Abstract
The spatio-temporal reinfestation patterns by Triatoma infestans following a blanket insecticide spraying in the rural community of Amamá in northwestern Argentina were analyzed using a geographic information system, satellite imagery, and spatial statistics. Domestic and peridomestic reinfestation by triatomine bugs was monitored from 1993 to 1997. Triatoma infestans was detected at least once in 75% of 2,110 sites evaluated. The prevalence of sites positive at least once for T. infestans during the study period increased sharply from 1993–1995 (0.6–2.9%) to November 1997 (32%). The initial source of T. infestans was a pig corral in southern Amamá one year post-spraying. Subsequent infestations were clustered around this initial focus at a distance of approximately 400 meters starting in 1995. In 1996, clustering was maximized in sites within the same or in neighboring compounds at distances of 25–175 meters. An effective control program on the community level will be based on the spraying of actual epicenters and sites within 450 meters of these epicenters to prevent the propagation of T. infestans.
INTRODUCTION
The geographic distribution of human Chagas disease in rural endemic areas is associated with poor housing and poverty. In the Southern Cone countries, the main vector Triatoma infestans is the target of an elimination program based on the residual application of pyrethroid insecticides and entomologic surveillance. This program has already shown great success in Uruguay, Brazil, and Chile,1 and in parts of Argentina, Bolivia, and Paraguay. In Argentina, the control of T. infestans has been historically more difficult in the northwestern province of Santiago del Estero.2 Triatoma infestans was not eliminated in this region following a district-wide residual spraying of deltamethrin of all rural houses and peridomestic sites, surveillance, and repeated treatment of the reinfested houses over five-year periods.3–5 Peridomestic sites were the first to be recolonized, and they sustained more abundant populations of T. infestans than domestic sites and showed an increase in the risk of domiciliary reinfestation during the first years of the surveillance phase.5 The main failures of control actions occurred in peridomestic sites, where in the absence of sensitive bug detection procedures,6 residual foci later reinfested the whole area.7 To date, long-term studies of the process of reinfestation by T. infestans (or other triatomines) in a well-defined area have not been reported.
Geographic information systems (GISs) allow the integration of spatial and temporal information with satellite data to describe, understand, and predict the potential distribution of arthropod vectors and transmission of pathogens.8–10 Analysis of spatial data of vector-borne diseases using global and local spatial statistics can identify clustering of vector distribution and disease cases.11–13 Using fine-resolution satellite imagery, a GIS, and spatial statistics tools, we describe the spatial reinfestation patterns by T. infestans in a rural community following a residual spraying with insecticides. A focal spatial statistic is used to test whether different infested sites were potential sources for subsequent reinfestation, allowing for a time-lag in the detection of T. infestans. This is part of a longitudinal study of the epidemiology and control of Chagas disease in northern Santiago del Estero initiated in 1985. The ultimate aims of the study are to elucidate the mechanisms underlying the process of reinfestation by triatomine bugs; to produce risk maps and predictions for reinfestation that may be used by vector control programs to evaluate and prioritize control efforts, and to add a spatial component to a mathematical model of Trypanosoma cruzi transmission.14
MATERIALS AND METHODS
Study area
Field studies were carried out in the rural village of Amamá (27°12′33″S, 63°02′10″W) in the Province of Santiago del Estero in Argentina. This community is located in a semiarid plain with hardwood forest of “quebracho” (Schinopsis lorentzii and Aspidosperma quebracho-blanco) undergoing intensive exploitation. The area and the history of infestation by T. infestans have been described previously.5,15
Most houses are made of adobe walls and thatched roofs, with one or two adjacent bedrooms and a front veranda 5–10 meters wide. These areas share a common roof and will be referred to hereafter as domestic or domiciliary areas. Poultry, pigs, and goats are raised by villagers for subsistence in the compound around the domiciliary area. This peridomestic area consists of a patio and 3–8 structures (store rooms, kitchen, corrals, wood piles, trees) separated from human habitations and were described by Canale and others.16 All houses were identified with a numbered plaque and mapped in 1992, and new and abandoned structures were continuously recorded.
Field surveys
All houses in the study area were sprayed with deltamethrin by the Servicio Nacional de Chagas (NCS) in 1985,15 and then in 1992 when the effectiveness of the spraying was also assessed.7 In Amamá and other nearby villages, the surveillance phase spanned from December 1992 to 2002 and included a strong community participation component.5,17,18 The study was reviewed and approved by the Ethical Review Committee of the National Chagas Institute Dr. Mario Fatala Chaben of the Argentine Ministry of Health and Social Welfare. The study objectives were explained to residents and all participants signed an informed consent form.
During each visit, householders were asked about recent housing improvements, the domestic use of insecticides, the place where chickens usually nested, and the numbers of fowl and corralled animals (goats, sheep, cows, horses, and mules) owned by each family. Each domestic and peridomestic site in Amamá was searched for triatomine bugs from October 1993 to October 2002, except in 2001 when only infested peridomestic structures were searched for triatomine bugs for other purposes (Ceballos LA and others, unpublished data). The vector collection methods used were described by Gürtler and others.18 The reinfestation of each house was evaluated by skilled bug collectors from the NCS using timed manual collections with 0.2% tetramethrin (Icona, Buenos Aires, Argentina) as an irritant agent (the flushing-out method); by householders’ collections starting in May 1993 when a labeled self-sealing plastic bag was provided to each household; by domestic sensor boxes (Biosensor; Biocientífica de Avanzada; Buenos Aires, Argentina) placed in bedrooms in mid-December 1992; and by knockdown collections after using one or two insecticide fumigant canisters (Aguvac, Buenos Aires, Argentina) per bedroom or spraying with deltamethrin (25 mg of active ingredient/meter2) (K-Othrina; Agrevo, San Isidro, Argentina).
Every 12 months from October 1993 to December 1997, two persons searched bedrooms while another person searched peridomestic sites for 30 minutes per site (one person-hour and 0.5 person-hours, respectively). Additional searches for bugs were carried out in peridomestic sites during May 1995, 1996, and 1997 (0.5 person-hours per house). All bugs were identified to species and stage at the field laboratory,16 and individually examined for T. cruzi infection.17 From 1993 to 1995, only sites with at least one T. infestans adult or nymph, but not with other triatomines, were treated selectively with deltamethrin (25 mg of active ingredient/meter2) by NCS staff as before. Sixteen domiciles in Amamá were treated with one or two insecticide fumigant canisters per bedroom or sprayed with deltamethrin at the standard dose in late 1995. Surveillance activities were transferred to the communities between late 1995 and 1996. Starting in 1996, the capture of one T. infestans bug of any stage prompted treatment of all domestic and peridomestic areas of each house, but most of the focal insecticide sprays were made after the November survey in 1997.17
Mapping and geospatial processing
An Ikonos satellite image (Space Imaging, Atlanta, GA) with 1-meter2 panchromatic and 4-meter2 multi-spectral resolution acquired in October 2002 was georeferenced using global positioning system (GPS) (Trimble GeoExplorer II; Trimble Navigation Ltd., Sunnyvale, CA) readings from landmarks in the field. The image was used to digitize structures that were not located originally with the GPS. The exact location of 636 structures was overlaid on the image using sketch maps from each house. The entomological database from Amamá was associated with x and y coordinates (in Universal Transverse Mercator, Zone 20S, WGS1984 datum) of each identified structure using ArcGIS 8.1 (Environmental Systems Research Institute, Redlands, CA).
Statistical analysis
We restricted the analysis of the reinfestation process to 1993–1997 because only 89 selective residual insecticide treatments were carried out, most of them (72% of the sites) by late 1997; thus, the system was less perturbed than thereafter. The effects of selective insecticide spraying during both periods will be presented elsewhere. The prevalence and abundance of infestations were calculated for ecotopes with at least one infested site detected during 1993–1997. Global (weighted K-function) and local (Gi[d]) spatial statistics were used to detect clustering of bugs within the study area and to identify epicenters of infestation. The weighted K-function was used to analyze the spatial distribution patterns of abundance of T. infestans among all locations (sites) in the study area after the exclusion of two distant and not frequently inhabited houses (A119 and A114).19 A local spatial statistic, such as Gi(d),12 can be used as a focal statistic when the weight of the point being evaluated is not included in the calculation.20,21 Gi(d) was used as a focal spatial statistic to measure spatial clustering of T. infestans abundance around known and suspected sources of T. infestans reinfestation and to calculate the range of distances over which such reinfestation occurred. For example, the site that was an epicenter in time t was considered with the data for other sites in the following year (t + 1) and up to four years later (t + 4). In this way, we represented the temporal aspect in the process of reinfestation and the probable effect of a time delay in the detection of infestation of a site. When using Gi[d], we considered more than one site as a potential source, and we corrected for multiple comparisons by adopting the significance levels listed by Ord and Getis.22 Spatial analyses were performed using Point Pattern Analysis software (San Diego State University, San Diego, CA).23
RESULTS
A total of 515 T. infestans was collected from 118 (6%) of 2,110 domestic or peridomestic sites inspected during 1993–1997 (Table 1). Only kitchens or storerooms, mud ovens, and goat and pig corrals reached a mean number of T. infestans per infested site > 5, but their prevalence of infestation (4.3–9.1%) was lower than domiciles and chicken coops (11.5–12.5%). Of these 2,110 sites, 531 (25%) belonged to ecotope types where no T. infestans was detected even once (cow or horse corrals, trees with chickens, sheds with only a roof, corn storage rooms, and rabbit coops), and these are excluded from the rest of the analysis. In the remaining 1,579 sites, the prevalence of T. infestans increased sharply from 0.6–2.9% in 1993–1995 to 32% in November 1997 (χ2 = 59.6, degrees of freedom [df] = 1, P < 0.01), and each year, with the exception of 1994, was higher in October-November than in May (Figure 1A). The total catch of T. infestans increased from 1 to 15 in 1993–1995 to 329 triatomine bugs in 1997 (H = 5.39, df = 2, n = 86; P = 0.07, by the Kruskall-Wallis test) and was always greater in October-November than in May surveys. The percentage of infested sites correlated positively and significantly with the mean number of T. infestans per infested site collected in each survey (r = 0.71, n = 9; P = 0.03).
Table 1.
Prevalence of infestation and mean number of Triatoma infestans per infested site by types of ecotope from October 1993 to November 1997 in Amamá, Argentina
| Ecotope | No. of sites inspected | No. of sites positive (%) | Mean number of bugs per infested site | No. of T. infestans |
|---|---|---|---|---|
| Domicile | 485 | 56 (11.5) | 2.8 | 155 |
| Pig corral | 222 | 17 (7.7) | 5.8 | 98 |
| Wood pile | 117 | 9 (7.7) | 2 | 18 |
| Goat corral | 173 | 8 (4.6) | 6.3 | 50 |
| Kitchen or store room | 187 | 8 (4.3) | 11.1 | 89 |
| Chicken coop | 48 | 6 (12.5) | 1.5 | 36 |
| Mud oven | 66 | 6 (9.1) | 9.3 | 56 |
| Latrine | 57 | 3 (5.3) | 2.3 | 7 |
| Chicken house or nest | 67 | 2 (3.0) | 1.5 | 3 |
| Tree without chickens | 126 | 2 (1.6) | 1 | 2 |
| Orchard fence | 31 | 1 (3.2) | 1 | 1 |
| Cow or horse corral | 59 | 0 (0) | – | 0 |
| Tree with chickens | 434 | 0 (0) | – | 0 |
| Others* | 38 | 0 (0) | – | 0 |
| Total | 2,110 | 118 (5.6) | 4.4 | 515 |
Other structures included sheds with only a roof, corn storage rooms, and rabbit coops.
Figure 1.
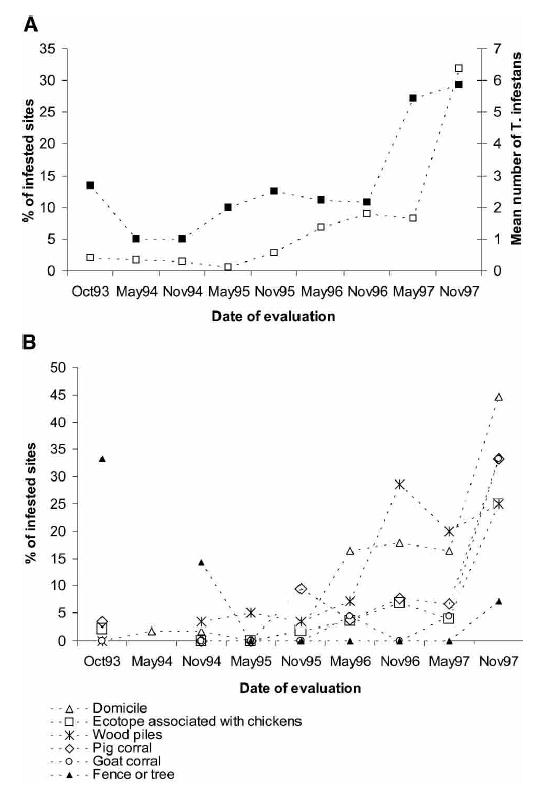
A, Percentage of infested sites (▪) and mean number of Triatoma infestans per infested sites from 1993 to 1997 in Amamá, Argentina. B, Percentage of infested sites in Amamá by ecotope and date of evaluation from 1993 to 1997.
In addition to domiciles, T. infestans occupied a wide range of peridomestic ecotopes including pig corrals, goat corrals, wood piles, trees without chickens, and additional sites associated with chickens (store rooms, mud ovens, chicken coops, latrines, and nests) during 1993–1997. From 1993 to 1995, the ecotope-specific prevalence of infestation increased significantly in domiciles from 2% to 45% (χ2 = 39.4, df = 1, P < 0.01), in pig corrals from 9% to 33% (χ2 = 6.2, df = 1, P < 0.05), in goat corrals from 0% to 33% (χ2 = 10.7, df = 1, P < 0.01), and in ecotopes associated with chickens from 2% to 25% (χ2 = 15.7, df = 1, P < 0.01), but the increase in wood piles from 5% to 25% was not significant (χ2 = 2.35, df = 1, P = 0.1) (Figure 1B). The prevalence of infestation decreased only in fences or trees from 7% to 3%, but this decrease was not significant (χ2 = 0.19, df = 1, P = 0.7).
The relative contribution of each type of ecotope to total infested sites from 1993–1995 to 1996–1997 increased for domiciles (from 21% to 51%), ecotopes associated with chickens (from 14% to 22%), and goat corrals (from 0% to 8%), but decreased in pig corrals (from 29% to 13%), wood piles (from 21% to 6%), and in fences or trees (from 14% to 1%). However, the mean abundance of T. infestans per site increased in all types of ecotope, and peaked in 1997 in ecotopes associated with chickens (mean ± SD = 9.4 ± 9.43), followed by pig (7.5 ± 9.96) and goat corrals (6.4 ± 10.98) (Table 2).
Table 2.
Mean number of Triatoma infestans per site and number of infested sites by year of survey from 1993 to 1997 in Amamá, Argentina
| Mean number of bugs per site (number of infested sites) by year
|
|||||
|---|---|---|---|---|---|
| Ecotope | 1993 | 1994 | 1995 | 1996 | 1997 |
| Domicile | 0 (0) | 1 (2) | 1 (1) | 1 (19) | 4 (34) |
| Pig corral | 6 (1) | 0 (0) | 3 (3) | 3 (3) | 8 (10) |
| Goat corral | 0 (0) | 0 (0) | 0 (0) | 5 (1) | 6 (7) |
| Wood pile | 0 (0) | 1 (1) | 2 (2) | 1 (3) | 3 (3) |
| Ecotope associated with chickens | 1 (1) | 0 (0) | 4 (1) | 4 (6) | 9 (17) |
| Fence and tree | 1 (1) | 1 (1) | 0 (0) | 0 (0) | 1 (1) |
| Total | 3 (3) | 1 (4) | 2 (7) | 2 (32) | 6 (72) |
The spatial distribution of T. infestans abundance per site during 1993–1997 is shown in Figure 2. The reinfestation process began in southern Amamá and by 1997 (five years post-spraying) spread throughout the community, despite sporadic focal spraying efforts. The abundance of T. infestans did not show global clustering for any year between 1994 and 1997. However, it showed a significant local clustering from 25 to 150 meters in 1995, from 25 to 350 meters in 1996, and from 25 to 75 meters and at 225 meters in 1997 (Gi*[d] > 3.71, P < 0.01).
Figure 2.
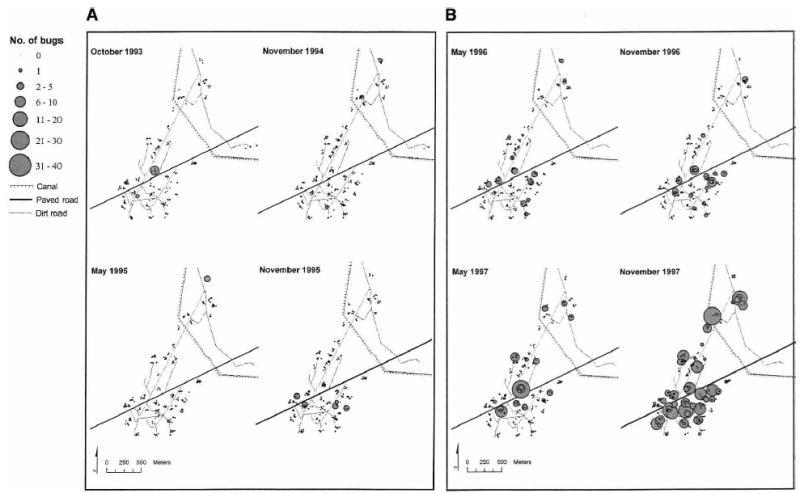
Total numbers of Triatoma infestans in domestic (collections by flushing out, sensor boxes, and householders) and peridomestic (collections by flushing out) sites from A, 1993 to 1995 and B, 1996 to 1997 in Amamá, Argentina.
After the blanket insecticide spray, the first source of T. infestans was detected in a pig corral of house A116 in southern Amamá in 1993 one year post-spraying (Figure 3). This pig corral was larger than others in the village and had numerous pigs and chickens (Figure 4). Following the capture of five T. infestans adults and one nymph, the pig corral was immediately sprayed by the NCS (Table 3). Two peridomestic sites located within approximately 430 meters from the primary pig corral were concurrently infested, each with one female T. infestans. A significant focal clustering of bug abundance was detected around the primary pig corral at a distance of 450–475 meters in 1995 (Gi[d] > 2.23, P < 0.05). In 1996, significant clustering was detected up to 425 meters (within a compound or including neighboring compounds), and decreased rapidly beyond the peak clustering at a distance of 25–150 meters (Figure 5A). No clustering was detected in 1994 or 1997.
Figure 3.
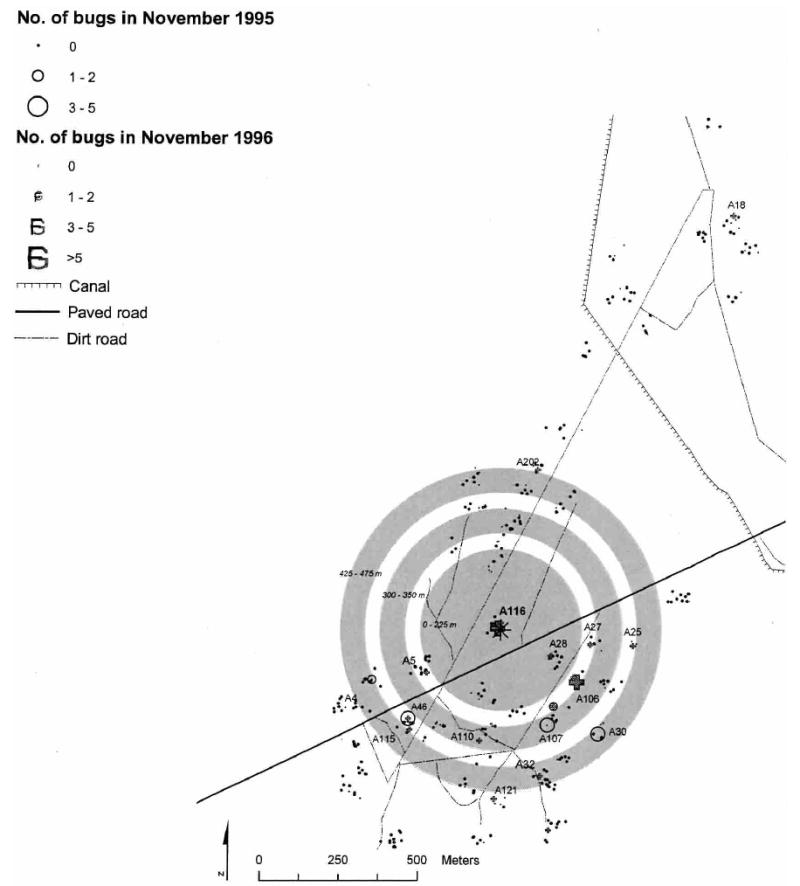
Location of infested source in 1993 (pig corral of house A116), and numbers of Triatoma infestans per site in 1995 and 1996 in Amamá, Argentina. Shaded areas represent significant clustering distances around the pig corral source in 1993. The asterisk represents the source of reinfestation in 1993.
Figure 4.

Pig corral of house A116, the initial source of the Triatoma infestans reinfestation in Amamá, Argentina.
Table 3.
Number of Triatoma infestans by stage, fate of bug population, and first detection of first and secondary potential sources of T. infestans from 1993 to 1996
| No. of T. infestans by stage
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Ecotope | House | First date of detection | I–III | IV–V | Female/Male | Total | No. of dates infested | Fate of bug population |
| Pig corral | 116 | Oct 1993 | 1 | 0 | 0/5 | 6 | 4 | Sprayed and reinfested in May–Nov 1996, May 1997 |
| Pig corral | 107 | Nov 1995 | 2 | 0 | 1/0 | 3 | 1 | Sprayed |
| Pig corral | 46 | Nov 1995 | 0 | 3 | 1/0 | 4 | 3 | Sprayed and reinfested again in Nov 1996, May 1997 |
| Store room | 30 | Nov 1995 | 4 | 0 | 0 | 4 | 1 | Sprayed and dismantled before May 1996 |
| Chicken coop | 116 | May 1996 | 4 | 3 | 0/0 | 7 | 2 | Infested in Nov 1996 |
Figure 5.
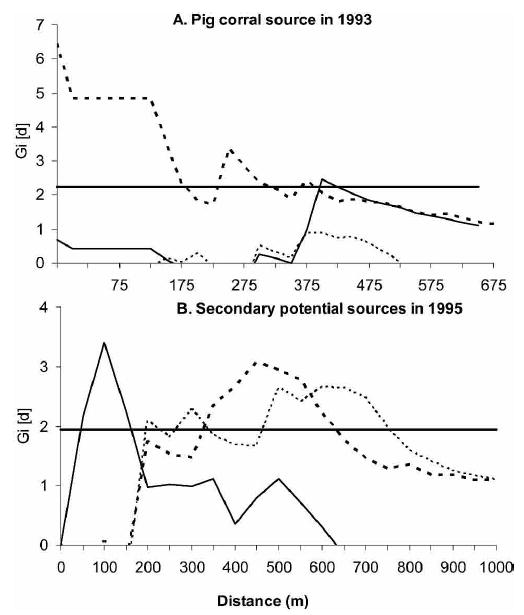
Focal clustering (- Gi[d] for P < 0.05) of bug abundance around A, the 1993 pig corral source in 1995 (solid line), 1996 (dashed line), and 1997 (dotted line), and B, around the store room of A30 in November 1996 (dotted line), pig corral A107 in November 1996 (dashed line), and pig corral A46 in November 1997 (solid line).
Apart from the primary pig corral, different potential secondary sources were detected in 1995 and 1996 in southern Amamá, but only significant sources are shown in Table 3. These sources had 3–6 T. infestans including at least one nymph, and most of them were infested more than once. Pig corrals predominated, and although their building materials were similar, they varied in size, location, and number of hosts. The significant clustering of bug abundance detected in 1996 and 1997 around these potential secondary sources fell within the range of the clustering around the primary 1993 pig corral source (Figure 3). Indeed, this same pig corral was a secondary source in May 1996. Thus, all foci in 1995 and 1996 appear to have originated from the 1993 primary source, and do not appear to be independent sources of reinfestation.
The numbers of T. infestans per site in 1997 were significantly clustered (Gi[d] > 2.32, P < 0.01) at a distance of 100–150 meters around another pig corral (A46) where infestation was detected in 1995 (Figure 5B). However, this range of distances is within the compound level and does not explain the spatial pattern of bug abundance in 1997. Other potential sources in 1996 were tested in an attempt to explain the 1997 spatial pattern, but no significant clustering was detected around them. Indeed, the spatial pattern of T. infestans abundance at five years post-spraying seems to have arisen by several continuous and simultaneous sources of T. infestans instead of from one single source. The reinfestation dynamics in northern Amamá was not explained by the A116 pig corral or other potential sources; its origin seemed to be independent of the southern sources.
DISCUSSION
Reinfestation by T. infestans appears to have originated from one main source in southern Amamá, while in the north reinfestation originated from other independent sources. Wing geometric morphometry of 254 T. infestans captured in Amamá in 2000 also suggested that the northern infestation source may be considered independent of the southern sources.24 A site in northern Amamá was not grouped with other sites from the southern section, while all five sites located within 400 meters from each other in the southern section were grouped together.24 Northern Amamá sites are separated from southern sites by a canal; therefore, distance between the northern and southern sections, the canal, or both could be acting as barriers to the propagation of T. infestans within the community. Microsatellite molecular markers are being developed to provide concluding evidence on the actual origin of T. infestans populations at a micro scale.
The scale at which a health problem is studied should reflect an understanding of the disease process and likely causative factors.25 In our study, we georeferenced all sites within a village to create the distance matrices needed to apply spatial statistics for the identification of sources of reinfestation by T. infestans. This scale of spatial analysis allowed us to incorporate environmental heterogeneity (e.g., different ecotopes) while considering the biological mechanisms that underlie the reinfestation process. Triatoma infestans can disperse actively by flight or walking and passively through accidental carriage by humans and their belongings.26,27 Flight initiation of T. infestans was female biased and dispersing adults were found up to 550 meters from release point in salt flats.28 In our study area, the number of flight-dispersing T. infestans males was positively associated with the abundance of adult T. infestans in peridomestic sites within 200 meters around each light trap, and the very few fifth-instar nymphs attracted to the light traps probably walked 29 to 42 meters from the nearest infested sites.29 These distances are within the range of significant clustering (up to 475 meters) that we detected around the 1993 pig corral source two and three years later. Moreover, the 1993–1995 period was characterized by very low abundance of T. infestans and higher frequency of peridomestic sites infested with only adults or fifth-instar nymphs in comparison with subsequent years. Thus, T. infestans flight dispersal appeared to be the main mechanism driving the observed spatial pattern of reinfestation during the early surveillance phase following spraying. On the other hand, dispersal of stage IV and V nymphs by walking could contribute to increased clustering, within and among neighboring compounds, close to the epicenter.
Focal statistics confirmed that the high abundance of T. infestans per site in 1995 and 1996 was significantly clustered around the 1993 pig corral source. By 1995, the sites located some 450 meters around this source probably became infested through the invasion of flight-dispersing triatomines. Whether the deltamethrin spray promoted a repellent or flushing-out effect on the surviving triatomines infesting the pig corral remains unclear. During the surveillance phase, when bug abundance is very low, the flushing-out method fails to detect many peridomestic triatomine populations.6 This explains in part why the newly infested sites originating from the primary pig corral were detected two years after it was sprayed. The distance from the pig corral to other compounds may also have contributed to the time delay in detecting T. infestans in its neighborhood. The pig corral source, located near the center of the community where houses were also more concentrated, was large, crowded with hosts, and thus difficult to treat. This pig corral had a residual focus of T. infestans detected only two months after the 1992 blanket spraying,7 and had the highest frequency of reinfestation and bug abundance during the surveillance phase.
The peridomestic environment offers a wide range of hosts, refuges, and climatic conditions for successful development of triatomine populations. However, not all peridomestic ecotopes appear to be equally suitable for T. infestans or play the same role during the reinfestation process. The role of animal corrals as sources for reinfestation during the surveillance phase is reinforced by an empirical model that predicted that the probability of T. infestans flight initiation would peak in summer from pig and goat corrals but not from ecotopes associated with chickens in the Amamá area (Ceballos LA and others, unpublished data). Pig corrals became infested earlier (October 1993) and reached a higher prevalence of infestation (up to May 1997) and mean abundance of T. infestans than goat corrals. Although pig corrals were the most important ecotopes that promoted reinfestation in 1993–1995, their relative contribution to infestation decreased from 1993 to 1997, while the contribution of goat corrals and ecotopes associated with chickens increased. We conclude that wooden pig corrals with permanent resident hosts, in which insecticide spraying has limited effectiveness, were key sources of T. infestans during the initial stage of the reinfestation process in Amamá.
Goat or sheep corrals have long been known to support abundant populations of T. infestans in the dry Chaco region, and had higher prevalence of T. infestans before and after residual spraying with insecticides than other ecotopes.30 In Amamá, however, during the first years following a blanket spraying, goat corrals were not a detectable source and their prevalence of infestation increased slowly. In general, the local patterns of reinfestation are affected by demographic and environmental variables. In Amamá, changes in land ownership and use since 1990 determined that the village was surrounded by a wire fence that restricted the movement of goats and access to the scarce grass available. This affected householders’ management practices of goats and other domestic animals, and probably impacted on host availability for triatomine bugs.
The ecotopes associated with chickens supported small T. infestans populations with high nutritional status and thus a very low flight dispersal potential in spring-summer (Ceballos LA and others, unpublished data). However, since peridomestic chicken nesting sites differed in physical structure and stability throughout the year, in the absence of chickens the local bug populations would likely die out or disperse elsewhere and serve as sources for reinfestation. The ecotopes associated with chickens had an increasing prevalence and abundance of T. infestans in Amamá during the late surveillance phase. In light of the heterogeneity of rural endemic areas in the Chaco region, the key peridomestic ecotopes for reinfestation may vary largely between zones and perhaps over time. Taking into account the unique characteristics of the local environment is crucial for improved vector control.
Focal spatial statistics were used to recommend control strategies for schistosomiasis in Kenya,21 and to analyze the vector distribution of T. guasayana in Amamá (Vazquez-Prokopec GM and others, unpublished data). To explain the observed spatio-temporal patterns of reinfestation and develop risk maps at the village level, integration of data on the local numbers of animals, type of construction material, focal insecticide spraying, and vector distribution into a GIS is needed. Geographic information systems, satellite imagery, and spatial statistics need to be applied to eco-epidemiological research on Chagas disease to provide scientifically based, improved tactics to control programs of T. infestans. The combined effects of spraying all peridomestic sites, as well as residual foci during and immediately after the blanket insecticide spraying, active surveillance with community participation, and regular removal of triatomines contributed to slow down the rate of domiciliary reinfestation in Amamá in 1992–1997 compared with that recorded in 1985–1992.5,15 Even more important, the set of control actions also reduced to marginal levels the transmission of Trypanosoma cruzi to T. infestans17 and dogs taken as sentinels of domestic risk of infection.31 Although T. infestans was not eliminated at the community level, a sustained interruption of transmission has been achieved until 2002 (Gürtler RE and others, unpublished data). Our results suggest that residual spraying with insecticides of the colonized site and all sites within a radius of 450 meters will be effective in preventing the subsequent propagation of T. infestans after a community-wide residual spraying with insecticides. Targeted surveillance of key peridomestic sites, such as goat and pig corrals, using low-cost sensing devices,32 and improved treatment regimens are recommended. Such detection boxes may also be used to distinguish a recent invasion from an established bug colony and to indicate the local extinction of T. infestans foci. Understanding the spatio-temporal population dynamics of T. infestans in domestic and peridomestic habitats may help improve the effectiveness of control efforts.
Acknowledgments
We thank Dr. Roberto Chuit and Abel Hurvitz and his staff at the National Control Service (Argentina) for providing active support during fieldwork; María Moyano and Omar Sitatti for field accommodation; Amamá residents for their participation in this effort; Janet Thornhill for assistance in the digitizing phase, and Delmi Canale for her long-term support. The Amamá database is the product of a sustained collaborative effort between researchers from the University of Buenos Aires (Ricardo E. Gürtler, Directorate of Epidemiology, Minister of Health and Social Action, the Argentina-National Chagas Service (Roberto Chuit), and Rockefeller University (Joel E. Cohen) between 1992 and 2000.
Footnotes
Financial support: This study was supported by awards from the Natonal Institutes of Health/National Science Foundation Ecology of Infectious Disease program award R01 TW05836 funded by the Fogarty International Center and the National Institute of Environmental Health Sciences to Uriel Kitron and Ricardo E. Gürtler), the Agencia Nacional de Promoción Científica y Técnica (Argentina), and the University of Buenos Aires to Ricardo E. Gürtler. Ricardo E. Gürtler and Maria C. Cecere are members of the Consejo Nacional de Investigaciones Científicas y Técnicas Researcher’s Career.
References
- 1.Anonymous Chagas disease interrupted in Chile. TDR News. 2000;61:10. [Google Scholar]
- 2.Segura EL, Cura EN, Sosa Estani S, Andrade J, Lansetti JC, De Rissio AM, Campanini A, Blanco SB, Gürtler RE, Alvarez M. Long-terms effects of a nation-wide control program on the seropositivity for Trypanosoma cruzi infection in young men from Argentina. Am J Trop Med Hyg. 2000;62:353–362. doi: 10.4269/ajtmh.2000.62.353. [DOI] [PubMed] [Google Scholar]
- 3.Paulone I, Chuit R, Pérez A, Wisnivesky-Colli C, Segura EL. Field research on a epidemiological surveillance alternative of Chagas’ disease transmission: the Primary health care (PHC) strategy in rural areas. Rev Argent Microbiol. 1988;20(Suppl):103–105. [PubMed] [Google Scholar]
- 4.Chuit R, Paulone I, Wisnivesky-Colli C, Bo R, Pérez A, Sosa-Estani S. Results of a first step toward community-based surveillance of transmission of Chagas disease with appropriate technology in rural areas. Am J Trop Med Hyg. 1992;46:444–450. doi: 10.4269/ajtmh.1992.46.444. [DOI] [PubMed] [Google Scholar]
- 5.Cecere MC, Gürtler RE, Canale D, Chuit R, Cohen JE. Effects of partial housing improvement and insecticide spraying on the reinfestation dynamics of Triatoma infestans in rural northwestern Argentina. Acta Trop. 2002;84:101–116. doi: 10.1016/s0001-706x(02)00183-3. [DOI] [PubMed] [Google Scholar]
- 6.Gürtler RE, Vazquez Prokopec GM, Ceballos LA, Lund Petersen C, Salomón OD. Comparison between two artificial shelter units and timed manual collections for detecting peridomestic Triatoma infestans (Hemiptera: Reduviidae) in rural northwestern Argentina. J Med Entomol. 2001;38:429–436. doi: 10.1603/0022-2585-38.3.429. [DOI] [PubMed] [Google Scholar]
- 7.Cecere MC, Gürtler RE, Canale D, Chuit R, Cohen JE. The role of peridomiciliary area in the elimination of Triatoma infestans from rural Argentine communities. Pan Am J Public Health. 1997;1:273–279. doi: 10.1590/s1020-49891997000400003. [DOI] [PubMed] [Google Scholar]
- 8.Clarke KC, McLafferty SL, Tempalski BJ. On epidemiology and geographic information systems: a review and discussion of future directions. Emerg Infect Dis. 1996;2:85–92. doi: 10.3201/eid0202.960202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitron U. Landscape ecology and epidemiology of vector-borne diseases: tools for spatial analysis. J Med Entomol. 1998;35:435–445. doi: 10.1093/jmedent/35.4.435. [DOI] [PubMed] [Google Scholar]
- 10.Hay SI, Randolph SE, Rogers DJ, 2000. Remote Sensing and Geographical Information Systems in Epidemiology London: Academic Press.
- 11.Getis A, Ord JK. The Analysis of Spatial Association by Use of Distance Statistics. Geogr Anal. 1992;24:189–206. [Google Scholar]
- 12.Getis A, Ord JK, 1996. Local Spatial Statistics: an Overview. Longley P, Batty M, eds. Spatial Analysis: Modeling in a GIS Environment Cambridge, United Kingdom: Geoinformation International, 261–277.
- 13.Morrison AC, Getis A, Santiago M, Rigau-Perez JG, Reiter P. Exploratory space-time analysis of reported dengue cases during an outbreak in Florida, Puerto Rico, 1991–1992. Am J Trop Med Hyg. 1998;58:287–298. doi: 10.4269/ajtmh.1998.58.287. [DOI] [PubMed] [Google Scholar]
- 14.Cohen JE, Gürtler RE. Modeling household transmission of American trypanosomiasis. Science. 2001;293:694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- 15.Gürtler RE, Petersen RM, Cecere MC, Schweigmann NJ, Chuit R, Gualtieri JM, Wisnivesky-Colli C. Chagas disease in north-west Argentina: risk of domestic reinfestation by Triatoma infestans after a single community-wide application of deltamethrin. Trans R Soc Trop Med Hyg. 1994;88:27–30. doi: 10.1016/0035-9203(94)90483-9. [DOI] [PubMed] [Google Scholar]
- 16.Canale DM, Cecere MC, Chuit R, Gürtler RE. Peridomestic distribution of Triatoma garciabesi and Triatoma guasayana in north-west Argentina. Med Vet Entomol. 2000;14:383–390. doi: 10.1046/j.1365-2915.2000.00254.x. [DOI] [PubMed] [Google Scholar]
- 17.Cecere MC, Castañera MB, Canale DM, Chuit R, Gürtler RE. Trypanosoma cruzi infection in Triatoma infestans and other triatomines: long-term effects of a control program in rural northwestern Argentina. Pan Am J Public Health. 1999;5:392–399. doi: 10.1590/s1020-49891999000500003. [DOI] [PubMed] [Google Scholar]
- 18.Gürtler RE, Cecere MC, Canale DM, Castañera MB, Chuit R, Cohen JE. Monitoring house reinfestation by vectors of Chagas disease: a comparative trial of detection methods during a four-year follow-up. Acta Trop. 1999;72:213–234. doi: 10.1016/s0001-706x(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 19.Getis A. Interaction modeling using second-order analysis. Environ Plann A. 1984;16:173–183. [Google Scholar]
- 20.Kitron U, Jones CJ, Bouseman JK, Nelson JA, Baumgartner DL. Spatial analysis of the distribution of Ixodes dammini (Acari:Ixodidae) on white-tailed deer in Ogle county, Illinois. J Med Entomol. 1992;29:259–266. doi: 10.1093/jmedent/29.2.259. [DOI] [PubMed] [Google Scholar]
- 21.Clennon JA, King CH, Muchiri EM, Curtis Kariuki H, Ouma JH, Mungai P, Kitron U. Spatial patterns of urinary schistosomiasis infection in a highly endemic area of coastal Kenya. Am J Trop Med Hyg. 2004;70:443–448. [PubMed] [Google Scholar]
- 22.Ord JK, Getis A. Local spatial autocorrelation statistics: distributional issues and an application. Geogr Anal. 1995;27:286–306. [Google Scholar]
- 23.Chen D, Getis A, 1998. Point Pattern Analysis San Diego: Department of Geography, San Diego State University.
- 24.Schachter-Broide J, Dujardin J-P, Kitron U, Gürtler RE. Spatial structuring of Triatoma infestans (Hemiptera, Reduviidae) populations from northwestern Argentina using wing geometric morphometry. J Med Entomol. 2004;41:643–649. doi: 10.1603/0022-2585-41.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cromley and McLafferty, 2002. GIS and Public Health New York: The Guilford Press.
- 26.Zeledón R, Rabinovich JE. Chagas’ disease: an ecological appraisal with special emphasis on its insect vectors. Annu Rev Entomol. 1981;26:101–133. doi: 10.1146/annurev.en.26.010181.000533. [DOI] [PubMed] [Google Scholar]
- 27.Schofield CJ, Matthews JNS. Theoretical approach to active dispersal and colonisation of houses by Triatoma infestans. J Trop Med Hyg. 1985;88:211–222. [PubMed] [Google Scholar]
- 28.Schofield CJ, Lehane MJ, McEwen P, Catala SS, Gorla DE. Dispersive flight by Triatoma infestans under natural climatic conditions in Argentina. Med Vet Entomol. 1992;6:51–56. doi: 10.1111/j.1365-2915.1992.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 29.Vazquez-Prokopec GM, Ceballos LA, Kitron U, Gürtler RE. Active dispersal of natural populations of Triatoma infestans (Hemiptera: Reduviidae) in rural northwestern Argentina. J Med Entomol. 2004;41:614–621. doi: 10.1603/0022-2585-41.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gürtler RE, Canale DM, Spillman C, Stariolo R, Salomon DO, Blanco S, Segura EL. Effectiveness of residual spraying of peridomestic ecotopes with deltamethrin and permethrin on Triatoma infestans in rural western Argentina: a district-wide randomized trial. Bull World Health Organ. 2004;82:196–205. [PMC free article] [PubMed] [Google Scholar]
- 31.Castañera MB, Lauricella MA, Chuit R, Gürtler RE. Evaluation of dogs as sentinels of the transmission of Trypanosa cruzi in a rural area of north-west Argentina. Ann. Trop Med Parasitol. 1998;9:671–683. doi: 10.1080/00034983.1998.11813327. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez-Prokopec GM, Ceballos LA, Salomón OD, Gürtler RE. Field trials of an improved cost-effective device for detecting peridomestic populations of Triatoma infestans (Hemiptera: Reduviidae) in rural Argentina. Mem Inst Oswaldo Cruz. 2002;97:971–977. doi: 10.1590/s0074-02762002000700008. [DOI] [PubMed] [Google Scholar]


