Abstract
Signaling via Jak2/STAT3 is critically important for normal dendritic cells (DC) differentiation. In addition, we have previously demonstrated that hyper-activation of the Jak2/STAT3 pathway induced by tumor-derived factors (TDF) may be responsible for abnormal DC differentiation in cancer. Here, using a novel selective inhibitor of Jak2/STAT3 JSI-124, we investigated the mechanism of Jak2/STAT3 effect on DCs and the possibility of pharmacological regulation of DC differentiation in cancer. Our experiments have demonstrated that JSI-124 overcomes the differentiation block induced by TDF and promotes the differentiation of mature DCs and macrophages. Surprisingly, inhibition of Jak2/STAT3 signaling resulted in dramatic activation of immature DCs generated in the presence of TDF as well as in control medium. This activation manifested in up-regulation of MHC class II, co-stimulatory molecules and a dramatic increase in the ability to stimulate allogeneic or antigen-specific T cells. Inhibition of Jak2/STAT3 signaling resulted in activation of transcription factor NF-κB. This up-regulation was not due to a conventional pathway involving IκBα but likely due to a block of the dominant negative effect of STAT3. This indicates that Jak2/STAT3 play an important role in negative regulation of DC activation and pharmacological inhibition of Jak2/STAT3 pathway can be used to enhance DC function.
Introduction
DCs are specialized antigen presenting cells (APCs) that recognize, acquire, process, and present antigens to naï resting T cells for the induction of an antigen-specific immune response (1-3). DCs are critically important for the induction and maintenance of antitumor immune responses both spontaneously developed and induced as a result of immunotherapy. Inadequate function of the host immune system may render all attempts to use immunotherapy ineffective. Data from many different laboratories obtained during the past few years indicate that defects in the DC system is one of the main factors responsible for tumor escape (rev. in (4). Recent studies have demonstrated an important role of Jak2/STAT3 pathway in DC differentiation under physiological condition and cancer. Laouar and colleagues reported that STAT3 is required for FLT3-ligand dependent DC differentiation (5). At the same time, we have demonstrated that hyper-activation of Jak2/STAT3 signaling is directly involved in the abnormal Dc differentiation in cancer (6, 7). Myloid cells maintaining high levels of Jak2 and STAT3 activity were not able to differentiate into DCs in vitro (7).
Janus family tyrosine kinases (Jaks) and signal transducer and activator of transcription (STAT) proteins are critical components of diverse signal-transduction pathways that are actively involved in cellular survival, proliferation, differentiation and apoptosis (8). Jaks are consitutively associated with many cytokine and growth factor receptors, including those implicated in defective DC differentiation (rev.(9)). Activated Jaks eventually induce phosphorylation of STATs, followed by their translocation into the nucleus where they modulate expression of traget genes. We hypothesized that inhibition of tumor-induced Jak2/STAT3 hyperactivation in myeloid cells may improve DC differentiation and function, and ultimately antitumor immune response.
To test this hypothesis we used new selective inhibitor of Jak2/STAT3 pathway JSI-124 (cucurbitacin I). We have previously demonstrated that JSI-124 selectively inhibited the activation of Jak2 and STAT3 but not Src, Akt, Erk, and Jnk (10). JSI-124 inhibited the growth of tumors with consitutively active STAT3 but did not affect tumors without STAT3 hyperactivation (10).
This study, for the first time, demonstrates that inhibition of Jak2/STAT3 signaling dramatically improves differentiation of DC. Surprisingly, inhibition of Jak2/STAT3 resulted in dramatic activation of DCs. This effect was observed in control DCs as well in the cells generated in the presence of TDF. It appears that the main mechanism of the effect of STAT3 activation inhibitors on DC activation was up-regulation of NF-κB but not through a conventional mechanism involving phosphorylation and degradation of IκB but rather through elimination of the dominant-negative effect of STAT3 on NF-κB.
Methods
Reagents, drug, and cell culture. RPMI 1640, DMEM, fetal bovine serum (FBS) and antibiotics were obtained from Gibco BRL (Grand Island, NY), recombinant murine GM-CSF and IL-4 from RDI (Flanders, NJ), lipopolysaccharides (LPS) and Conconovalin A (ConA) from Sigma (St.Louis, MO), Low-Tox rabbit complement and Lympholyte M from Cedarlane Laboratories (Hornby, Ontario, Canada). Lysine-fixable FITC-dextran (FITC-DX, MW 40,000) were obtained from Molecular Probes (Eugene, OR) and dissolved in PBS. All peptides were purchased from SynPep Corporation (Dublin, CA). The following antibodies were obtained from BD Pharmingen (San Diego, CA): anti-Gr-1 (anti-Ly-6G), anti-CD11b, anti-CD11c, anti-I-Ab, anti-I-Ab, anti-CD86, anti-CD40, anti-I-A/I-E, and anti-CD3. Anti-F4/80 antibody were from Serotec Inc (Raleigh, NC). JSI-124 (cucurbitacin I) was obtained from NCI and. For in vivo experiments Cucurbitacin I was obtained from Indofine Chemicals Inc (Hillsborough, NJ). It was dissolved in DMSO.
Murine NIH-3T3 fibroblasts and CT26 colon carcinoma cell line were obtained from ATCC (Manassas, VA). Cells were grown in DMEM supplemented with 10% FBS and antibiotics. MethA (methylcholantrene-induced) sarcoma cell line was obtained from Dr. Lloyd J. Old. MethA tumor was developed in BALB/c mice and passaged in vivo as an ascitic tumor. To generate conditioned medium (CM) cells were kept in medium with reduced (3%) FBS concentration. After 48 hr supernatants were collected, filtered and used in experiments.
Generation of DCs and isolation of cells. Bone marrow (BM) cells were obtained from the femurs and tibias of mice and red cells were eliminated using ACK buffer. Cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 20 ng/ml GM-CSF, 10 ng/ml IL-4, and 50 μM 2-mercaptoethanol alone or in the presence of control (from 3T3 fibroblasts) or tumor cell (from CT26) CM. Half of the medium was replaced every 2 days. Cells were collected on day 5 followed by isolation of CD11c-positive DCs. To investigate the effect of JSI-124 on hematopoietic progenitor cells (HPCs) differentiation, BM cells obtained from mice were initially enriched for HPCs by depletion of lineage-specific cells using antibody cocktail against T cells (TIB 210 and TIB-207), B cells (B220), macrophages and DCs (TIB-120), granulocytes (Gr-1), and red cells (Ter-119) as described earlier (11). HPCs were cultured for 5-7 day as described above for BM culture. Gr-1 or CD11c positive cells were isolated from spleens of tumor-bearing or control mice using magnetic beads separation technique and MidiMACS according to the manufacturer's protocol (Miltenyi Biotec, Auburn, CA). Purity of Gr-1-positive or CD11c-positive populations was more than 95% as determind by flow cytomertry.
Evaluation of T cell proliferation and cytokine production. Murine CD11c DCs were used as stimulators in allogeneic mixed leukocyte reaction. T cells isolated from spleens of allogeneic mice using T cell enrichment columns (R&D systems, Minneapolis, MN) were used as responders. Cells were mixed at different ratios and incubated in triplicates in U-bottom 96-well plates for 4 days. 1 μCi of [3H]-Thymidine (Amersham, Arlington Heights, IL) was added per well 18h prior to cell harvest. [3H]-Thymidine incorporation was measured using liquid scintillation counter. In some experiments splenocytes were cultured for 4 days in the presence of 1 or 5 μg/ml of ConA, or 0.5 μg/ml anti-CD3 antibody. Antigen-specific T cell response was evaluated using MHC class II (I-Ad)-restricted (SFERFEIFPKE) Influenza virus hemagglutinin (HA)-derived peptide. Murine DCs were cultured with autologous T cells isolated from spleens of TCR transgenic mice in the presence of 12.5 μg/mL HA-derived peptide. In some experiments after 2 days of incubation cells were collected and intracellular level of INFγ, IL-10, and IL-2 was determined in the population of CD4+ T cells by flow cytometry using Manufacturer's protocol. (BD Pharmingen). Cells were analyzed on FACSCalibur using CellQuest software.
To evaluate whether the direct contact or soluble factors were responsible for induction of T cell response, DCs were cultured in U-bottom 96-well plates mixed together with splenocytes or on the top of 0.2μm Transwell inserts (Nunc, Naperville, IL). After 4 days in culture cells were pulsed with BrdU for 1 hrs, collected, labeled with anti-CD3 antibody, fixed and permeabilized using BrdU Flow Kit (BD Pharmingen) followed by staining with anti-BrdU-FITC antibody and 7-AAD. Proportion of CD3+ T cells in S phase of cell cycle was evaluated by flow cytometry.
FITC-Dextran (FITC-DX) assay was performed as described in (12). Cells were collected and resuspended in RPMI 1640 medium buffered with 25 mM HEPES and supplemented with 10% FBS. FITC-DX was added at the final concentration of 1 mg/ml and cells were then incubated at 37°C or on ice. After 45 min cells were washed with ice cold PBS containing 1% FBS and analyzed on FACSCaliber (Becton Dickinson). For each sample the background (mean value of fluorescence of cells pulsed at 4°C) was subtracted from the mean value of fluorescence of cells incubated at 37°C.
ELISA. CD11c+ DCs were plated in concentration 106 cells/mL and treated with JSI-124 or VC for 48 hrs. After that time supernatants were collected and the levels of IL-12 (p70) and RANTES was measured using ELISA kits (Pierce Endogen and Biosource, respectively). Sensitivity for both assays was >5 pg/mL.
ELISPOT assay was performed as described previously (13). Briefly, MultiScreen-HA plates (Millipore, Berford, MA) were precoated with anti-mouse IFN-γ antibody (BD PharMingen) by overnight incubation at 4°C. Two hundred thousand splenocytes were plated in quadruplicates in each well and cultured for 24 h at 37°C in the presence of MHC class I matched (H2Kd) control p53-derived (KYICNSSCM) or specific HA-derived (IYSTVASSL) peptides (10 μg/ml). Cells were then washed with PBS containing 0.1% Tween, and plates were incubated overnight at 4°C with biotinylated anti-IFN-γ antibody (BD PharMingen). Results were visualized using avidin-alkaline phosphatase and 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate (Sigma-Aldrich). The number of spots was calculated on CTL analyzer (CTL Analyzers, Cleveland, OH) using ImmunoSpot 2.8 version software (Cellular Technology, Ltd). Results are presented as number of spots per 1 × 106 cells.
Electrophoretic mobility shift assay (EMSA) and Western blotting. EMSA was performed as described previously (11). Specific bands were visualized by overnight exposure to X-ray films (Fuji, Stamford, CT) at -70°C. Quantitation of band intensity was performed on Storm phosphor imager using ImageQuant software (Molecular Dynamics, Sunnyvale, CA). For Western blotting cells were lysed in RIPA buffer. Equal amount of protein was run on SDS-PAGE gel followed by transfer into nitrocellulose membrane. Membrane was blocked in 5% milk in TBS/T for 1 hr at room temperature and probed for overnight at +4°C with the following primary antibodies: anti- p65, cRel, Rel B, IkBα, or β-actin (Santa Cruz Biotechnology, Santa Cruz, CA), phosphoSTAT3 or STAT3 (Cell Signaling Technology, Beverly, MA), or anti-mouse IA/IE-biotin antibody (BD Pharmingen). Membranes were then washed with TBS/T, incubated with corresponding secondary antibodies (all obtained from Santa Cruz excluding anti-biotin-HRP antibody, which was purchased from Cell Signaling), and then washed again with TBS/T. Specific bands were visualized using chemiluminescence reagent kit (ECL, Amersham Bioscience, Piscataway, NJ) and their intensity was quantitated using ImageQuant software.
Immunoprecipitation. Cells were collected, washed twice with ice cold PBS and lyzed in the following buffer: 10 mM Tris-Hcl pH 7.4, 150 mM NaCl, 1% deoxycholate, 1% Triton X-100, 0.1% SDS, 2 mM Na3VO4, 2 mM PMSF, and 1:100 protease inhibitor cocktail (Sigma, St.Louis, MO). STAT3 antibody was added to lysates (500 μg of protein per sample) and incubated with end-over-end rotation for 2 hrs at 4°C followed by the addition of protein A Sepharose beads (Amersham Bioscience) and incubation for another 1 hrs at the same conditions. Protein/antibody/beads mix was then washed 5 times with lysis buffer, re-suspended in Laemmli SDS sample buffer and denatured at 95°C for 5 min. Samples were resolved in 8% SDS-PAGE followed by transfer to PVDF membrane. After blocking membrane was probed with p65, Rel B, cRel, or STAT3 antibodies. Membrane was developed using ECL detection kit.
Luciferase assay was performed to evaluate NF-κB transcriptional activity in DCs as described (11). Briefly, 5×106 CD11c+ DCs were electroporated with either 6xINF-γtkLuc plasmid containing luciferase reporter gene under NF-κB-dependent promotor or control pGL3-basic plasmid containing only luciferase gene. Co-transfection with pRL-TK plasmid containing Renilla luciferase gene was performed to measure the transfection efficiency. After that cells were treated with JSI-124 or VC for 36 hrs followed by luciferase assay using Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Values of luciferase activity for specific and control plasmid were normalized to Renilla luciferase activity.
Polymerase chain reaction (PCR). Total cellular RNA was extracted using RNeasy Mini kit (Qiagen, Santa Clara, CA). First strand cDNA was synthesized with 1 μg RNA using SuperScript II reverse transcriptase (Invitrogen) and oligo(dT) primers according to the manufacturer's protocol. PCR was performed using CIITA promoter I, and promoter III specific primers. Primers were a kind gift from Dr. K. Wright (Moffitt Cancer Center). Amplification of hprt gene served as an internal control. Each PCR reaction carried appropriate positive and negative controls. The products were electrophoresed on 1.5% agarose gel and photographed.
Confocal microscopy. Cells were collected on slides using cytospin centrifugation, fixed in 4% paraformaldehyde in PBS for 10 min, washed three times with PBS followed by permeabilization in 0.5% TritonX-100 for 5 min and blocking for 30 min in 2% BSA in PBS. After that slides were incubated in a humid chamber for 1 hr with primary biotinylated anti-IA/IE antibody followed by incubation with streptavidin-FITC antibody. Slides were analyzed on the Zeiss LSM 510 confocal microscope.
Statistics. Statistical analysis was performed using JMP software (SAS Institute, Cary, NC).
Results
Inhibition of Jak-2/STAT3 pathway improves differentiation of DCs from HPCs
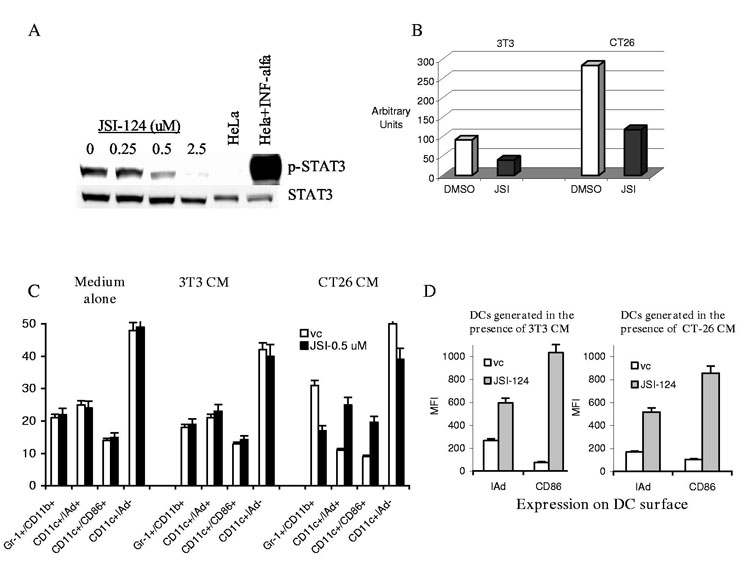
Previous studies have determined that JSI-124 inhibits STAT3 activation in tumor cells (10). Here, we have investigated whether this compound exerts similar effects on STAT3 activation in DCs. HPCs isolated from control mice were grown in the presence of control (3T3) or tumor cell (CT26) conditioned medium (CM) for 5 days. CD11c+ DCs were isolated and treated with 0.5 μM JSI-124 or DMSO (vehicle control, vc) for 24 hrs. This dose was selected after preliminary experiments. It significantly inhibited STAT3 activity (the level of pSTAT3) (Fig. 1A) and provided more than 95% viability of DCs (data not shown). Consistent with our previous observations (7), STAT3 activity in DCs generated in the presence of TDF was substantially higher than those generated in the presence of control CM. JSI-124 significantly reduced the level of phospho-STAT3 in both groups of cells (Fig. 1B).
Figure 1.
JSI-124 decreases the levels of phospho-STAT3 and promotes DC differentiation. (A) DC were generated from HPCs in the presence of tumor cell (CT26) CM for 5 days. CD11c+ DCs were isolated and treated with different concentrations of JSI-124 for 24 hrs. Proteins were evaluated by Western Blotting. HeLa cells treated with IFN-α were used as positive control for STAT3 activation. (B) HPCs were grown in the presence of control (3T3) or tumor cell (CT26) CM for 5 days. CD11c+ DCs were isolated and treated with 0.5 μM JSI-124 or vc for 24 hrs. Intensity of specific bands was quantitated using ImageQuant software. Intensity of the signals from pSTAT3 was normalized to the one from total STAT3. (No differences in the level of total STAT3 between the cells were observed). (C) HPCs were isolated from mice BM and cultured in medium alone (No CM) or in the presence of 3T3 or CT26 CM. Cells were treated with JSI-124 or DMSO (vc) starting from day 3. On day 7 cells were collected. The proportions of different cell populations were calculated. Mean results of four experiments are shown. (D) CD11c+ cells from the experiment described in Fig. 1B were gated and the level of expression of MHC class II (IAd) and B7-2 (CD86) molecules was evaluated. Mean results of four experiments are shown.
Accumulation of immature myeloid cells (IMC) and their inability to differentiate into DCs is one of the hallmarks of immunological abnormalities in tumor-bearing hosts. We asked whether inhibition of STAT3 signaling might affect differentiation of these cells. Enriched HPCs isolated from BM of control mice were cultured for 7 days with GM-CSF and IL-4. Medium alone or CM from CT26 tumor cells or control 3T3 fibroblasts was added on day 0 and JSI-124 (0.5 μM) or DMSO on day 3. As expected, the presence of TDF significantly increased the proportion of Gr-1+CD11b+ IMC and decreased the proportion of CD11c+IAd+ and CD11c+CD86+ DCs (Fig. 1C). Treatment with JSI-124 did not affect the cell populations generated in the presence of medium alone or 3T3 CM. However, it significantly decreased the proportion of IMCs and increased the proportion of DCs generated in the presence of CT26 tumor cell CM. These data were consistent with our previous observations made using dominant negative STAT3 construct (7) and suggested that inhibition of Jak2/STAT3 signaling allowed IMCs to differentiate towards DCs. However, the larger differences were observed when we evaluated the level of expression of MHC class II and CD86 within the gated population of CD11c+ cells. Treatment with JSI-124 dramatically increased the expression of these molecules (Fig. 1D). This suggested that JSI-124 might not only promote DC differentiation but also activate DCs.
Inhibition of Jak2/STAT3 signaling activates DCs
To directly evaluate this possibility DCs were obtained by isolation of CD11c+ cells after 5-day culture of HPC with GM-CSF and IL-4 in the presence of 3T3 or CT26 CM. These DCs were then cultured with medium alone, 3T3 or CT26 CM for an additional 4 days in the presence of JSI-124 or DMSO. JSI-124 induced substantial (2-4-fold) up-regulation of the expression of MHC class II, B7-2 (CD86), and CD40 molecules on the surface of DCs (Fig. 2 A,B). Expression of surface markers and the effect of JSI-124 was the same in cells cultured in medium alone and 3T3 CM (data not shown). JSI-124 dramatically up-regulated the ability of DCs to stimulate allogeneic T cells (Fig. 2C) or antigen-specific T cells (Fig. 2D). Inhibition of Jak2/STAT3 pathway in DCs resulted in loss of the ability to take up soluble antigen. DCs generated in the presence of CT26 CM had a significantly higher level of FITC-dextran uptake than those generated in the presence of 3T3 CM, which reflects their immature state. JSI-124 dramatically down-regulated FITC-dextran uptake in both cases (Fig. 2E). Taken together these observations indicate that inhibition of Jak2/STAT3 pathway resulted in activation/maturation of DCs. We evaluated the ability of DCs to induce peptide-specific immune response in vivo. DCs generated from BM precursors were cultured for 4 days with either DMSO or JSI-124 and then pulsed BALB/c mice twice with 10 days interval. Seven days after the second immunization mice were sacrificed and peptide-specific response was measured in IFN-γ ELISPOT assay. Treatment of DCs with JSI-124 substantially increased their ability to induce CD8+ T-cell response (Fig. 2F).
Figure 2.
Jak/STAT3 pathway inhibitor JSI-124 activates DCs. DCs were generated from mice BM cells in the presence of control 3T3 or CT26 CM. CD11c+ DCs were isolated using magnetic beads separation technique. Cells were then treated with 0.5 μM JSI-124 or vc, and cultured again with 3T3 or CT26 CM. On day 4 cells were collected and their phenotype and function was evaluated. The level of surface markers expression was evaluated by flow cytometry. Mean results of three performed experiments (A) and typical example (B) are shown. MFI- mean fluorescence intensity. Dotted line represents CD11c+ DCs cultured for 4 days with DMSO, solid line − treated with JSI-124. Expression of MHC class II (IAd), CD86, and CD40 molecules was evaluated by flow cytometry as described in Materials and Methods. Cells were also used as stimulators of allogeneic T cells in MLR (C) and syngenic T cells in the presence of HA peptide (D). Two experiments with the same results were performed. (E) - DC ability to process antigen was evaluated by uptake of FITC-Dextran. MFI- mean fluorescence intensity. The level of FITC-dextran uptake at 4°C was subtracted from uptake at 37°C. Mean results of three performed experiments are shown. (F). DCs were generated from bone marrow HPC using GM-CSF and IL-4. CD11c+ cells were isolated and treated for 4 days with either 0.5 μM JSI-124 or DMSO (vc). After that time DCs were pulsed with 10 μg/ml of H2Kb matched peptide (IYSTVASSL). Naïve BALB/c mice were immunized s.c. with 2×105 DCs. Immunization was repeated once 10 days later. Seven days after the second immunization mice were sacrificed, splenocytes isolated and stimulated with either control (C.P.) or specific peptide (S.P.). The number of IFN-γ producing cells was measured in ELISPOT assay. Each group included 3 mice and each experiment was performed in quadruplicates. Naïve − non-immunized mice. Mean ± St. Dev. are shown. (G, H) Human DCs were generated in vitro from peripheral blood MNC with GM-CSF and IL-4 and purified by Metrizamide gradient centrifugation. Cells were then treated with 0.5 μM JSI-124 or DMSO (vc) for 5 days. (G) After that DCs were collected and labeled with PE-conjugated lineage cocktail antibody (Anti-CD3, CD14, CD19, CD56), PerCP-conjugated anti-HLA-DR antibody, and FITC-conjugted anti-CD86, CD40, CD83 antibodies. Cells were analyzed by flow cytometry (FACSCalibur). Fluorescence intensity was measured within the population of lin- HLA-DR+ DCs. MFI- mean fluorescence intensity. (H) DCs treated with JSI-124 or DMSO (vc) were mixed in triplicates in U-bottom 96-well plates at different ratios with 105 MNC from a different donor. Cells were incubated for 5 days. 3H-Thymidine was added 18 hr before cell harvesting. cpm − count per minute. Mean results of two experiments are shown. Spontaneous proliferation of MNC was less than 3,000 CPM.
The presence of endotoxin in the JSI-124 preparation could activate DCs and thus affect the interpretation of the results. JSI-124 is a pure natural product and not a bacterial product; therefore its contamination by endotoxins is highly unlikely. We tested the level of endotoxin contamination directly. In Limulus amebocyte lysate (LAL) test (CapeCode, InC, East Falmouth, MA) undiluted 0.5 μM solution of JSI-124 showed endotoxin activity <0.03 EU/ml (detection limit).
Next we asked whether the inhibition of Jak2/STAT3 signaling had effect on human DCs. Mononuclear cells were obtained from peripheral blood of healthy volunteers and DCs were generated using GM-CSF and IL-4 as previously described (14). DCs were treated with 0.5 μM JSI-124 or DMSO for 5 days. Cells were collected and their phenotype and function were analyzed. DCs were defined as lineage cocktail negative (CD3, CD14, CD19, CD56) and HLADR positive cells. JSI-124 induced a significant increase in the expression of CD40 and CD83 molecules on DC surface, but an especially dramatic increase was observed in the expression of CD86 (Fig. 2G). DCs treated with JSI-124 had considerably higher ability to stimulate allogeneic T cells than control cells (Fig. 2H). These data indicate that JSI-124 exerts similar effects on DCs in humans as it does in mice.
Activated DCs exert their effect on T cells via direct cell-cell contact
Previous studies have demonstrated that inhibition of STAT3 in macrophages and tumor cells with dominant negative forms of STAT3 or conditional knockout results in increased production of different cytokines, most prominently RANTES (6, 15). In this study we measured the level of RANTES and IL-12 (p70), the cytokine critically important for DC function. JSI-124 increased RANTES production by DCs more than 5-fold (Fig. 3A). However, inhibition of Jak2/STAT3 signaling in DC did not affect IL-12 production. It remained below detectable levels in all performed experiments (data not shown). We asked whether increased functional activity of JSI-124 treated DCs was due to soluble factors released by these cells. CD11c+ DCs generated from BM cells of BALB/c mice were treated with JSI-124 or DMSO for 4 days. Splenocytes from syngeneic BALB/c mice were placed in the bottom chamber of 96-well Transwell plates and stimulated with 0.5 μg/ml anti-CD3 antibody. DCs were added either directly into the bottom chamber or in the top chamber. JSI-124 treated DC increased anti-CD3 induced T-cell proliferation more than 6-fold (Fig. 3B). However, this effect was observed only when DCs were placed in direct contact with T cells but not in the top chamber of the Transwell plates. To make sure that our data are accurate we used different assay (BrdU pulse-labeling of responder cells) and different experimental system with antigen-specific T cells. LN cells isolated from HAtransgenic mice were placed in the bottom chamber of 96-wells. DCs were added either directly into the bottom chamber or the upper chamber. LN cells were stimulated with HA-derived specific peptide and T-cell proliferation was evaluated 4 days later. JSI-124 treated DCs significantly increased antigen-specific T-cell response. This augmentation was observed only when DCs were added directly to the LN cells but not when DCs were separated by semi-permeable membrane (Fig. 3C). This indicates that direct cell-cell contact was required for JSI-124 treated DCs to exert their effect on T-cell activation.
Figure 3.
Activated by JSI-124 DCs exert their effect via direct cell-cell contact. (A) RANTES concentration was measured in triplicates in supernatants obtained after 48 hr treatment of CD11c+ DC with JSI-124 or VC as described in Material and Methods. (B) Splenocytes were isolated from syngeneic BALB/c mice and 2×105 cells were placed in the bottom chamber of U-bottom 96-well Transwell plates. Cells were stimulated with 0.5 μg/ml anti-CD3 antibody. DCs treated with DMSO (vc) or JSI-124 (JSI) were added either to the bottom chamber (2×103 cells per well) (mix) or to upper chamber (4×103 cells per well) (transwell). 3H-thymidine was added 18 hr prior to the cell harvest and its incorporation was measured on scintillation counter as described in Material and Methods. All experiments were performed in triplicates and repeated at least once to assure reproducibility of the results. (C) LN cells were isolated from HA-TCR transgenic mice and were placed into the bottom chamber of Transell wells (105 cells per well). Cells were stimulated with 12.5 μg/ml specific HA-derived peptide. DCs were placed into the bottom chamber (103 per well) or into the upper chamber (2×103) of the Transwell. After 4-day culture upper chamber was removed and cells were were pulsed for 1 hr with BrdU. The proportion of CD3+ T cells in S phase of cell cycle was evaluated by flow cytometry. Each experiment was performed in triplicates. Two experiments with the same results were performed.
Mechanism of MHC class II up-regulation on DCs by Jak2/STAT3 inhibitor
As we demonstrated above DC activation induced by JSI-124 manifested in a dramatic up-regulation of MHC class II on the cell surface. There are two potential mechanisms of this effect: inhibition of Jak2/STAT3 could result in increased synthesis of MHC class II molecules or MHC class II molecules could be transferred from the cytoplasm to the cell surface. To address this question CD11c+DCs were generated from BM cells and treated with 0.5 μM JSI-124 or DMSO. Total RNA was extracted from cells cultured for 24 and 48 hrs followed by RT-PCR with primers specific for MHC class II transactivator (CIITA) promoter I and III. Two-day treatment of DCs with JSI-124 completely blocked CIITA promoter I expression. As expected CIITA promoter III expression was barely detected in myeloid DCs (Fig. 4A). These data were confirmed by the analysis of MHC class II expression in Western blotting with anti-I-A/I-E antibodies (Fig. 4B) and indicated that JSI-124 not only did not stimulate MHC class II transcription and synthesis but in fact blocked MHC class II transactivator, which eventually results in shutting down MHC class II transcription. Experiments with intracellular staining of DCs with MHC class II antibody showed that JSI-124 induced translocation of MHC class II molecules from intracellular compartments to the cell surface (Fig. 4C).
Figure 4.
JSI-124 induces translocation of MHC class II to the surface of DCs. Murine BM HPCs were cultured with GM-CSF and IL-4 to generate DCs. After 5-day culture CD11c+ cells were isolated using magnetic beads separation technique and were then treated with 0.5 μM JSI-124 or DMSO (vc). (A) Total RNA was isolated from cells cultured for 24 and 48 hrs followed by RT-PCR as described in Material and Methods. (B) Cells were collected after 48 hrs in culture and Western blotting was performed using I-A/I-E antibody as described in Materials and Methods. (C) After 48 hrs in culture, cells were collected to cytospin slides. Slides were fixed, and stained with either isotype control IgG (control) or biotinylated anti-I-A/I-E antibody followed by streptavidin-FITC and slides were analyzed by confocal microscopy (x1000).
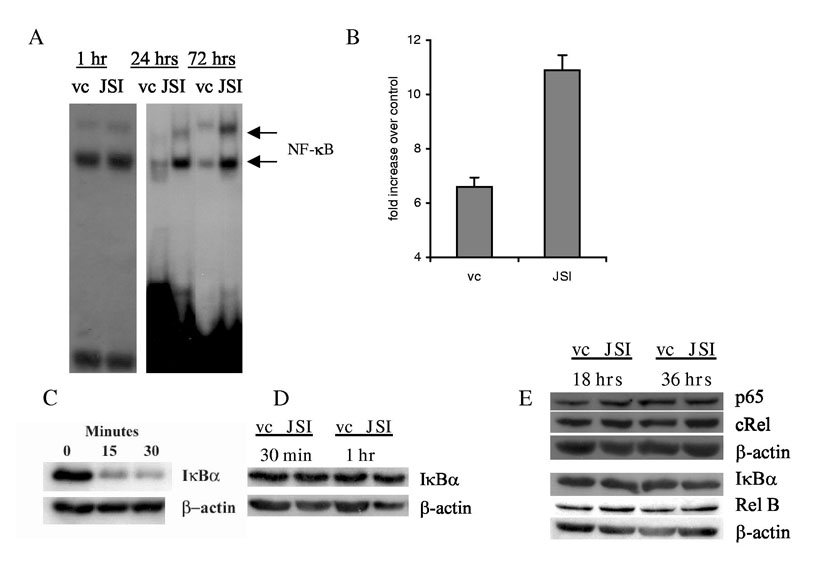
Transcription factor NF-κB is one of the major factors responsible for DC maturation. In our experiments JSI-124 induced activation of NF-κB, as measured by DNA binding in an EMSA assay (Fig. 5A) and transcriptional activity of reporter gene (Fig. 5B). Importantly, strong activation became evident only 72 hr after the start of the treatment (Fig. 5A). The main conventional pathway of NF-κB activation is the phosphorylation and degradation of IkBα. Figure 5C illustrates the effect of LPS on IκBα degradation in DCs. However, JSI-124 did not affect IκBα (Fig. 5D). It also did not affect the expression of NF-κB subunits (Fig. 5E). Thus, it appears that JSI-124 did not activate NF-κB via IκBα degradation or increased synthesis of NF-κB subunits.
Figure 5.
Inhibition of Jak2/STAT3 signaling activate NF-κB in DCs. CD11c+DCs were generated as described in Fig. 4 and treated with either JSI-124 or DMSO (vc). (A) DCs were collected at time point indicated and EMSA with NF-κB specific probe was performed. (B) CD11c+ cells were co-transfected with NF-κB or control plasmid and plasmid carrying Renilla luciferase gene, and then treated with JSI-124 or DMSO (vc) for 36 hrs. Values of luciferase activity (LA) for specific and control plasmid were normalized to Renilla LA. Data presented is the fold increase of LA in cells transfected with plasmid containing NF-κB−responsive element over the LA in cells transfected with control plasmid. Mean results of three performed experiments are shown. (C) DCs were treated with 5 μg/ml LPS for 15-30 min. The level of IkBα was evaluated by Western blotting as described in Material and Methods; (D) - Level of IκBα in DCs after 30 min and 60 min of treatment with JSI-124; (E) - DCs were treated with JSI-124 or DMSO (vc) for 18 hr and 36 hr, whole cell lysates were prepared and the levels of different NF-κB subunits and IκBα was determined in Western blotting.
Mechanism of NF-κB activation by Jak2/STAT3 inhibition
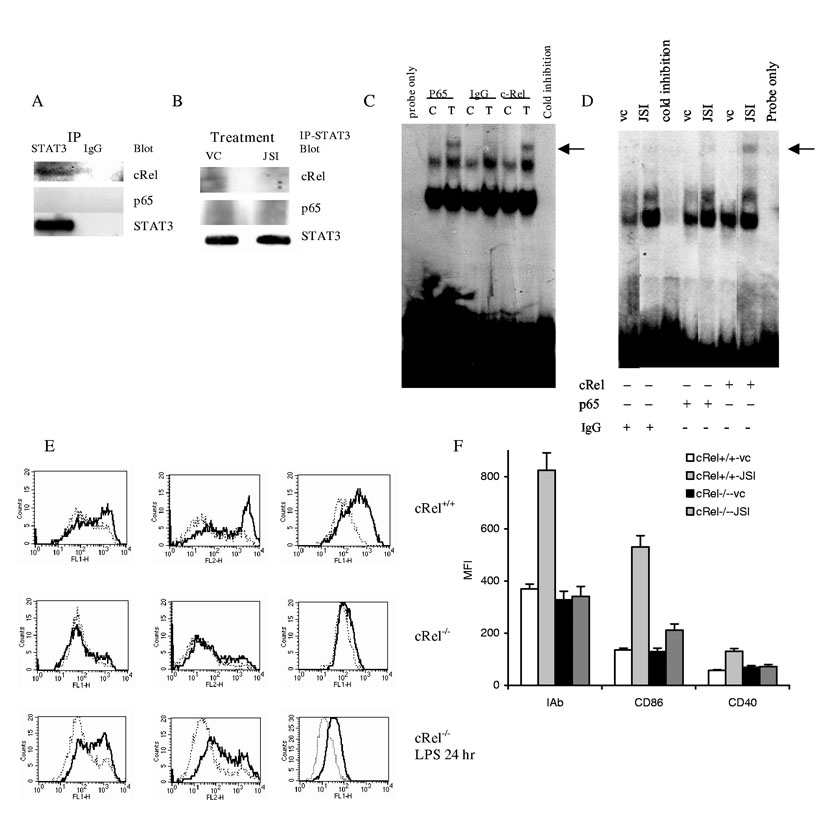
One of the possible mechanisms that may regulate NF-κB activity was suggested recently. STAT3 was shown to bind p65 in the cytoplasm and thus, prevent its translocation to nuclei and DNA binding (16). We tested the possibility of physical interaction between STAT3 and NF-κB subunits in DCs and the effect of JSI-124 on this process. DCs were generated in the presence of tumor-derived factors as described above and then were stimulated withknown activator of NF-κB TNFα. STAT3 were pulled-down and membranes were then probed with antibodies against different members of NF-κB family. We could detect co-precipitation of STAT3 with cRel but not with p65 (Fig. 6A) or RelB (data not shown). Next, DCs were treated instead of TNFα with DMSO or JSI-124. Treatment of DCs with JSI-124 completely eliminated the presence of cRel from the STAT3 complex (Fig. 6B). These experiments although suggestive demonstrated rather low level of cRel protein co-precipitated with STAT3. To independently verify these data we performed a supershift EMSA with antibodies against-p65 and c-Rel. Consistent with previous data stimulation of DCs with TNFα resulted in nuclear translocation of both p65 and cRel subunits of NF-κB (Fig. 6C). These subunits were absent in unstimulated DCs treated with DMSO (Fig. 6D). However, treatment of DCs with JSI-124 induced marked increase in DNA binding of c-Rel and very little of p65 (Fig. 6D). These data further suggest that STAT3 may interact with cRel in DCs and therefore inhibition of STAT3 phosphorylation by JSI-124 may prevent the formation of such complexes.
Figure 6.
Mechanism of JSI-124 induced activation of NF-κB in DCs. (A) CD11c± DCs were generated as described above and cells were stimulated with 20 ng/ml TNFα for 40 min. Immunoprecipitation was performed with anti-STAT3 antibody or control rabbit IgG as described in Material and Methods. Membranes were then probed with antibodies against cRel and STAT3. (B) CD11c± DCs treated with JSI-124 or DMSO (VC) for 36 hrs were collected, cell lysates were prepared. STAT3 was precipitated using anti-STAT3 antibody. Membranes were then probed with antibodies against cRel, p65, and STAT3. (C) CD11c±DCs were generated as described in Fig. 4 and treated for 20 min with 20 ng/ml TNFα (T). In control (C) cells were cultured in medium alone. Nuclear extracts were prepared and EMSA was performed with NF-κB specific probe in the presence of 5 μg of rabbit IgG or rabbit polyclonal antibodies against p65 or cRel (Santa Cruz). Arrow points on the place of supershift. Cold inhibition − EMSA performed in the presence of x100 excess of unlabeled probe. Probe only − EMSA performed without nucleoproteins. (D). CD11c±DCs were treated with either JSI-124 or DMSO (VC) for 48 hr. Nuclear extracts were prepared and EMSA was performed as described above. Arrow points on the place of supershift. Cold inhibition − EMSA performed in the presence of x100 excess of unlabeled probe. Probe only − EMSA performed without nucleoproteins. (E, F) DCs were generated from BM HPCs obtained from cRel-/- or control cRel+/+ mice. CD11c+ cells were isolated using magnetic beads separation technique and treated with 0.5 μM JSI-124 (solid line) or VC (dotted line) for 5 days. Cells then were collected and expression of MHC class II and co-stimulatory molecules was analyzed by flow cytometry. Typical example (E) and cumulative results (F) of three performed experiments are shown. In the bottom row labeled “cRel-/- LPS 24 hr”, DCs generated from cRel-/- HPCs were isolated using CD11c marker and were immediately activated for 24 hr with 5 μg/ml LPS. In that case solid line represent cells treated with LPS.
To test the functional significance of this hypothesis we investigated the effect of JSI-124 on DCs isolated from cRel deficient mice. These mice do not have a defect in DC production and DCs generated from these mice have normal response to LPS (17). In our experiments CD11c+DCs were generated from BM HPCs and then were treated with JSI-124 or DMSO for 5 days. No significant differences in the level of the expression of MHC class II, B7-2, or CD40 were observed in DMSO treated DCs obtained from cRel-/- or cRel+/+ mice. In cRel+/+ mice JSI-124 induced a significant increase in the expression of MHC class II and CD86 and slightly lower but still significant increase in the expression of CD40. However, JSI-124 was not able to increase the expression of MHC class II, CD86, or CD40 in cRel deficient DCs (Fig. 6 E, F). In a separate set of experiments we have confirmed that DCs isolated from cRel-/- mice indeed respond to 24 hr treatment with 5 μg/ml LPS (Fig. 6E). These data suggest that STAT3 may regulate NF-κB activation in DCs via direct binding with at least one NF-κB subunit. Furthermore, the experiments demonstrate that the ability of JSI-124 to induce DC maturation requires a functional cRel.
Discussion
Here we report that Jak2/STAT3 may play a role as a negative regulator of DC activation under normal conditions and cancer. Previous studies have demonstrated a possible critical role of Jak2/STAT3 hyperactivation in abnormalities observed in DC differentiation in cancer (6, 7). Therefore, inhibition of Jak/STAT3 pathway may be an attractive therapeutic approach to improve the differentiation and function of DCs in cancer. To test this hypothesis we used JSI-124, a recently discovered selective JAK/STAT3 signaling pathway inhibitor with potent antitumor activity against human tumors in immune deficient as well as immune competent mouse models (10). JSI-124 (cucurbitacin I) is a member of the cucurbitacin family of compounds that are isolated from various plant families such as the Cucurbitaceae and Cruciferae. Previous study has demonstrated that JSI-124 inhibits the cellular levels of phosphotyrosine-STAT3 and phospho-Jak2, but not phospho-ERK1/2, phospho-JNK, and phospho-Akt (10). Importantly, although JSI-124 is very effective at suppressing the levels of tyrosine-phosphorylated STAT3 and JAK2, it is unable to directly inhibit Src or Jak2 kinase activities in vitro, whereas as AG-490 (known inhibitor of Jak kinases) inhibited kinase activity of both Jak1 and Jak2 (10). Therefore, JSI-124 could be a good candidate to evaluate the role of Jaak2/STAT3 signaling in DCs.
Consistent with previous observations from different groups (rev. in (4)) TDF significantly inhibited generation of DCs from HPCs and increased accumulation of IMCs. JSI-124 substantially reduced the proportion of IMC and increased the presence of DCs. Recent study from Laouar et al.(5) has demonstrated using conditional knockout mice that STAT3 is necessary for normal DC differentiation. In other studies this group reported accumulation of myeloid cells in STAT3-deficient mice (18). We believe that there is no contradiction between our results. In conditional knockout mice STAT3 was targeted on early stages of myeloid cell differentiation. STAT3 activity in early progenitors is critically important for the development of DCs. In our experiments STAT3 inhibitor predominantly targeted population of IMC, which is represented by mixed group of myeloid cells primarily in the late stages of myeloid cell differentiation. It is likely that the effect of STAT3 on myeloid cells depends on the stage of cell development. At present, the molecular mechanisms of the effect of STAT3 inhibition on myeloid cell differentiation are under investigation.
Most strikingly, inhibition of Jak2/STAT3 caused dramatic activation of DCs. This DC activation resulted in significant increase in the DC ability to stimulate antigen-specific T-cell response. These particular findings are not entirely unexpected since similar observations were made previously using conditional knockout STAT3 mice and Jak inhibitor of AG490 (6, 15, 18). However, in this study, we for the first time have clarified the mechanism of this activation. We have demonstrated that enhanced ability of DCs to stimulate T cells was caused not by increased production of cytokines but by direct cell-cell contact apparently due to dramatic increase in the expression of surface molecules including MHC class II and co-stimulatory molecules. There are several potential mechanisms of increased expression of MHC class II on the surface of DCs induced by JSI-124. First, it is possible that JSI-124 treatment results in increased transcription and biosynthesis of MHC class II molecules. The main factor controlling MHC class II genes is expression of transcriptional co-activator CIITA. The gene encoding CIITA (C2ta in mice) is controlled by three independent promoters: pI, pIII, and pIV (19). pI is a myeloid promoter driving CIITA and MHC class II expression in macrophages and DCs, whereas, pIII is primarily lymphoid promoter essential for the direction of MHC class II expression in B cells and plasmacytoid DCs (20). JSI-124 completely inhibited expression of CIITA pI while not affecting CIITA pIII. This is consistent with the classical pathway of myeloid DC differentiation. The second possible mechanism of up-regulation of MHC class II on the surface is translocation of MHC class II molecules from intracellular compartments to cell surface (21, 22). Our experiments demonstrated the presence of MHC class II molecules inside DCs and their migration to the surface after the treatment with JSI-124. Thus, JSI-124 induced DC maturation via classical pathway that involved loss of the ability to take up soluble antigens, shutdown of MHC class II transcription and translocation of these molecules on cell surface.
Since the main mechanism of classical DC maturation involves activation of NF-κB we have investigated the effect of STAT3 inhibition on NF-κB. NF-κB is present as an inactive complex in the cytoplasm of DCs bound to members of the IκB family of inhibitory proteins. Activation of NF-κB involves serine phosphorylation, dissociation, and degradation of IκB, followed by the release and nuclear translocation of NF-κB. This activation can be induced by a variety of stimuli, including LPS, TNF, and CD40L (23). Using DNA binding assay and transcription of luciferase reporter gene we found that JSI-124 indeed activated NF-κB. However, strong NF-κB activation was not observed during first 24 hr of culture. This activation did not involve degradation of IκBα or changes in the amount of NF-κB subunits. This kinetic makes participation of other member of IκB family unlikely. Several recent studies have suggested possible direct link between STAT3 and NF-κB. STAT3 was shown to bind the p65 subunit of NF-κB and inhibit NF-κB activity (16). The potential role of a direct interaction between STAT3 and cRel was shown in a recent study by Hoentjen and colleagues (24). In our experiments we observed weak but clearly detectable association between STAT3 and cRel. Treatment of DCs with JSI-124 eliminated this association suggesting that this mechanism may be involved in the effect of JSI-124. To verify the potential role of such association we used cRel-/- mice. Despite lacking one member of the NF-κB family, these mice had normal development of DCs and they responded normally to LPS stimulation (17). We reasoned that if the interaction of STAT3 with cRel is important for the JSI-124 mediated effects on DC activation, then in the absence of this protein the effect of JSI-124 would be reduced. Our experiments confirmed this hypothesis. Control (cRel+/+) mice responded to JSI-124 by up-regulating MHC class II, B7-2, and CD40, whereas cRel-/- mice did not. This data suggest the potential mechanism for the DC activation following STAT3 inhibition. However, it does not preclude alternative explanation like modification of chromatin by STAT3 suggested in previous studies (24). More studies are needed to clarify the relationship between STAT3 and NF-κB.
Thus, this study has demonstrated that selective inhibition of Jak2/STAT3 pathway with the pharmacological agent JSI-124 significantly activates DCs. Here, we for the first time have clarified the possible mechanism of DC activation induced by Jak2/STAT3 inhibition and suggested that pharmacological inhibition of the Jak2/STAT3 pathway may be potentially useful in cancer immunotherapy. This study was focused on the mechanism of the effects of Jak2/STAT3 inhibition on DCs. Verification of potential usage of this approach in cancer immunotherapy will require more vigorous analysis in vivo.
Glossary
- TDF
tumor-derived factors
- BM
bone marrow
- LN
lymph nodes
- IMC
immature myeloid cells
Footnotes
This work was supported by NIH grants CA 84488 and CA 100062 to DG and 5P01 CA 78038 to SMS. YN was supported by NIH fellowship F32 CA103393. This work has been supported in part by the Flow Cytometry Core and Molecular Imaging Core at H. Lee Moffitt Cancer Center.
References
- 1.Steinman RM. Some interfaces of dendritic cell biology. Apmis. 2003;111:675. doi: 10.1034/j.1600-0463.2003.11107802.x. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka KA. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 4.Gabrilovich D. The mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 5.Laouar Y, Welte T, Fu XY, Flavell RA. STAT3 Is Required for Flt3L-Dependent Dendritic Cell Differentiation. Immunity. 2003;19:903. doi: 10.1016/s1074-7613(03)00332-7. [DOI] [PubMed] [Google Scholar]
- 6.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 7.Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, Gabrilovich D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 8.Rane SG, Reddy ES. Janus kinases:components of multiple signaling pathways. Oncogene. 2000;19:5662. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- 9.Imada K, Leonard WJ. The JAK-STAT pathway. Mol. Immunol. 2000;37:1. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 10.Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (Cucurbitacin I), a Selective Janus Kinase/Signal Transducer and Activator of Transcription 3 Signaling Pathway Inhibitor with Potent Antitumor Activity against Human and Murine Cancer Cells in Mice. Cancer Res. 2003;63:1270. [PubMed] [Google Scholar]
- 11.Cheng P, Zlobin A, Volgina V, Gottipati S, Osborne B, Miele L, Gabrilovich DI. Notch-1 regulates NF-kappa B activity in hematopoietic progenitor cells. J. Immunol. 2001;167:4458. doi: 10.4049/jimmunol.167.8.4458. [DOI] [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Chen HL, Girgis KR, Cunningham T, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nature Medicine. 1996;2:1096. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 13.Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich DI. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441. [PubMed] [Google Scholar]
- 14.Pisarev V, Yu B, Salup R, Sherman S, Altieri DC, Gabrilovich DI. Full-Length Dominant-Negative Survivin for Cancer Immunotherapy. Clin Cancer Res. 2003;15:6523. [PubMed] [Google Scholar]
- 15.Cheng F, Wang HW, Cuenca A, Huang M, Ghansah T, Brayer J, Kerr WG, Takeda K, Akira S, Schoenberger SP, Yu H, Jove R, Sotomayor EM. A critical role for Stat3 signaling in immune tolerance. Immunity. 2003;19:425. doi: 10.1016/s1074-7613(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 16.Yu Z, Zhang W, Kone BC. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor kappaB. Biochem J. 2002;367:97. doi: 10.1042/BJ20020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouaaz F, Arron J, Zheng Y, Choi Y, Beg AA. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity. 2002;16:257. doi: 10.1016/s1074-7613(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 18.Welte T, Zhang SS, Wang T, Zhang Z, Hesslein DG, Yin Z, Kano A, Iwamoto Y, Li E, Craft JE, Bothwell AL, Fikrig E, Koni PA, Flavell RA, Fu XY. STAT3 deletion during hematopoiesis causes Crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci U S A. 2003;100:1879. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu. Rev. Immunol. 2001;19:331. doi: 10.1146/annurev.immunol.19.1.331. [DOI] [PubMed] [Google Scholar]
- 20.LeibundGut-Landmann S, Waldburger JM, Reis e Sousa C, Acha-Orbea H, Reith W. MHC class II expression is differentially regulated in plasmacytoid and conventional dendritic cells. Nat Immunol. 2004;5:899. doi: 10.1038/ni1109. [DOI] [PubMed] [Google Scholar]
- 21.Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman RM, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 22.Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, Mellman I. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288:522. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 23.Liou HC, Baltimore D. Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Current Opinion in Cell Biology. 1993;5:477. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- 24.Hoentjen F, Sartor RB, Ozaki M, Jobin C. STAT3 regulates NF-{kappa}B recruitment to the IL-12p40 promoter in dendritic cells. Blood. 2005;105:689. doi: 10.1182/blood-2004-04-1309. [DOI] [PubMed] [Google Scholar]