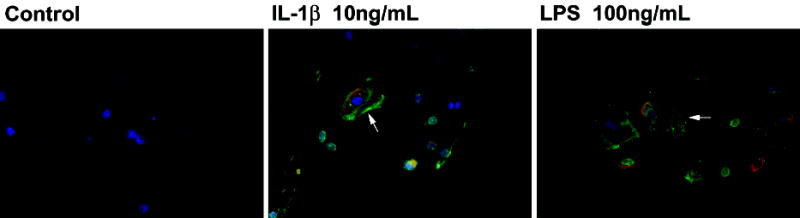
Co-localization of BMP2 and PGE2 in amnion cells that were treated with cytokines or LPS. Both the green immunofluorescence for BMP2 and red immunofluorescence for PGE2 are found in the cytoplasm of scattered amnion cells (arrows) treated with IL-1β and LPS (X 400).
COMMENT
The principal finding of this study was that BMP2 is up-regulated in the chorioamniotic membranes in labor and histologic chorioamnionitis. This is a novel observation.
The clinical and biological significance of this study is that genome-wide screening is a valuable tool in the identification of candidate molecules associated with human parturition that had not previously been implicated in this process with the use of conventional approaches. Having established that the mRNA and protein expression of BMP2 are increased in spontaneous parturition at term and preterm labor with intact membranes, regardless of the presence or absence of chorioamnionitis, the next step is to determine the specific role of BMP2 in the mechanism of parturition.
A strength of the study is that it was carried out with well-characterized human samples from patients with spontaneous labor at term, preterm labor (with and without intact membranes), and with and without histologic chorioamnionitis. The study design allowed the examination of a physiologic process (labor at term) from pathologic labor. A limitation of this study is that we focused on the changes in mRNA and protein for BMP2, and have only begun to examine the functional aspects of this cytokine in parturition. BMP2 expression was not increased in preterm PROM. It is unclear whether this is due to the sample size of this study or to the differences in the biology of parturition in patients with ruptured membranes.
This is the first study to examine the expression of BMP2 in parturition in any mammalian species. Thus, we are not able to compare our results with those of others. BMPs are also expressed in placental tissues. Placental BMP and growth differentiation factor 15 were related to placental calcification, while other BMPs were not.13 BMP2 signaling is mediated through transmembrane serine/threonine kinase receptors (types I and II), which transduce the signal to the nucleus by Smad proteins. In the nucleus, Smad heterodimers recruit DNA binding proteins and co-activator/co-repressor proteins, which in turn regulate the expression of target genes.17 We observed nuclear translocation of Smads 1, 5, and 8 following BMP2 treatment (data not shown), an indication that a functionally intact BMP2 signaling pathway is present in human amnion cells.
Functional studies of BMP2 in uterine contractility, cervical ripening, chorioamniotic membrane apoptosis and matrix-degrading enzymes are necessary to explore the role of this cytokine in parturition. It would be important to determine whether the administration of BMP2 to pregnant animals can induce parturition. Such studies will be important to determine whether the increased BMP2 expression reported herein is linked causally to or is the consequence of parturition.
Acknowledgments
We thank Ms. Enola Cushenberry and Ms. Stella DeWar for expert technical assistance in histological sample preparation, Mr. Pat Schoff for expert graphic assistance in the manuscript and Mrs. Julie Powers for editorial support of the manuscript.
References
- 1.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–29. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 2.Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002;23:257–73. doi: 10.1053/plac.2001.0782. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. In: Critchley H, Bennett P, Thornton S, editors. In Preterm Birth. London: RCOG Presss; 2004. p. 28–60.
- 4.Esplin MS, Henebold J, Adashi EY. The use of microarray technology to identify genes uniquely expressed by the laboring myometrium. Am J Obstet Gynecol. 2000;182:S131. Ref Type: Abstract. [Google Scholar]
- 5.Muhle RA, Pavlidis P, Grundy WN, Hirsch E. A high-throughput study of gene expression in preterm labor with a subtractive microarray approach. Am J Obstet Gynecol. 2001;185:716–24. doi: 10.1067/mob.2001.117183. [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Kuivaniemi H, Tromp G. Functional genomics and proteomics in term and preterm parturition. J Clin Endocrinol Metab. 2002;87:2431–34. doi: 10.1210/jcem.87.6.8689. [DOI] [PubMed] [Google Scholar]
- 7.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003;24 (Suppl A):S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 8.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Romero R. Spontaneous labor at term is characterized by a genomic signature of acute inflammation in the chorioamniotic membranes but not in the systemic circulation. Am J Obstet Gynecol. 2004;191(6):S138. Ref Type: Abstract. [Google Scholar]
- 9.Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–38. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- 10.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–86. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 12.Erickson GF, Fuqua L, Shimasaki S. Analysis of spatial and temporal expression patterns of bone morphogenetic protein family members in the rat uterus over the estrous cycle. J Endocrinol. 2004;182:203–17. doi: 10.1677/joe.0.1820203. [DOI] [PubMed] [Google Scholar]
- 13.Hromas R, Hufford M, Sutton J, Xu D, Li Y, Lu L. PLAB, a novel placental bone morphogenetic protein. Biochim Biophys Acta. 1997;1354:40–44. doi: 10.1016/s0167-4781(97)00122-x. [DOI] [PubMed] [Google Scholar]
- 14.Zhao GQ, Hogan BL. Evidence that mouse Bmp8a (Op2) and Bmp8b are duplicated genes that play a role in spermatogenesis and placental development. Mech Dev. 1996;57:159–68. doi: 10.1016/0925-4773(96)00543-6. [DOI] [PubMed] [Google Scholar]
- 15.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 16.Tromp G, Kuivaniemi H, Romero R, Chaiworapongsa T, Kim YM, Kim MR, et al. Genome-wide expression profiling of fetal membranes reveals a deficient expression of proteinase inhibitor 3 in premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1331–38. doi: 10.1016/j.ajog.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]


