Abstract
Following increasing reinfestation with Triatoma infestans after insecticide spraying, the household incidence of infection with Trypanosoma cruzi in children was positively related to the domestic abundance of infected T. infestans and the presence or proportion of infected dogs or cats in Amamá, a rural village in northwestern Argentina. Seven (12.1%) children seronegative for antibodies to T. cruzi at baseline, with no history of travel or blood transfusion, seroconverted after three years. Six incident cases lived in houses heavily infested with T. infestans, with high proportions of bugs infected with T. cruzi and having fed on humans or dogs. The remaining incident case occurred under a very light domestic infestation detected only at the endpoint, and most bugs had fed on humans. Dogs had a 17 times greater force of infection than children (4.3% per year). Sustained vector surveillance is crucially needed in high-risk areas for Chagas disease such as the Gran Chaco.
INTRODUCTION
Trypanosoma cruzi, the causative agent of Chagas disease, is widely prevalent in the Gran Chaco, a semi-arid landscape extending over Argentina, Bolivia, Paraguay and southwestern Brazil. Triatoma infestans, the main or only domestic vector of T. cruzi in this region, has long been the target of control programs that produced a strong decrease in human prevalence and incidence of T. cruzi.1,2 In the Gran Chaco, however, symptomatic acute cases of Chagas disease are still reported3 in spite of recurrent insecticide campaigns, and they have long been known as a small fraction of all cases occurring.
Theoretical models and empirical findings indicate that the household prevalence and incidence of T. cruzi infection are mainly determined by domestic bug abundance, the prevalence of infection, the infectiousness of humans, dogs, and cats to bugs, and host-specific feeding frequencies.4 The household prevalence5,6 and incidence7,8 of human infection with T. cruzi are closely related to the presence and number of domestic triatomines collected per person-hour. The few longitudinal studies relating incidence of T. cruzi to entomologic variables either dealt with a few heavily infested, selected households7,9 or a well-defined rural community sprayed with insecticides during the follow-up,8 thereby obscuring the relationship between vector abundance and transmission of T. cruzi. A fundamental question still unresolved is whether there is any threshold domestic bug abundance under which transmission to humans is zero.
After a single community-wide application of deltamethrin in rural northwestern Argentina, failure to set up a community-based surveillance system run by householders themselves allowed domestic reinfestation to return to pre-treatment levels within 3–7 years post-spraying.10 Screening for new cases of infection with T. cruzi showed a recently infected child who was referred for treatment.11 Operational constraints determined that the study village was resprayed by the Argentinian National Chagas Service (NCS) three years later, when we re-examined the cohort of previously seronegative children, referred the new cases for treatment, and assessed the incidence of infection according to several variables measured at the household level. As part of a larger project aimed at building an empirically based mathematical model of T. cruzi transmission and control,4 we sought to provide a solid quantitative basis to answer questions of public health relevance. First, in the absence of surveillance after a community-wide insecticide spraying conducted by professional sprayers, when and under what conditions did seronegative children acquire infections with T. cruzi? Second, can the future appearance of a vector-mediated incident case be predicted, and if so, what is the best and simplest risk predictor?
MATERIALS AND METHODS
Study area and design
Studies were undertaken in the rural village of Amamá (27°12′33″S, 63°02′10″W), Santiago del Estero, Argentina. In Amamá, 88–96% of houses had bedroom areas infested by T. infestans prior to being sprayed with deltamethrin (2.5% suspension concentrate, which is the type of formulation, also termed SC) by the NCS in September 1985 and October 1992.10 Other rural villages in the Moreno Department were not sprayed with residual insecticides until 1994–1996.
The study comprised a two-panel, house-to-house census, questionnaire, and entomologic and serologic surveys whose procedures and results were previously reported.6,10–15 From 1988 and 1989 to 1992, Amamá experienced an increase in the numbers of inhabited houses (from 41 to 48) and people (from 204 to 260), the percentage of houses with cracked walls (from 42.9% to 78.8%), the prevalence of domestic infestation by T. infestans (from 54–70% to 96%), the percentage of domestic T. infestans infected with T. cruzi (from 21% to 41%), and the seroprevalence for T. cruzi among dogs (from 40% to 65%). Little change occurred in child seroprevalence (from 29.6% to 26.5%), the use of domestic insecticides by householders (62% versus 60%), and the type of thatched roofs (roofs made of the grass simbol [Pennisetum sp.] were less favorable for infestation).
Triatomine surveys
A two-person team collected domestic triatomines by timed manual searches after spraying walls and roof repeatedly with a dislodging agent (0.2% tetramethrin) in December 1988 (four person-hours per house) and March 1992 (one person-hour per house), and after applying one γ-hexachlorocyclohexane (benzene hexachloride) fumigant tablet (Gammexane, Duperial, Buenos Aires, Argentina) per bedroom in March 198910,16 or spraying with deltamethrin in October 1992. Immediately after timed collections, insecticide fumigant canisters (Aguvac; Aguvac, Buenos Aires, Argentina) were used in two heavily infested houses in 1988 and in all houses in 1992 to reduce infestations and to calibrate the timed method. One month before, matched pairs of triatomine bug sensor boxes and paper sheets had been nailed to the bedroom walls of each house, and all the devices were inspected for numbers of fecal smears, eggs, bugs, and exuviae just before timed collections. The average number of triatomine fecal smears per house-month in each type of device was taken as an index of triatomine abundance. Householders were asked in October 1986, November 1987, March 1990, and October 1991 whether their bedroom areas had many, few, or no T. infestans. Peridomestic sites sustained very low bug infection rates with T. cruzi, and thus were excluded from consideration regarding domestic transmission.
Triatomines were identified to species, counted by instar, and a 30–50% sample of third instars and larger stages was microscopically examined for T. cruzi infection at a magnification of 400× within 10 days of capture.11,13 The individual blood meals of triatomines were identified by agar double-diffusion tests using five family-specific antisera (human, dog, cat, chicken, and goat-sheep).17
Serologic and parasitologic diagnosis
The Ethical Review Committee of the National Chagas Institute Dr. Mario Fatala Chaben of the Argentine Ministry of Health and Social Welfare reviewed and approved the study protocol in 1992. The study objectives were explained to house residents and all participants signed an informed consent form. Blood samples were obtained by venipuncture from 93 dogs ≥ 2 months old in December 1988, from 98 children < 16 years old (infants by finger prick) in March 1989, and from 83 dogs and 225 people examined in March 1992. These numbers corresponded to 77%, 90%, 66%, and 87%, respectively, of the registered population. Movement of residents to a different home in or out of the study area was noted. Each child’s mother was asked for individual travel histories between 1989 and 1992, including destination, length of time spent, whether there were triatomine bugs in the houses visited, and whether children showed clinical signs compatible with acute Chagas disease.
Seroreactivity of human sera for T. cruzi was demonstrated by titers ≥ 1:32 in an indirect hemagglutination (IHA) test and immunofluorescent antibody tests (IFAT) in both 1989 and 1992, and by an enzyme-linked immunosorbent assay (ELISA) in 1992.1 Seroreactivity of dog sera for T. cruzi was demonstrated by IHA and IFAT titers ≥ 1:16 and ELISA readings ≥ 0.2 in 1992.18 For human and dog sera, seropositive refers to samples reactive by at least two different serologic tests in any one year. New human serum samples obtained in 1993 and 1994 (after deltamethrin spraying and elimination of domestic infestations) were used for a definitive diagnosis of a few cases that had been serologically discordant or were absent in March 1992. Trypanosoma cruzi was detected by xenodiagnosis in 29% of seropositive people and 85% of seropositive dogs in 1992.12 Seven recently infected children and their mothers were transported to the Hospital Independencia in Santiago del Estero in January 1993, when seropositivity for T. cruzi by IHA and IFAT was confirmed in every case before treatment. Children who had been seronegative in March 1989 and had permanent residence in Amamá during 1989–1992, with no travel history, were considered as potential candidates to acquire an autochthonous infection with T. cruzi.
Data analysis
Data were scrutinized for discrepancies among census, interview, and entomologic data and corrected as reported.13 Assuming that the incidence of infection is constant with time and age, the instantaneous per capita rate of seroconversion from negative to positive (λ) may be estimated prospectively using a catalytic model with serorecovery rate set to 0,19 reflecting the previous absence of specific chemotherapy. λ was computed as −ln (1 minus; p)/t, where p is the proportion of seroconverted children after a three-year exposure period (t). λ was also estimated retrospectively from age-specific dog seroprevalence data using maximum likelihood procedures (Matlab 6.3; The MathWorks, Natick, MA), and the catalytic model λ = −ln (1 − pa)/a, where pa is the proportion of seropositive individuals within the age class whose midpoint is a.
The relationship between child incidence of T. cruzi (the response variable) and potential predictors was studied using maximum likelihood logistic multiple regression analysis in EGRET software.20 The logistic-binomial random effects model for distinguishable data included as predictors the numbers of T. infestans collected in bedroom areas in 1988–1989 and 1992; the number or proportion of domestic triatomines infected with T. cruzi on both dates; the proportion of reactive T. infestans with human blood meals (human blood index) and the chicken blood index; each child’s age in 1992 and sex; the total number of domestic hosts (people, dogs, cats, and chickens); the household presence of at least one dog or cat infected or the proportion of dogs or cats infected in 1988–1989 and 1992; and the presence of at least one child seropositive for T. cruzi in 1989 or 1992. Two analyses were conducted: one based on only 1988–1989 data, and another using only 1992 data. Backward and forward stepwise procedures were used to obtain the most parsimonious model that retained predictors at the 10% nominal significance level. Interaction terms were then added to this model and tested for significance. The probability used for nominal statistical significance was 5% unless otherwise stated.
RESULTS
The median domestic abundance of T. infestans increased from 1 bug per four person-hours (first–third quartile = 0–10) in December 1988 to 11 bugs per person-hour (1–40) in March 1992. Domestic bug abundance fluctuated dramatically only in four heavily infested houses treated with insecticide fumigant canisters or with indoor bedroom walls completely plastered between surveys. Infection rates of T. cruzi in domestic T. infestans in 1992 were consistently higher than in 1988–1989, except in three houses with few bugs examined at baseline. The log-transformed numbers of domestic T. infestans collected by timed searches and after applying insecticide fumigant canisters in March 1992 were significantly correlated (r2 = 0.855) although non-linearly, as shown by a slope > 1 (Figure 1A). In addition, the log-transformed total number of domestic T. infestans collected per person-hour (adjusted for linearity using equation in Figure 1A) and knockdown at each house (x) correlated positively and significantly with the log-transformed mean number of triatomine fecal smears per house-month (y) in sensor boxes (Figure 1B) or paper sheets (y = 0.3126 x + 0.0045, r2 = 0.473, number of houses = 43).
Figure 1.
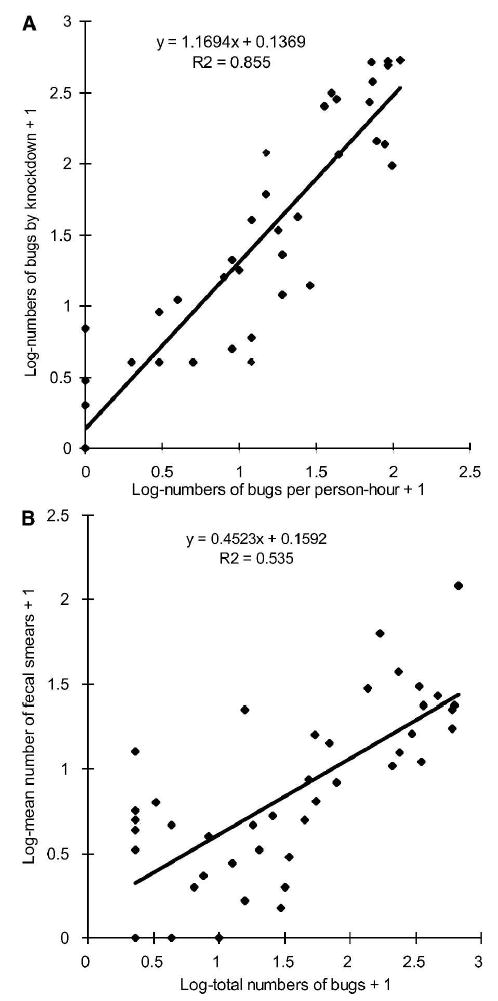
Linear regressions of the log-transformed numbers of domestic Triatoma infestans by timed manual and knockdown collections (A), and of mean triatomine fecal smears per house-month in sensor boxes and total numbers of domestic bugs by timed (adjusted for linearity using the equation in A) and knockdown collections (B) in Amamá, Argentina, March 1992.
Of 98 children examined serologically in March 1989, 19 (19%) who emigrated temporarily or definitively by 1992 were considered lost to follow-up (Table 1). An emigrated child seronegative at baseline was found seropositive for T. cruzi in 1994 while living in an infested house in another village. Children seropositive in 1989 emigrated as frequently (16%, 4 of 25) as seronegative children (18%, 12 of 68); children serologically discordant for T. cruzi in 1989 were excluded from these migration estimates. All 58 children serologically negative or discordant for T. cruzi in 1989 and reexamined in 1992 were reported by their parents and the local health care agent to have permanent residence at their homes during 1989–1992; no child received a blood transfusion. Seven (12.1%) children seroconverted between 1989 and 1992 in the absence of overt specific clinical symptoms, yielding an annual force of infection (λ) of 4.3% (SE = 1.25%). The age of incident cases ranged from 4 to 12 years in 1992 (median age = 6 years), and four were girls. Five cases were positive by two or three serologic methods, and the two cases positive by a single method later were positive by xenodiagnosis.
Table 1.
Serologic follow-up for Trypanosoma cruzi infection of children in March 1989 and March 1992 in Amamá, Argentina*
| Serodiagnosis in 1992
|
|||||
|---|---|---|---|---|---|
| Serodiagnosis in 1989 | Positive | Negative | Discordant | Lost to follow-up | Total |
| Positive* | 18 | 0 | 3 | 4 | 25 |
| Negative | 5† | 49 | 2† | 12 | 68 |
| Discordant | 0 | 2‡ | 0 | 3 | 5 |
| Total | 23 | 51 | 5 | 19 | 98 |
Children were ≤ 16 years old in March 1989. Seropositive refers to samples reactive by at least two different serologic tests in any one year. Discordant refers to samples reactive by only one test in any one year.
Confirmed as seropositive in a subsequent blood sample.
Both were seronegative in a subsequent blood sample.
All incident cases occurred in five houses that had few or many cracked walls, thatched roofs more favorable for T. infestans infestation (no simbol), indoor-nesting chickens, 6–8 people, and 2–5 dogs. The crude incidence of T. cruzi infection among children was greater in houses less than 10 years old (21% of 28 children versus 3% of 30 children tested); in those with a domiciliary area < 80 meters2 (21% of 29 children versus 3% of 29 children tested); in those with ≤ 2 peridomestic structures (18% of 40 children versus 0% of 18 children tested); in those with < 10 bovines, pigs, or equines (15% of 39 children versus 5% of 19 children tested), and in those with < 40 chickens or ducks (21% of 28 children versus 3% of 30 children tested), measures that are all associated with household wealth. None of the odds ratios was statistically significant, probably because of the limited sample size.
Assuming that the onset of new infections occurred at the midpoint between March 1989 and March 1992, the incident cases occurred on average 2–3 years (based on the earliest finding of domestic infestation by any method, including reports of householders; range = 1–3 years) after domestic reinfestation by T. infestans was initially detected in their homes. All reports of householders of domestic reinfestation in 1986–1987 were accompanied or followed by positive findings of T. infestans 1–2 years later.
The household incidence of T. cruzi increased with the number of domestic T. infestans collected per person-hour in 1988 and 1992 (Figure 2A). Six of the seven incident cases occurred in four houses with 42–111 domestic bugs per person-hour (11–30 triatomine dejecta per house-month) and large numbers of domestic bugs infected with T. cruzi in 1992 (range = 32–66 infected bugs per person-hour) (Figure 2B). Only one of these four houses was already in such a condition in December 1988, whereas the remaining three developed very light or moderate infestations (1 to 10–23 bugs per person-hour) by March 1989. The percentages of domestic T. infestans fed on dogs, humans, and cats were high and widely variable among households in both surveys. The remaining seventh incident case inhabited a house with only three, non-infected bugs collected in March 1989, and a light infestation in 1992 (14 bugs per person-hour, 7 triatomine dejecta per house-month) with low numbers of infected bugs (1.2 infected bugs per person-hour), no blood meals on dogs or cats, and most of the bugs fed on humans (88%).
Figure 2.
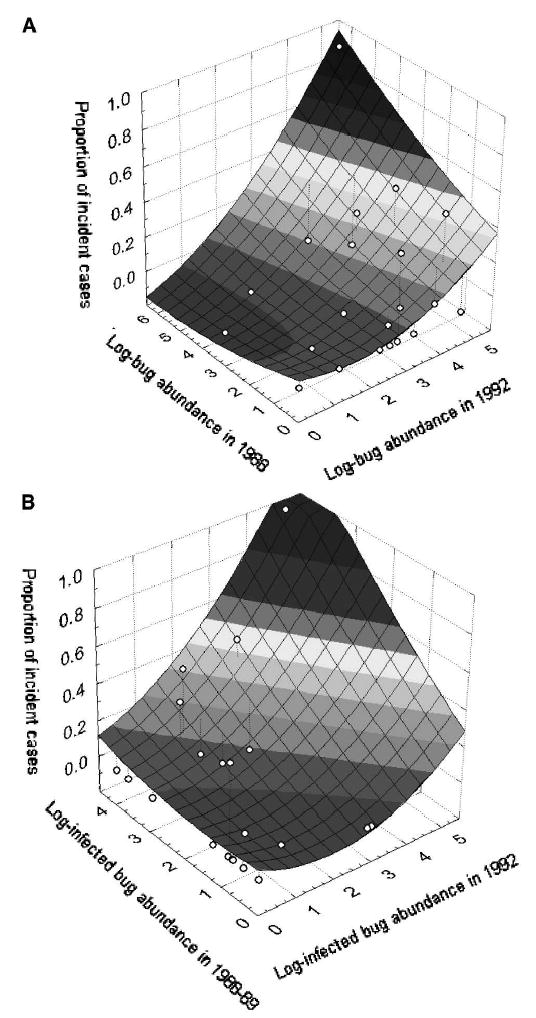
Relationships between the incidence of cases of infection with Trypanosoma cruzi in children ≤ 16 years old and numbers of domestic Triatoma infestans collected in 1988 (four person-hours) and 1992 (one person-hour) (A) and numbers of T. cruzi-infected domestic bugs collected in 1988–1989 (maximum catch) and 1992 (B) in Amamá, Argentina, 1988–1989 and March 1992.
Domestic T. infestans fed more frequently on humans and cats in those households with an incident case both in 1988–1989 and October but not March 1992 (Table 2). Blood meals on chickens were consistently more frequent in households with an incident case. Blood meals on dogs were very high and of similar prevalences in both types of households in all surveys.
Table 2.
Comparison between households with and without human incident cases with respect to the host-feeding patterns of domestic Triatoma infestans in Amamá, Argentina, 1988–1989 and 1992
| Median host blood indices (first–third quartile)
|
||||||
|---|---|---|---|---|---|---|
| Date of survey | Households with incident cases | No. of bugs tested | Humans | Dogs | Chickens | Cats |
| 1988–1989 | Yes | 82 | 61 (32–92) | 87 (78–91) | 66 (47–78) | 28 (20–33) |
| No | 69 | 45 (27–83) | 80 (22–100) | 50 (25–71) | 7 (0–34) | |
| March 1992 | Yes | 155 | 33 (21–44) | 52 (7–55) | 54 (25–85) | 4 (3–14) |
| No | 246 | 67 (26–76) | 45 (26–58) | 21 (0–50) | 2 (0–25) | |
| October 1992 | Yes | 35 | 82 (72–89) | 72 (66–74) | 25 (20–28) | 13 (11–17) |
| No | 31 | 50 (0–50) | 100 (63–100) | 0 (0–0) | 0 (0–0) | |
In dogs, the seroprevalence of T. cruzi infection in 1992 increased from 47.1% in dogs < 1 year of age to 88.9% and 70% in those 4–5 and ≥ 6 years old, respectively (Figure 3). The mean age of infected dogs < 1 year of age was 4.6 months. λ was 72.7% per year (95% confidence interval [CI] = 17.7–100% per year). Older infected dogs were less frequently observed than predicted by a time- and age-independent incidence model.
Figure 3.
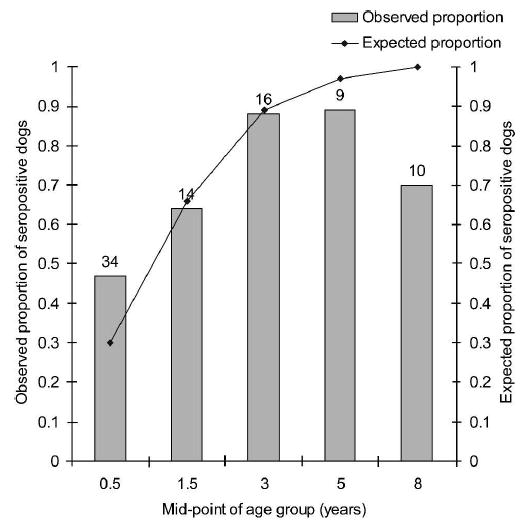
Observed (bars) and expected (line) age-specific sero-prevalence rates for Trypanosoma cruzi infection in dogs from Amamá, Argentina, March–October 1992. The line is the fit of the catalytic model with constant force of infection over time and age. Numbers above the bars represent the numbers of dogs examined for infection.
Households with at least one infected dog in both surveys had a higher crude incidence of human T. cruzi infection (23.1%; λ = 8.7%) than households with at least one infected dog in either survey (0–5.3%; λ = 0–1.8%) or in no survey (0%) (Figure 4A). Relative to numbers of infected dogs or cats per household in 1992, the crude incidence of T. cruzi infection increased from 0% (of 13 children tested) to 5.6% (of 18) to 19% (of 21) when there were 0–1, 2, and 3–5 infected dogs or cats, respectively. All of the incident cases co-occurred with 2–3 T. cruzi-infected dogs per household; 93% of 14 dogs or cats examined were infected, and 9 (82%) of those tested by xenodiagnosis were infectious to T. infestans.
Figure 4.
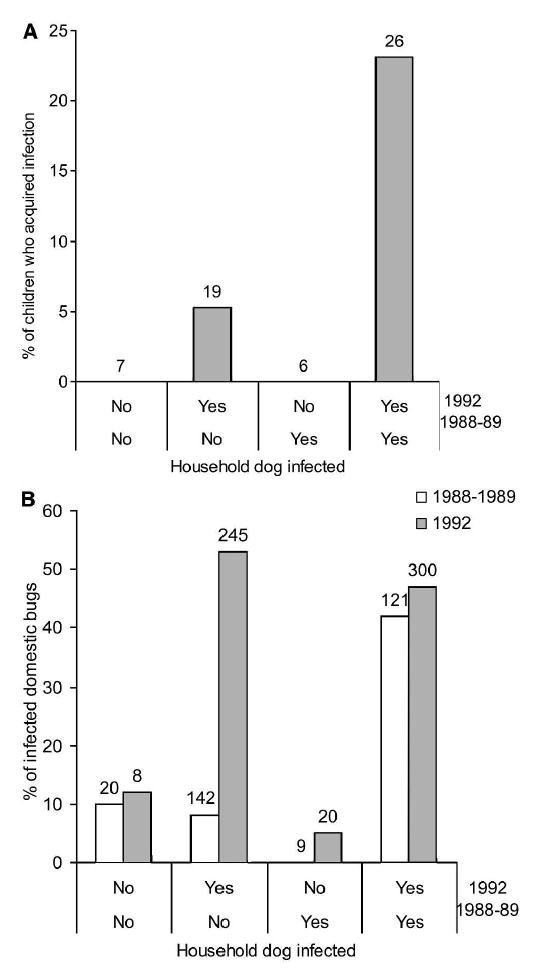
Incidence of Trypanosoma cruzi infection in A, children ≤ 16 years old and B, proportions of T. cruzi-infected domestic Triatoma infestans according to the household presence of infected dogs in 1988–1989 and 1992 in Amamá, Argentina. Numbers above the bars represent the numbers of children seronegative for T. cruzi in March 1989 with permanent residence at the source home (A) or bugs that were examined for infection (B).
Households with one or more infected dogs in both surveys had persistently higher proportions of infected bugs (42–47%) than households with no infected dog (10–12%), which also had very few bugs throughout (Figure 4B). Households with one or more infected dogs detected only in 1992 had bug infection rates (53%) similar to those of houses with infected dogs in both surveys, coinciding with the increasing trend in domestic bug abundance and prevalence of infection in dogs.
Multiple logistic regression analysis showed that both the domestic bug abundance in 1988 and the maximum bug abundance in 1988–1989 were significant predictors of the odds of being a future incident case (Table 3). Addition of the presence of at least one infected dog in the household significantly improved the fit of both models, whereas the percent of infected domestic bugs did not. The best fitting and most parsimonious model included the maximum domestic abundance of infected T. infestans in 1988–1989. The relative odds of being a future incident case was 1.071 (95% CI = 1.022–1.122) per unit increase in the maximum number of infected bugs collected. Using only the 1992 entomologic data, we observed that the number of domestic bugs per person-hour and the infected domestic bug abundance were highly significant predictors of the odds of currently being an incident case. Adding the proportion of infected dogs or cats improved significantly the fit of both models. The separate addition to every model (in 1988–89 or 1992) of terms that represented the (current or previous) presence of at least one infected child in the household, the age and sex of the child, host numbers, the human or chicken blood indices (in the data subset that included observations with no missing data), and the random effects parameter was not significant. Two indices of transmission computed as the product of domestic bug abundance (or infected bug abundance) per host times the human blood index performed worse than the model including infected domestic bug abundance and the proportion of infected dogs or cats.
Table 3.
Logistic multiple regression analysis of the models that regressed the proportion of candidate children who acquired Trypanosoma cruzi infection (the response variable) on the domiciliary abundance of Triatoma infestans, the abundance of bugs infected with T. cruzi per person-hour, the number or proportion of infected dogs or cats, the presence of at least one infected dog or child, the human blood index, and the household effects parameter (the predictor variables), Amamá, Argentina, 1988–1992*
| Data | Terms in model† | Deviance | Degrees of freedom | Likelihood ratio statistic | P |
|---|---|---|---|---|---|
| 1988–1989, Intercept | 42.72 | 0 | |||
| Bug abundance in 1988 | 38.03 | 1 | 4.69 | 0.03 | |
| + % infected bugs | 36.29 | 1 | 1.74 | 0.19 | |
| + at least 1 dog positive | 33.51 | 1 | 4.52 | 0.03 | |
| Maximum bug abundance in 1988–1989 | 37.39 | 1 | 5.33 | 0.02 | |
| + % infected bugs | 35.72 | 1 | 1.67 | 0.20 | |
| + at least 1 dog positive | 33.18 | 1 | 4.21 | 0.04 | |
| + at least 1 child positive | 36.81 | 1 | 0.57 | 0.45 | |
| Maximum infected bug abundance in 1988–1989 | 32.70 | 1 | 10.02 | 0.002 | |
| + at least 1 dog positive | 30.86 | 1 | 1.85 | 0.17 | |
| + at least 1 child positive | 32.15 | 1 | 0.55 | 0.46 | |
| 1992, Intercept | 42.72 | 0 | |||
| Bug abundance | 30.79 | 1 | 11.93 | < 0.001 | |
| + % infected bugs | 30.78 | 1 | 0.01 | 0.92 | |
| + % dogs positive | 26.46 | 1 | 4.33 | 0.04 | |
| Infected bug abundance | 31.66 | 1 | 11.06 | < 0.001 | |
| + % dogs positive | 25.44 | 1 | 6.22 | 0.01 | |
| + at least 1 child positive in 1989 | 31.60 | 1 | 0.06 | 0.81 | |
Addition of child age and sex, number of domestic hosts, human or chicken blood indices, and the random effects parameter to every model was not significant. This includes cases with no missing data for the variables in the model.
Predictor variables preceded by a + sign were added separately and independently, not cumulatively with others preceded by a + sign, to the above predictor variable not preceded by a + sign; for example, Bug abundance in 1988 alone had a deviance of 38.03, Bug abundance in 1988 plus % infected bugs had a deviance of 36.29, and Bug abundance in 1988 plus “at least 1 dog positive” (not including “% infected bugs”) had a deviance of 33.51.
Of 30 children born in Amamá after the 1985 deltamethrin spraying and examined serologically for T. cruzi only in 1989, a 10-month-old seropositive girl born to a seronegative mother was living in a heavily infested house in 1988 (97 infected bugs per four person-hours, 27% of the bugs fed on humans and 87% fed on dogs, two infected dogs). Of 38 Amamá-born children ≤ 7 years old examined only in 1992, two (5.3%) born to seronegative mothers were seropositive for T. cruzi: a three-year-old girl with no travel history living in a heavily infested house with an incident case, and a five-year-old boy in 1992 whose house had been heavily infested in 1988 (151 bugs per four person-hours but none infected, two of four children seropositive, three dogs seronegative) just before being treated with insecticide fumigant canisters. The mother of the five-year-old boy recalled that the child presented a persistently swollen eye at eight months of age (in 1988, when we detected no infected bugs) after staying approximately two weeks at the infested home of his grandparents in another village. In March 1989, the home of the child had 50 bugs collected by knockdown (10% infected, 75% of bugs fed on humans and 44% fed on dogs), and in 1992 there was a light infestation in his newer house (9 bugs per person-hour, 47% infected, 33% of the bugs fed on humans, and 100% fed on dogs or cats, 1 of 4 children seropositive, and 2 of 4 dogs and 1 cat infected).
DISCUSSION
In the absence of vector surveillance, infection of first child with T. cruzi occurred in a dilapidated hut approximately three years after residual spraying with deltamethrin, and most incident cases probably occurred between two and three years after domestic reinfestation by T. infestans was initially perceived by householders or detected by manual collections. The increasing trend in transmission suggests that the onset of child infections was more likely biased toward 1992, in which case the force of infection averaged over three years would underestimate the actual rate at the time in which most cases appeared. The high pressure of domestic recolonization by T. infestans from persistent peridomestic foci21 and high prevalence of T. cruzi infection in dogs and humans set the stage for rapid resurgence of domestic transmission.
An unexpected observation was that one of the seven incident cases occurred in a house with very low domestic infestation and infected numbers of bugs, and a high human-bug contact rate. An explanation could be the relative lack of sensitivity and precision of timed manual collections, or nights spent away from the usual residence, as observed in Brazil.8,22 Unlike these studies, we rule out the possibility that dispersing adult bugs flew into the house without forming colonies2 because T. infestans is a highly domiciliated species in Santiago del Estero and other triatomine species were rarely found infected with T. cruzi. In our study, all incident cases had permanent residence at their homes and no travel history, and all indices of domestic bug abundance were quantitatively consistent. Sensor boxes were very sensitive for detecting domestic infestations at low bug densities.16 Results of the 1993–1996 follow-up of children serologically negative or discordant for T. cruzi in 1992 rule out the possibility that existent latent infections were not detected. In Santiago del Estero, most symptomatic acute cases of T. cruzi occurred in children < 16 years of age in spring and summer.23 The evidence therefore suggests that the threshold domestic abundance of T. infestans below which transmission of T. cruzi to humans is unlikely was very low, if any threshold exists at all, and undetectable within the imprecision of vector sampling methods.
Measurement of a threshold human biting rate for transmission is additionally fraught with other sources of inaccuracy regarding human-bug contact rate, host exposure, and the probability of human infection given a feeding contact with an infected bug (b).24 In domestic T. infestans, the human blood index varies with the availability of domestic animal hosts, and reflects human-bug contact over an undefined period of time because blood meals are large and remain detectable for 3–4 months depending on temperature, stage, and additional blood meals.17 The daily feeding rate of T. infestans is measurable through the temperature-adjusted occurrence of transparent urine assessed shortly after capture.25 The product of human blood index and daily feeding rate estimates human-bug contact rates,25,26 but it is not a daily rate because of the undefined period of time upon which the bugs have fed on a given host species and whether they fed once or several times. In contrast, in most anopheline mosquitoes the occurrence and detectability of blood meals are tightly linked within each gonotrophic cycle. Since both events are dimensionally consistent, the product of the human blood index and daily biting rate yields a daily human biting rate. The large variability in bug feeding rates26 and host blood indices17 among houses and seasons implies the need of recurrent labor to catch and process a sufficient number of bugs to achieve a given precision. For regular monitoring purposes in vector control programs, these indices are operationally complex and did not show added predictive power. The number of domestic bugs collected per person-hour, whether infected or not, and other proportional correlates of bug abundance constitute suitable indices that may be measured routinely by vector control programs for risk assessment and for prioritizing field operations.
Human and animal exposure to domestic T. infestans varied markedly among and within households or individual hosts over seasons. Humans reduced their exposure to domestic bugs when infestations were irritating or weather was hot by sleeping in verandas or patios, as reflected in lower human blood indices in late summer. However, the actual exposure patterns of hosts are hard to measure in the field. Local villagers sometimes reported extended visits to relatives elsewhere where they became exposed to domestic triatomines, as illustrated by the seropositive child with symptoms compatible with acute Chagas disease. Some householders reported having no dog or cat or chicken nesting indoors but actually had them, as also determined through the host-feeding patterns of the bugs.13,14 Since nearly all domestic animals moved freely and houses did not have fences, animals listed at a household may not be available during the normal feeding periods of the bugs. Heterogeneity in host exposure may result in a non-random distribution of potentially infective feeding contacts between bugs and hosts, thereby violating the random assumption upon which estimates of basic reproduction number and b are based.24 A fundamental and still unresolved question is whether the thousands of potentially infective contacts delivered by a large domestic triatomine population annually are distributed uniformly among individuals of a given host species, or tend to be concentrated on uninfected or infected hosts. Such host-feeding choices modify greatly the risk of infection and reinfection.
Transmission of T. cruzi through contamination with bug feces has generally been considered very ineffective. The probability that a seronegative child would contract an infection with T. cruzi given a potentially infective contact with infected domestic T. infestans (b) was estimated between 0.001 and 0.004 in households with incident cases, although this was regarded as an underestimate.9 In medical entomology, estimates of b depend on several untestable assumptions and are derived from entomologic and parasitologic measurements combined.24 Several of these measurements are affected by significant errors further compounded by multiplication, which therefore increase the variance of b. Experimental studies showed that blood meal size of triatomine bugs correlated negatively with time to first defecation, thereby suggesting that low-density bug populations with increased feeding success would pose the greatest risk of T. cruzi transmission.27 Indeed, human cases of acute T. cruzi transmitted by dispersing adult triatomines flying into houses in Acapulco or the Amazon2 and the United States,28 among others, or by low-density T. infestans populations in Paraguay29 and our study, demonstrate that the probability of transmission b is considerable and should leave little room for complacency. Given the multiple sources of uncertainty underlying the estimation of b and insufficient empirical data, we propose a more prudent approach promoting no tolerance of domestic triatomine infestations in areas highly endemic for Chagas disease.
The household incidence of T. cruzi infection among children was strongly associated with the presence and number of dogs infected with T. cruzi, as was human and bug infection.6,11 Infected dogs served both as an antecedent and or concurrent risk factor for increased transmission to children and bugs in all of the incident cases. This pattern emerges because dogs are highly infectious to bugs and preferred over humans by domestic T. infestans,12,13,17 and most dogs living in infested houses became infected in a few months and much faster than children. Some incident infections in dogs were probably not recorded because of high incidence and high death rates among pups. Cats were less abundant than dogs and their role is less well-defined but probably significant under certain circumstances.5,30 Dogs and chickens also play an outstanding role as risk factors for the household transmission of T. cruzi in other rural areas in Argentina.26
Households with at least one incident case consistently had indoor-resting chickens and more domestic bugs fed on chickens than households without incident cases. The presence of brooding or nesting chickens in bedroom areas increased chicken blood indices and the domestic abundance of T. infestans, whether infected or not.13–15 Although all birds are refractory to T. cruzi, indoor-resting chickens increase the risk of human or bug infection, and may eventually contribute to propagating infestations within a village.4
All incident cases occurred in newer and smaller houses that were more favorable for domestic infestation, with chickens indoors and many dogs or cats, fewer peridomestic structures, and fewer corral animals. Some of these attributes were associated with larger domestic infestations and higher sero-prevalence of T. cruzi in humans.6,15 Human incidence of T. cruzi was therefore aggregated and linked inversely to household wealth.
In conclusion, the evidence herein provided does not support tolerance of light domestic infestations because T. cruzi transmission may occur at low abundance of T. infestans in the context of sporadic insecticide spraying and high host prevalence of infection. Light infestations may first lead to an inapparent incidence of T. cruzi among dogs,31 which will increase rapidly the risk of infection for triatomine bugs and other household members. Disorganized decentralization of vector control programs in the early 1980s followed by diminishing operational capacity (staff, vehicles) since the 1990s led to the current scenario of persistent peridomestic infestation with recurrent domestic recolonization by T. infestans in the most affected regions. Sustained, open-ended vector surveillance is crucially needed in high-risk areas such as the Gran Chaco.
Acknowledgments
We thank Abel Hurvitz and his staff at the Servicio Nacional de Chagas (Argentina), and Nicolás Schweigmann and Diego P. Vázquez for their support. Helpful comments were provided by Richard Reithinger. Dr. Oscar Ledesma Patiño kindly gave us support at the Hospital Independencia in Santiago del Estero. Ricardo E. Gürtler thanks the Latin American Network for Research on the Biology and Control of Triatominae (ECLAT) for helpful discussions. Ricardo E. Gürtler, María C. Cecere, and Elsa L. Segura are members of the Researcher’s Career (Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina).
Footnotes
Financial support: This study was supported by grants from the Rockefeller Foundation (New York) to Rockefeller University (New York) for a collaborative research project on modeling transmission dynamics and control of Chagas’ disease in Argentina (principal investigators: Joel E. Cohen, Roberto Chuit, and Ricardo E. Gürtler) and from the University of Buenos Aires to Ricardo E. Gürtler. The participation of Joel E. Cohen was also supported, in part, by U. S. National Science Foundation Grant DEB-9981552. Joel E. Cohen thanks Mr. and Mrs. William T. Golden for hospitality during this work. The final stage of the study was supported by National Institutes of Health research grant # R01 TW05836 funded by the Fogarty International Center and the National Institute of Environmental Health Sciences (NIEHS) (principal co-investigators: Uriel Kitron and Ricardo E. Gürtler).
Reprint requests: Ricardo Esteban Gürtler, Laboratorio de Eco-Epidemiología, Departamento de Ecología, Genética y Evolución, Ciudad Universitaria, C1428EHA, Buenos Aires, Argentina, E-mail: gurtler@bg.fcen.uba.ar.
Authors’ addresses: Ricardo E. Gürtler, María C. Cecere, and Rosario M. Petersen, Departamento de Ecología, Genética y Evolución, Ciudad Universitaria, C1428EHA, Buenos Aires, Argentina. Marta A. Lauricella and Elsa L. Segura, Instituto Nacional de Parasitología Dr. Mario Fatala Chabén, Paseo Colón 568, 1063 Buenos Aires, Argentina. Roberto Chuit, Centro de Investigaciones Epidemiológicas, Academia Nacional de Medicina, Avenida Las Heras 3092, 1425 Buenos Aires, Argentina. Joel E. Cohen, Laboratory of Populations, Box 20, Rockefeller University and Columbia University, 1230 York Avenue, New York, NY 10021-6399.
References
- 1.Segura EL, Cura EN, Sosa Estani S, Andrade J, Lansetti JC, De Rissio AM, Campanini A, Blanco SB, Gürtler RE, Alvarez M. Long-terms effects of a nation-wide control program on the seropositivity for Trypanosoma cruzi infection in young men from Argentina. Am J Trop Med Hyg. 2000;62:353–362. doi: 10.4269/ajtmh.2000.62.353. [DOI] [PubMed] [Google Scholar]
- 2.Dias JCP, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America—A review. Mem Inst Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- 3.Ministerio de Salud y Acción Social, Argentina, 2003. Boletín Epidemiológico Nacional Buenos Aires: Ministerio de Salud y Acción Social.
- 4.Cohen JE, Gürtler RE. Modeling household transmission of American trypanosomiasis. Science. 2001;293:694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- 5.Mott KE, Muniz TM, Lehman JS, Jr, Hoff R, Morrow RH, Jr, Oliveira TS, Sherlock IA, Draper CC. House construction, triatomine distribution and household distribution of seroreactivity to Trypanosoma cruzi in a rural community in northeast Brazil. Am J Trop Med Hyg. 1978;27:1116–1122. doi: 10.4269/ajtmh.1978.27.1116. [DOI] [PubMed] [Google Scholar]
- 6.Gürtler RE, Chuit R, Cecere MC, Castañera MB, Cohen JE, Segura EL. Household prevalence of seropositivity for Trypanosoma cruzi in three rural villages of northwest Argentina: environmental, demographic and entomologic associations. Am J Trop Med Hyg. 1998;59:741–749. doi: 10.4269/ajtmh.1998.59.741. [DOI] [PubMed] [Google Scholar]
- 7.Minter DM. Triatomine bugs and the household ecology of Chagas’ disease. Trans R Soc Trop Med Hyg. 1978;72:85–93. [Google Scholar]
- 8.Piesman J, Sherlock IA, Mota E, Todd CW, Hoff R, Weller TH. Association between household triatomine density and incidence of Trypanosoma cruzi infection during a nine-year study in Castro Alves, Bahia, Brazil. Am J Trop Med Hyg. 1985;34:866–869. doi: 10.4269/ajtmh.1985.34.866. [DOI] [PubMed] [Google Scholar]
- 9.Rabinovich JE, Wisnivesky-Colli C, Solarz ND, Gürtler RE. Probability of transmission of Chagas disease by Triatoma infestans (Hemiptera, Reduviidae) in an endemic area of Santiago del Estero, Argentina. Bull World Health Organ. 1990;68:737–746. [PMC free article] [PubMed] [Google Scholar]
- 10.Gürtler RE, Petersen RM, Schweigmann NJ, Cécere MC, Chuit R, Gualtieri JM, Wisnivesky–Colli C. Chagas disease in north-west Argentina: risk of domestic reinfestation by Triatoma infestans after a single community-wide application of deltamethrin. Trans R Soc Trop Med Hyg. 1994;87:12–15. doi: 10.1016/0035-9203(94)90483-9. [DOI] [PubMed] [Google Scholar]
- 11.Gürtler RE, Cécere MC, Rubel DN, Petersen RM, Schweigmann NJ, Lauricella MA, Bujas MA, Segura EL, Wisnivesky-Colli C. Chagas disease in north-west Argentina: infected dogs as a risk factor for the domestic transmission of Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1991;85:741–745. doi: 10.1016/0035-9203(91)90440-a. [DOI] [PubMed] [Google Scholar]
- 12.Gürtler RE, Cecere MC, Castañera MB, Canale D, Lauricella MA, Chuit R, Cohen JE, Segura EL. Probability of infection with Trypanosoma cruzi of the vector Triatoma infestans fed on infected humans and dogs in northwest Argentina. Am J Trop Med Hyg. 1996;55:24–31. [PubMed] [Google Scholar]
- 13.Gürtler RE, Cohen JE, Cécere MC, Lauricella MA, Chuit R, Segura EL. Influence of humans and domestic animals on the household prevalence and abundance of the Chagas disease vector Triatoma infestans infected with Trypanosoma cruzi in northwest Argentina. Am J Trop Med Hyg. 1998;58:748–758. doi: 10.4269/ajtmh.1998.58.748. [DOI] [PubMed] [Google Scholar]
- 14.Cecere MC, Gürtler RE, Chuit R, Cohen JE. Effects of chickens on the prevalence of infestation and population density of Triatoma infestans in rural houses of north-west Argentina. Med Vet Entomol. 1997;11:383–388. doi: 10.1111/j.1365-2915.1997.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 15.Cecere MC, Gürtler RE, Canale D, Chuit R, Cohen JE. Factors limiting the domiciliary density of Triatoma infestans, vector of Chagas’ disease, in north-west Argentina: a longitudinal study. Bull World Health Organ. 1998;76:373–384. [PMC free article] [PubMed] [Google Scholar]
- 16.Gürtler RE, Chuit R, Cecere MC, Castañera MB. Detecting domestic vectors of Chagas disease: a comparative trial of six methods in north-west Argentina. Bull World Health Organ. 1995;73:487–494. [PMC free article] [PubMed] [Google Scholar]
- 17.Gürtler RE, Cohen JE, Cecere MC, Chuit R. Shifting host choices of the vector of Chagas disease Triatoma infestans in relation to the availability of hosts in houses in north-west Argentina. J Appl Ecol. 1997;34:699–715. [Google Scholar]
- 18.Lauricella MA, Castañera MB, Gürtler RE, Segura EL. Immunodiagnosis of Trypanosoma cruzi (Chagas’ Disease) infection in naturally infected dogs. Mem Inst Oswaldo Cruz. 1998;93:501–507. doi: 10.1590/s0074-02761998000400016. [DOI] [PubMed] [Google Scholar]
- 19.Muench H, 1959. Catalytic Models in Epidemiology Cambridge, MA: Harvard University Press.
- 20.EGRET, 1993. Epidemiological Graphics, Estimation and Testing Package Seattle, WA: Statistics and Epidemiology Research Corporation.
- 21.Cecere MC, Gürtler RE, Canale DM, Chuit R, Cohen JE. Effects of partial housing improvement and insecticide spraying on the reinfestation dynamics of Triatoma infestans in rural northwestern Argentina. Acta Trop. 2002;84:101–116. doi: 10.1016/s0001-706x(02)00183-3. [DOI] [PubMed] [Google Scholar]
- 22.Mota EA, Guimaraes AC, Santana OO, Sherlock I, Hoff R, Weller TH. A nine-year prospective study of Chagas’ disease in a well-defined rural population in northeast Brazil. Am J Trop Med Hyg. 1990;42:429–440. doi: 10.4269/ajtmh.1990.42.429. [DOI] [PubMed] [Google Scholar]
- 23.Romaña CA, 1963. Enfermedad de Chagas Buenos Aires: López Libreros Editores.
- 24.Dye C. The analysis of parasite transmission by bloodsucking insects. Annu Rev Entomol. 1992;37:1–19. doi: 10.1146/annurev.en.37.010192.000245. [DOI] [PubMed] [Google Scholar]
- 25.Catalá S, Crocco LB, Morales GF. Trypanosoma cruzi transmission risk index (TcTRI): an entomological indicator of Chagas disease vectorial transmission to humans. Acta Trop. 1997;68:285–295. doi: 10.1016/s0001-706x(97)00098-3. [DOI] [PubMed] [Google Scholar]
- 26.Catalá SS, Crocco LB, Munoz A, Morales G, Paulone I, Giraldez E, Candioti C, Ripol C. Entomological aspects of Cha-gas’ disease transmission in the domestic habitat, Argentina. Rev Saude Publica. 2004;38:216–222. doi: 10.1590/s0034-89102004000200010. [DOI] [PubMed] [Google Scholar]
- 27.Trumper EV, Gorla DE. Density-dependent timing of de-faecation by Triatoma infestans. Trans R Soc Trop Med Hyg. 1991;85:800–802. doi: 10.1016/0035-9203(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 28.Navin TR, Roberto RR, Juranek DD, Khanchit L, Mortenson EW, Clover JR, Yescott RE, Taclindo C, Steurer F, Allain D. Human and sylvatic Trypanosoma cruzi infection in California. Am J Public Health. 1985;75:366–369. doi: 10.2105/ajph.75.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rojas de Arias A, Ferro EA, Ferreira ME, Simancas LC. Chagas disease vector control through different intervention modalities in endemic localities of Paraguay. Bull World Health Organ. 1999;77:331–339. [PMC free article] [PubMed] [Google Scholar]
- 30.Gürtler RE, Cécere MC, Petersen RM, Rubel DN, Schweigmann NJ. Chagas disease in north-west Argentina: association between Trypanosoma cruzi parasitaemia in dogs and cats and infection rates in domestic Triatoma infestans. Trans R Soc Trop Med Hyg. 1993;87:12–15. doi: 10.1016/0035-9203(93)90400-k. [DOI] [PubMed] [Google Scholar]
- 31.Castañera MB, Lauricella MA, Chuit R, Gürtler RE. Evaluation of dogs as sentinels of the transmission of Trypanosoma cruzi in a rural area of north-west Argentina. Ann Trop Med Parasitol. 1998;92:671–683. doi: 10.1080/00034983.1998.11813327. [DOI] [PubMed] [Google Scholar]


