Abstract
An empirical model of flight initiation coupled with data from a longitudinal study predicted that the flight dispersal of Triatoma infestans from peridomestic sites was more likely to occur in late summer. To partially test this prediction, we operated 11–12 black light traps from 1945 to 2200 hours in March 2003 in two villages in northern Argentina. All peridomestic sites around the light traps were later inspected to assess the relative abundance and nutritional status of T. infestans at each site. Traps were located 19–94 m from the nearest infested site. A total of 2 female, 10 male, and 3 fifth-instar nymphs of T. infestans; 4 adult Triatoma garciabesi; and 1 Triatoma guasayana fifth-instar nymph were collected in 64 trap nights. Nearly two-thirds of the bugs arrived to the traps during the first hour after sunset, when ambient temperatures were 22–28°C; 80% of adults were unfed. The number of T. infestans that flew to the traps was significantly and negatively associated with wind speed, and the number of males positively associated with the abundance of adult T. infestans in peridomestic sites within 200 m around each light trap. This is the first successful application of light traps for collecting dispersing nymphal and adult T. infestans on a village-wide scale. We attribute this success to the placement of traps with consideration to spatial infestation patterns and seasonal variation in nutritional status of peridomestic triatomine populations.
Keywords: flight, light trap, vector ecology, Triatominae, Chagas’ disease
Triatoma infestans (Klug), the main vector of Chagas’ disease, infests almost exclusively domestic and peridomestic habitats (Zeledón and Rabinovich 1981, Noireau et al. 2000). Transmission of Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) mostly occurs in human habitations (Cohen and Gürtler 2001), but in the Gran Chaco region peridomestic foci of T. infestans are very frequent and persist after insecticide spraying (Cecere et al. 2002, Gürtler et al. 2004). Flight dispersal of T. infestans from infested residual foci is considered one of the mechanisms determining domestic reinfestation after control interventions (Carcavallo 1985, Schofield 1985).
Flight initiation by triatomine bugs was associated with low nutritional status (measured by the weight/length ratio, W/L) and high temperatures (Ekkens 1981, Lehane and Schofield 1982, Lehane et al. 1992). The probability of flight initiation of T. infestans in the laboratory was closely associated with their W/L and maximum temperature (Lehane et al. 1992), and so was the likelihood of flight initiation of laboratory-reared T. infestans bugs under natural climatic conditions (Lehane et al. 1992, Schofield et al. 1992). Flight dispersal of T. infestans was recorded at 1.5–2.0 km from their putative sources (Schweigmann et al. 1988). However, in a mark-release trial conducted in goat corrals, the high recapture rate of adult T. infestans suggested a very low dispersal rate during the following 1–4 mo (Ronderos et al. 1980). The lack of an effective and simple method for sampling flight-dispersing T. infestans accounts for the near absence of field reports, and hindered investigations on the determinants of house invasion by triatomines.
Poor nutritional status of bug populations can lead to more dispersive flights, and thus regulate triatomine population density (Schofield 1985). Higher bug densities per host would reduce the population nutritional state and increase the likelihood of flight initiation. In northwestern Argentina, the nutritional status and daily feeding rates of T. infestans varied among seasons in chicken coops (Lopez et al. 1999) and other peridomestic ecotopes (unpublished data). Fitting the observed W/L ratios and field maximum temperatures into the model by Lehane et al. (1992), the probability of flight initiation was predicted to peak in summer, when adult triatomines also peaked in abundance and reached the lowest W/L ratio (unpublished data). As a part of a wider study on the spatial and temporal patterns of reinfestation by T. infestans in northwestern Argentina, we conducted simultaneous light trap collections in two neighboring rural villages during the most likely flight dispersal period, and related the flight activity of T. infestans to weather and demographic variables.
Materials and Methods
Study Area
The study was carried out in the rural villages of Amamá and Trinidad (27° 12′ 33″S, 63° 02′ 10″W), Province of Santiago del Estero, Argentina (Fig. 1). Both villages are situated within 9 km of each other in semiarid hardwood, thorny forest habitat. Amamá houses are relatively close together and surrounded largely by grasses, whereas Trinidad houses are widely dispersed and surrounded by high trees, abundant cactaceous plants, and little grass. The climatic characteristics of this area were described previously (Vazquez-Prokopec et al. 2002). The peridomestic environment includes mostly storerooms, chicken coops, and corrals (Canale et al. 2000). The houses were illuminated by one to three kerosene lamps for a few hours after sunset. The villages were under community-based domestic triatomine surveillance (Cecere et al. 2002).
Fig. 1.
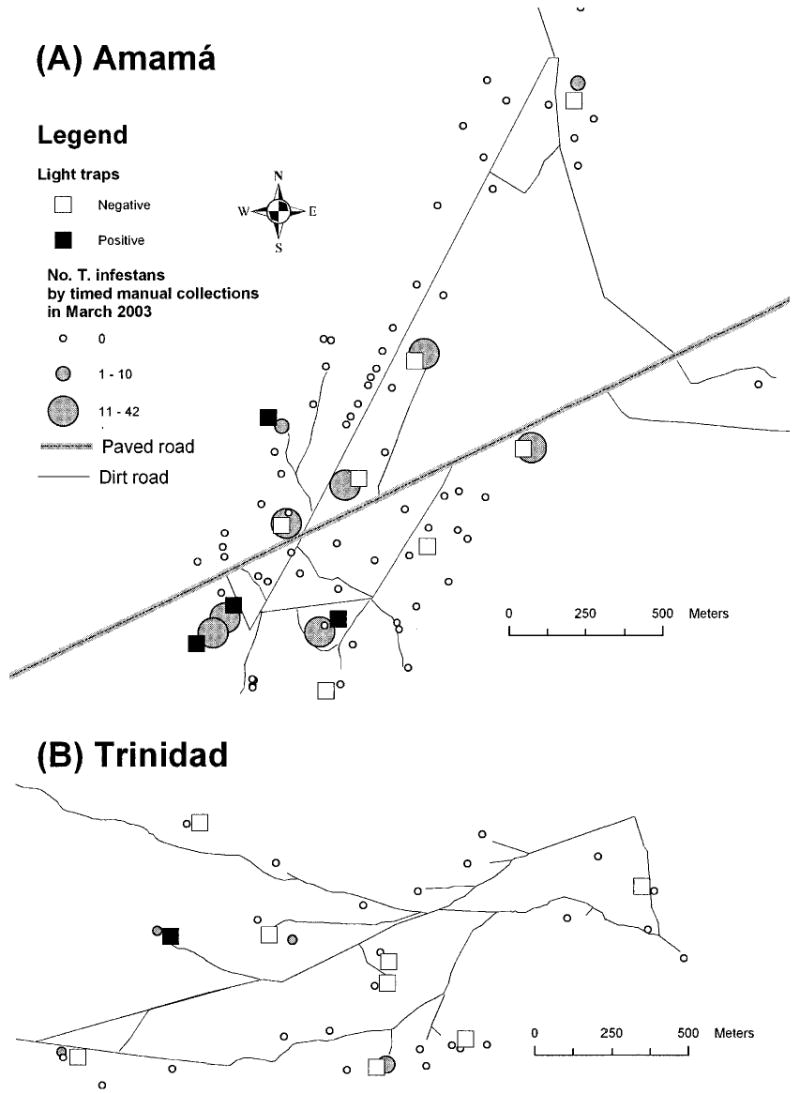
Map of Amamá (A) and Trinidad (B) indicating the location of light traps, whether they collected T. infestans or not, and the number of T. infestans collected by timed catches in March 2003.
Study Design
A pilot trial aimed at training local villagers in light trap collections was conducted in October 2002. A total of 9 light traps was operated in 9 Amamá houses for 4 nights. All 300 houses and peridomestic sites of Amamá and neighboring villages were searched for triatomines by timed manual collections with an irritant spray, and the bugs removed.
In March 2003, the light traps in Amamá and Trinidad were set up along two transects with five to six catch sites each (Fig. 1). The selected catch sites were close to peridomestic sites that had been infested by T. infestans in October 2002. Each light trap was set up in a way that enabled maximum visibility from all the surrounding structures. The mean distance from a light trap to the nearest peridomestic structure was 38 m (range 7–100 m) in Amamá and 52 m (range 17–102 m) in Trinidad. The light trap system consisted of a vertical white cloth (1.25 × 2 m) illuminated by a portable lantern (Energizer Multifunction Lantern Number 9450, Energizer, St. Louis, MO) with a 6 W black light tube (F6T5BLB; Satellite, Hong Kong, China).
After appropriate training, a householder, using disposable gloves and a flashlight, was left in charge of each light trap to collect the bugs in and around it; two to four family members usually accompanied him or her. All the insects collected during each 30-min interval were stored in separate plastic bags labeled with collection times. Light trapping was performed from 19:45 (15′ before sunset) to 22:00 h because the flight activity of T. infestans is known to peak during the first hour after sunset (Lehane and Schofield 1982).
In each village, all catch stations along a given transect were operated simultaneously on alternate nights. This design was chosen to increase the chance of collecting bugs, to compare catches between villages, and to cover the whole community. Only nights with temperatures above 20°C and no rainfall were selected for light trapping because of the well-known temperature-dependent flight dispersal of T. infestans and the inhibitory effects of rainfall. Appropriate light-trapping nights occurred on 10, 11, 17, 18, and 19 March 2003, with a total trapping effort of 58 night traps, and a mean number of 5.8 traps per village night. In addition, six light-trapping nights were performed in Amamá near high-density peridomestic sites on 3, 8, and 12 March to increase the chances of bug collection.
A weather station (Weather Monitor II, Davis, Baltimore, MD) located in Amamá measured temperature, relative humidity, wind speed and direction, barometric pressure, and rainfall at 15-min intervals during the trapping period. Moon phase during each light-trapping night was measured as the percentage of the moon’s surface that was lit. Each light trap and the surrounding domestic and peridomestic structures were located using a GPS (GeoExplorer II; Trimble, Sunnyvale, CA).
After the light-trapping period, two skilled bug collectors searched for triatomines in all peridomestic structures surrounding the light traps using 0.2% tetramethrin dislodgant (Icona, Buenos Aires, Argentina) for 30 min per house (timed manual collections or flushing-out method) to assess the relative abundance and nutritional status of T. infestans at each site. A total of 51 peridomestic structures from 11 Amamá houses and 33 structures from 6 Trinidad houses was inspected for infestation.
All triatomines collected were identified to species and counted by stage, as described by Canale et al. (2000). Each adult and fifth-instar nymph were weighed individually in an electronic balance (precision, 0.1 mg; OHAUS, Pine Brooks, NJ), measured from clypeus to abdominal tip with a hand-held vernier caliber accurate to 0.02 mm, and microscopically examined for T. cruzi infection at ×400. Females collected by light trapping were also dissected to determine whether they were inseminated or not, and to count the number of eggs in the ovaries. Because of the high number of females collected by timed collections, their reproductive state was determined indirectly by the number of eggs laid by each individual female after a 10-d period. The wings, legs, and head of all triatomines were stored in ethanol for other studies. The qualitative nutritional status of adult bugs was determined by direct observation of the volume and shape of the anterior midgut against a flashlight and classified as starved, or with scarce, good, or large blood contents (Montenegro 1983).
Statistical Analysis
W/L ratios and the mean maxima (26.2°C) or the absolute maxima (29.4°C) between 20 and 22 h during the light-trapping period (T) were used to estimate the probability of flight initiation of peridomestic bugs collected by timed catches (Pb) (Lehane et al. 1992): Logit (Pb) = −A + B (W/L) − C(W/L)2 + D(T), where Logit (Pb) = x; Pb = ex/(1 + ex); A = 18.37; B = 1.97; C = 0.18; D = 0.43.
As the exact source of the bugs that flew to each light trap was unknown, we pooled the abundance of T. infestans in all infested sites within a 200 m radius around each trap to assess the relationship between flight dispersal and bug abundance. This analysis was only performed for males because of the very low number of females collected by light trapping.
Results
In the pilot trial, 8 Triatoma guasayana, 1 Triatoma garciabesi and no T. infestans adults were collected by light trapping in October 2002. A total of 86 T. infestans (including 23 adult bugs) was collected by timed catches from 8 (21%) peridomestic sites around the light traps.
In March 2003, T. infestans bugs were collected by timed manual catches from 22% of Amamá peridomestic sites (totaling 175 bugs) and 6% of Trinidad peridomestic sites (26 bugs) (Table 1). A total of 15 T. infestans, 4 adult T. garciabesi, and 1 fifth-instar T. guasayana were collected during the 5 light-trapping nights (Table 1). None of the triatomines was infected by T. cruzi. The catch rate of T. infestans per light trap night in Amamá (0.40) was 13 times greater than that in Trinidad (0.03). All T. infestans except 1 fifth-instar nymph were collected in Amamá. Both the light trap and timed collections of T. infestans were significantly biased toward males (χ2 = 5.3, df = 1, P < 0.02; χ2 = 10.0, df = 1, P = 0.002, respectively). Seven (62.5%) of the T. infestans fliers and 2 of the 3 nymphs that walked to the traps did so within the first hour after sunset.
Table 1.
Collections of T. infestans by timed manual catches and light traps in Amamá and Trinidad, March 2003
| Village
|
|||
|---|---|---|---|
| Collection method | Variable | Amamá | Trinidad |
| Timed manual catches | % Sites infested (no. examined) | 22 (51) | 6 (33)a |
| No. collected | |||
| Males | 61 | 6 | |
| Females | 33 | 2 | |
| Nymphs | 81 | 18 | |
| Mean bug abundance per infested site | 14.6 | 6.5 | |
| Light trap catchesb | No. traps positive/No. trap nights | 4/35 | 1/29 |
| No. collected | |||
| Males | 10 | 0 | |
| Females | 2 | 0 | |
| Nymphsc | 2 | 1 | |
| No. bugs per trapping night | 0.40 | 0.03 | |
Excludes one house infested by T. infestans as notified by householders.
Excludes 4 T. garciabest (3 males, 1 female) and 1 fifth instar nymph T. guasayana.
Fifth instar nymphs.
The mean distance from each light trap to the nearest T. infestans-infested site was 39 m (range 19–82 m) in Amamá and 58 m (range 30–94 m) in Trinidad, whereas the mean distance from a T. infestans positive light trap to the nearest infested site was 50 m (range 30–82 m) and 42 m, respectively. The mean distance between T. infestans-positive light traps was 344 m (range 72–653 m) in Amamá. The three T. infestans fifth instars were collected in two light traps at 30 and 42 m from the nearest infested site.
Overall, 80% of male and 50% of female T. infestans that flew to the light traps were starved, whereas the remainder had scarce blood contents (Fig. 2). The time-collected bugs had a significantly better qualitative nutritional status than the light trap-collected bugs (Fisher test, P = 0.002), with only 31% of males and 23% of females unfed. Female and male T. infestans collected manually from peridomestic sites had a similar nutritional status (χ2 = 2.41, df = 1, P = 0.12). The three T. infestans fifth-instar nymphs that walked to the traps had scarce (one bug) or good blood contents (two bugs).
Fig. 2.
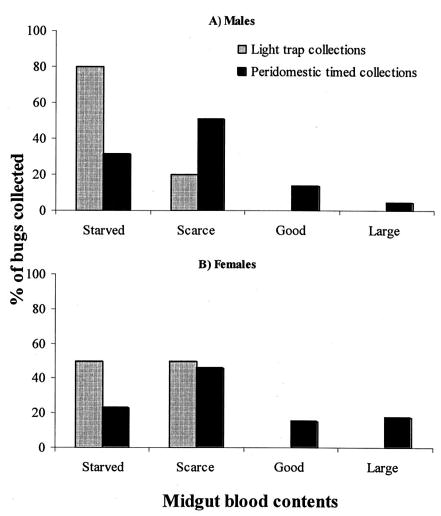
Qualitative nutritional status of light trap and time-collected male (A) and female (B) T. infestans determined by transparency of the anterior midgut. Amamá and Trinidad, March 2003.
The mean weight of the T. infestans male fliers was significantly lower than the time-collected males (t test, t = 3.92, df = 69, P = 0.0002). The two light trap-collected females (150.9 and 222.2 mg) were also lighter than the time-collected females (mean ± SD, 320.6 ± 88.4 mg). The length of light trap- and timed-collected males did not differ significantly (t = 1.45, df = 69, P = 0.15). Only one of the light trap-collected females was inseminated and had six eggs (two vitel-logenic and four chorionated), whereas 60% of time-collected females laid eggs (mean ± SD, 16.6 ± 8.7 eggs).
Male T. infestans fliers had a significantly lower mean W/L ratio than the time-collected males (t = 3.75, df = 69, P = 0.0004) (Fig. 3). The two females collected by light trapping had a lower W/L (5.9 and 8.2 mg/mm) than time-collected females (12.3 ± 3.3 mg/mm). The extreme W/L values for the light trap-collected T. infestans were 3.6–10.3 mg/mm (males) and 5.9–8.2 mg/mm (females), whereas for the time-collected bugs, the extreme W/L were 7.9–16.7 and 6.6–18.6 mg/mm, respectively. If the extreme W/L of each sex captured by light trapping is taken as the extreme value allowing flight, twice as many time-collected male (53.7%) as female T. infestans (25.7%) would be expected to fly.
Fig. 3.
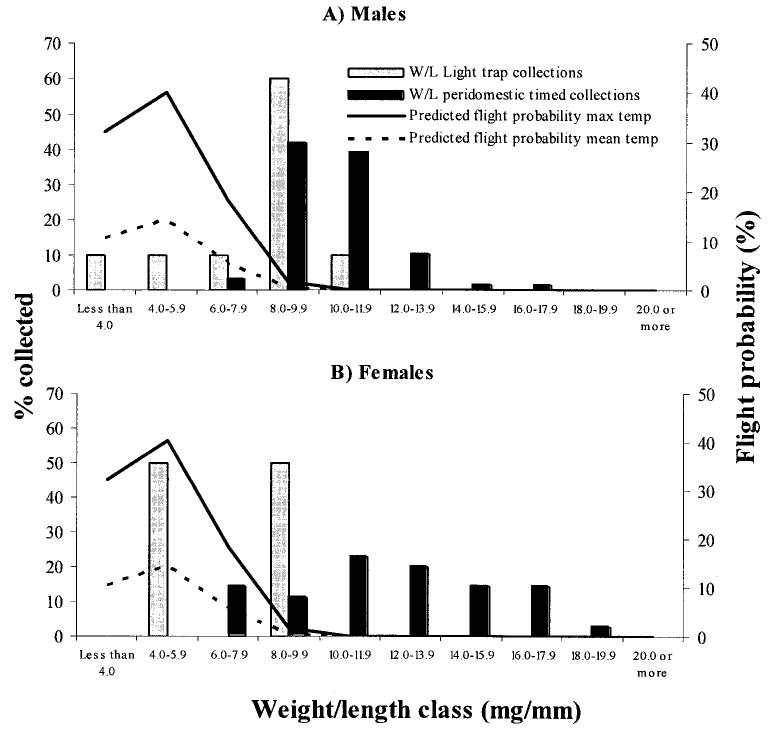
Percentage of male (A) and female (B) T. infestans collected by light trapping or in peridomestic sites according to their nutritional status measured by the W/L ratio (bars). The figure also shows the percentage of bugs predicted to fly according to the model by Lehane et al. (1992) using W/L ratios and the period mean maxima (26.2°C, – – –) or absolute maxima (29.4°C, —) during light trap collections. Amamá and Trinidad, March 2003.
The predicted percentage of T. infestans that would fly, according to the model of Lehane et al. (1992), parameterized with the observed W/L ratios and mean or absolute maximum temperature during the light trap period, is also shown in Fig. 3. The model predicted that 60% of males and 50% of females that actually flew to the light traps would not be physiologically apt for flying, and that nearly all of the time-collected bugs with a W/L >8.0 mg/mm would not be able to perform any dispersive flight at both temperatures. The observed number of T. infestans that flew to the traps was significantly higher than the expected number that would fly according to the model (χ2 = 27839, df = 4, P < 0.001).
Mean temperatures during the light-trapping nights were 22.3–27.8°C. The number of T. infestans that flew to the traps each night was significantly and negatively associated with wind speed (Pearson correlation coefficient (r) = −0.79, P = 0.02) (Fig. 4A), whereas no significant relationship was found with temperature (r = 0.15, P = 0.72) (Fig. 4B), relative humidity (r = −0.63, P = 0.09), barometric pressure (r = 0.12, P = 0.78), or phase of the moon (r = 0.49, P = 0.22). The number of T. infestans males collected by light trapping was significantly and positively associated with the total adult bug abundance within 200 m around each trap (lineal regression coefficient, β = 0.73, t = 4.2, P = 0.0008) (Fig. 4C). However, the statistical significance was caused by a single data point at high bug abundance.
Fig. 4.
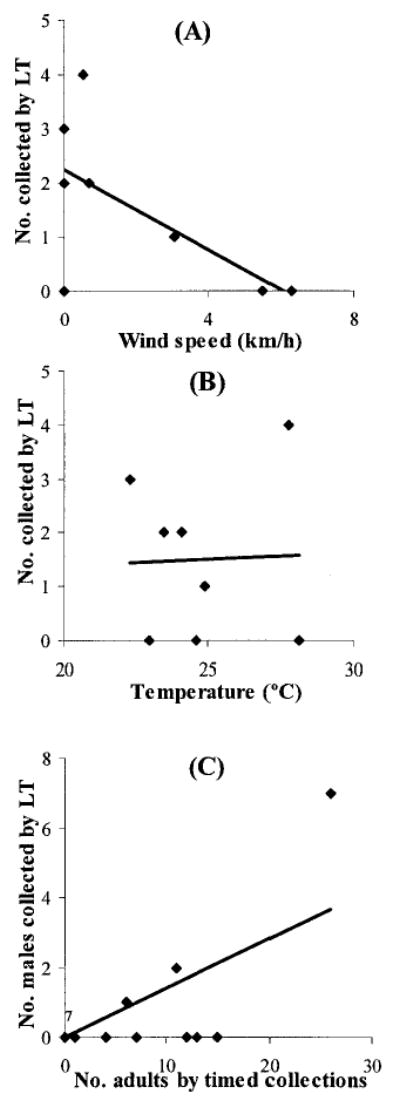
Number of adult T. infestans collected by light trapping (LT) on each night according to wind speed (A), maximum temperature (B), and the total number of adult T. infestans collected in all sites within a 200 m radius around the light trap (C). Numbers beside points represent repeated data points. Amamá and Trinidad, March 2003.
Discussion
This is the first report of successful collection of dispersing T. infestans using light traps in a well-defined rural area. We attribute our success to the placement of traps with consideration of spatial infestation patterns and temporal variation in nutritional status of peridomestic bugs. Flight dispersal was significantly associated with wind speed within temperatures adequate for flight and the peridomestic abundance of adult T. infestans in the vicinity of the light traps. Our study also revealed that T. infestans fifth-instar nymphs walked significant distances from the nearest infested sites.
The number of dispersing T. infestans caught by light trapping differed greatly between villages (14 bugs in Amamá versus 1 in Trinidad) as a consequence of the lower abundance and better nutritional status of peridomestic T. infestans at Trinidad. At higher densities, the population of T. infestans is expected to achieve a poorer nutritional status that would increase the probability of flight initiation (Schofield 1985), although the latter proved to be related inversely to bug densities under laboratory conditions (McEwen et al. 1993). However, our study suggested a positive bug density-dependent effect on the number of flight arrivals of male T. infestans to the light traps. Goat or pig corrals had more abundant T. infestans populations than other peridomestic ecotopes in March 2003; more variable microclimatic conditions (Vazquez-Prokopec et al. 2002); and a poorer nutritional status and higher probability of flight initiation in 2001–2002 (unpublished data). In the study villages between 1993 and 1996, the active invasion of domestic sites by adult T. infestans dispersing by flight was revealed by house-holders’ collections of adult bugs (Gürtler et al. 1999), and the occurrence of an infested peridomestic site in the house was involved as a significant risk factor of domestic reinfestation and invasion by T. infestans (Cecere et al. 2002). Although goat or pig corrals were the most likely sources of the bugs that flew to the light traps, molecular and morphometric studies (Schachter-Broide et al. 2004) underway may provide conclusive evidence on the source of the dispersants.
The sex ratio of light trap and timed collections was biased toward male triatomines. A similar male-biased sex ratio (15 male and 6 female T. infestans) was found among dead bugs lying outside an illuminated building, but not in nearby light trap collections (3 males and 4 females) in western Argentina (Schweigmann et al. 1988). Conversely, the probability of flight initiation of laboratory-reared T. infestans released in salt flats was female biased (Schofield et al. 1992), and 82% of female and 30% of male T. infestans dispersed by flight from open experimental huts (Canale and Carcavallo 1985). The joint effects of a good nutritional status and large fecundity of peridomestic female T. infestans populations (Figs. 2B and 3B) probably explain why, in our study, females flew much less frequently than males. However, different flight activity patterns by sex (Ekkens 1981) or a higher attractiveness of male triatomines to black lights (Carcavallo 1985) cannot be excluded.
Winds of at least 6 km/h apparently inhibited T. infestans-dispersive flights, as incidentally observed by Schofield et al. (1992). Under laboratory conditions, the flight initiation of T. infestans was positively correlated with temperature, but no association was found with barometric pressure (Lehane et al. 1992). In our study, however, the lack of any relationship between flight dispersal and temperature or other weather conditions probably resulted from the exclusion of colder nights in which T. infestans flight activity was not expected to occur. The arrival of T. infestans bugs to the light traps was not associated with the phase of the moon, suggesting that this factor did not interfere with the orientation of bugs. The presence of a variable number of bug collectors among catch stations may have added an extra source of variation and differential attractiveness to the traps.
The model of Lehane et al. (1992) predicted the proportion of flight initiations from a given source population (although some of these flights were trivial), whereas the light trap collections are the effective number of bugs arriving from one or more infested sites. This difference should be taken into account when comparing the model with field data. According to the model (Lehane et al. 1992), 50–60% of the T. infestans bugs that effectively flew to the light traps would not have flown, and this difference would have been more marked had we considered the mean rather than the period maximum temperature. These discrepancies may originate from the use of T. infestans colonies kept under laboratory conditions for many years and the range of temperatures used for parameterizing the model. Compared with the T. infestans used by Lehane and Schofield (1982) (Table 2, p. 502), male and female bugs in our study were significantly longer (ttests, P < 0.001, and P = 0.0003, respectively), male fliers were significantly heavier (P < 0.0001), and W/L ratios would be strongly modified by disproportionate differences in weight. As laboratory-reared T. infestans colonies are shorter and lighter than natural populations, the exact quantitative predictions of the model with natural bug populations need to be adjusted.
T. infestans fifth-instar nymphs walked from 29 to 42 m to arrive at the light traps, assuming that they came from the nearest infested site. Schofield (1985) also mentioned these nymphs walking up to 60 m. The walking distances recorded in this study are consistent with the finding, in bedroom areas, of T. infestans nymphs that have fed on goats or bovids enclosed 50–80 m away (Gürtler et al. 1996). The active dispersal of large nymphs possibly plays an important role in the local propagation of T. infestans within and between neighboring households.
At a village level, the spatial patterns of reinfestation and gene flow are determined by flight dispersal and may depend on distances between houses, the local abundance of bugs and hosts, and vegetation cover. Given a flight-dispersal capacity ranging from 200 to 2,000 m (Schweigmann et al. 1988, Schofield et al. 1992), T. infestans from a few peridomestic residual foci might reinfest the entire village. The spatial heterogeneity generated by the effects of landscape and vegetation cover may affect the spatial distribution of T. infestans infestations and the risk of house invasion if they act as a barrier for bug dispersal. In Amamá, the low vegetation cover allowed one trap to be seen 500–600 m away, whereas in Trinidad the visibility range of each trap was 100–200 m because of dense vegetation cover and higher trees. Thus, in addition to bug nutritional differences, differences in landscape and accessibility to the light traps may modify the numbers of T. infestans bugs that fly to the traps.
Acknowledgments
Carla Cecere, Juan Manuel Gurevitz, Paula Marcet, Marcela Orozco, and Francisco Petrocco kindly participated in field and laboratory work. We also thank Heinrich Zu Dohna, Judith Schachter-Broide, Alejandro Cittadino, Claudio Lazzari, and Daniel Salomón for their helpful comments; and the European Community–Latin American Network for Research on the Biology and Control of Triatominae (ECLAT) network for support. This project was supported by grants from the University of Buenos Aires and Agencia Nacional de Promoción Científica y Técnica (Argentina) to R.E.G., and National Institutes of Health Research Grant R01 TW05836, funded by the Fogarty International Center and the National Institute of Environmental Health Sciences to U.K. and R.E.G. R.E.G. is member of Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina Researcher’s Career.
References
- Canale DM, Cecere MC, Chuit R, Gürtler RE. Peridomestic distribution of Triatoma garciabesi and Triatoma guasayana in north-west Argentina. Med Vet Entomol. 2000;14:383–390. doi: 10.1046/j.1365-2915.2000.00254.x. [DOI] [PubMed] [Google Scholar]
- Canale, D. M., and R. U. Carcavallo. 1985.Triatoma infestans (Klug), pp. 237–250. In R. U. Carcavallo, J. E. Rabinovich, and R. J. Tonn (eds.), Factores biológicos y ecológicos en la enfermedad de Chagas. Ministerio de Salud y Acción Social de Argentina, Buenos Aires, Argentina.
- Carcavallo, R. U. 1985. Técnicas de estudio de triatominos en ambiente silvestre, pp. 49–52. In R. U. Carcavallo, J. E. Rabinovich, and R. J. Tonn (eds.), Factores biológicos y ecológicos en la Enfermedad de Chagas. Ministerio de Salud y Acción Social de Argentina, Buenos Aires, Argentina.
- Cecere MC, Gu rtler RE, Canale DM, Chuit R, Cohen JE. Effects of partial housing improvement and insecticide spraying on the reinfestation dynamics of Triatoma infestans in rural northwestern Argentina. Acta Trop. 2002;84:101–116. doi: 10.1016/s0001-706x(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Cohen JE, Gürtler RE. Modeling household transmission of American trypanosomiasis. Science. 2001;293:694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- Ekkens D. Nocturnal flights of Triatoma (Hemiptera: Reduviidae) in Sabino Canyon, Arizona. I. Light collections . J Med Entomol. 1981;18:211–227. [Google Scholar]
- Gürtler RE, Cecere MC, Vazquez DF, Chuit R, Cohen JE. Host-feeding patterns of domiciliary Triatomainfestans (Hemiptera: Reduviidae) in northwest Argentina: seasonal and instar variation. J Med Entomol. 1996;33:15–26. doi: 10.1093/jmedent/33.1.15. [DOI] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Canale D, Castañera MB, Chuit R, Cohen JE. Monitoring house reinfestation by vectors of Chagas’ disease: a comparative trial of detection methods during a four-year follow-up. Acta Trop. 1999;72:213–234. doi: 10.1016/s0001-706x(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Gürtler RE, Canale DM, Spillmann C, Stariolo R, Salomón OD, Blanco S, Segura EL. Effectiveness of residual spraying with deltamethrin and permethrin on peridomestic populations of Triatoma infestans in rural western Argentina: a district-wide randomized trial. Bull World Health Organ. 2004;82:196–205. [PMC free article] [PubMed] [Google Scholar]
- Lehane MJ, Schofield CJ. Flight initiation in Triatomainfestans (Klug) (Hemiptera: Reduviidae) Bull Entomol Res. 1982;72:497–510. [Google Scholar]
- Lehane MJ, McEwen PK, Whitaker CJ, Schofield CJ. The role of temperature and nutritional status in flight initiation by Triatoma infestans. Acta Trop. 1992;52:27–38. doi: 10.1016/0001-706x(92)90004-h. [DOI] [PubMed] [Google Scholar]
- Lopez AC, Crocco L, Morales G, Catalá S. Feeding frequency and nutritional status of peridomestic populations of Triatoma infestans from Argentina. Acta Trop. 1999;73:275–281. doi: 10.1016/s0001-706x(99)00039-x. [DOI] [PubMed] [Google Scholar]
- McEwen PK, Lehane MJ, Whitaker CJ. The effect of adult population density on flight initation in Triatoma infestans (Klug) (Hem Reduviidae) J Appl Entomol. 1993;116:321–325. [Google Scholar]
- Montenegro S. Determinación de las reservas alimenticias en Triatomainfestans Klug, 1834 (Hemiptera, Reduviidae) en base a caracteres externos. I Adultos Physis. 1983;41:159–167. [Google Scholar]
- Noireau F, Bastrenta B, Catalá S, Dujardin JP, Panzera F, Torres M, Perez R, Galvão C, Jurberg J. Sylvatic population of Triatoma infestans from the Bolivian Chaco: from field collection to characterization. Mem Inst Oswaldo Cruz. 2000;95 (Suppl I):119–122. doi: 10.1590/s0074-02762000000700020. [DOI] [PubMed] [Google Scholar]
- Ronderos RA, Schnack JA, Mauri RA. Resultados preliminares respecto de la ecología de Triatoma infestans (Klug) y especies congenéricas con referencia especial a poblaciones peridomiciliarias. Medicina. 1980;40:187–196. [PubMed] [Google Scholar]
- Schachter-Broide J, Dujardin JP, Kitron U, Gürtler RE. Spatial structuring of Triatoma infestans (Hemiptera, Reduviidae) populations from northwestern Argentina using wing geometric morphometry. J Med Entomol. 2004;41:643–649. doi: 10.1603/0022-2585-41.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield CJ. Population dynamics and control of Triatoma infestans. Ann Soc Belge Med Trop. 1985;65:149–164. [PubMed] [Google Scholar]
- Schofield CJ, Lehane MJ, McEwen PK, Catala S, Gorla DE. Dispersive flight by Triatoma infestans under natural climatic conditions in Argentina. Med Vet Entomol. 1992;6:51–56. doi: 10.1111/j.1365-2915.1992.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Schweigmann N, Vallvé S, Muscio O, Guillini M, Alberti A, Wisnivesky-Colli C. Dispersal flight by Triatoma infestans in an arid area of Argentina. Med Vet Entomol. 1988;2:401–404. doi: 10.1111/j.1365-2915.1988.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prokopec GM, Ceballos LA, Cecere MC, Gürtler RE. Seasonal variations of microclimatic conditions in domestic and peridomestic habitats of Triatoma infestans (Hemiptera: Reduviidae) in rural northwest Argentina. Acta Trop. 2002;84:229–238. doi: 10.1016/s0001-706x(02)00204-8. [DOI] [PubMed] [Google Scholar]
- Zeledón R, Rabinovich JE. Chagas’ disease: an ecological appraisal with special emphasis on its insect vectors. Annu Rev Entomol. 1981;26:101–133. doi: 10.1146/annurev.en.26.010181.000533. [DOI] [PubMed] [Google Scholar]


