Abstract
Hyolaryngeal elevation is essential for airway protection during swallowing and is mainly a reflexive response to oropharyngeal sensory stimulation. Targeted intramuscular electrical stimulation can elevate the resting larynx and, if applied during swallowing, may improve airway protection in dysphagic patients with inadequate hyolaryngeal motion. To be beneficial, patients must synchronize functional electrical stimulation (FES) with their reflexive swallowing and not adapt to FES by reducing the amplitude or duration of their own muscle activity. We evaluated the ability of nine healthy adults to manually synchronize FES with hyolaryngeal muscle activity during discrete swallows, and tested for motor adaptation. Hooked-wire electrodes were placed into the mylo- and thyrohyoid muscles to record electromyographic activity from one side of the neck and deliver monopolar FES for hyolaryngeal elevation to the other side. After performing baseline swallows, volunteers were instructed to trigger FES with a thumb switch in synchrony with their swallows for a series of trials. An experimenter surreptitiously disabled the thumb switch during the final attempt, creating a foil. From the outset, volunteers synchronized FES with the onset of swallow-related thyrohyoid activity (~225 ms after mylohyoid activity onset), preserving the normal sequence of muscle activation. A comparison between average baseline and foil swallows failed to show significant adaptive changes in the amplitude, duration, or relative timing of activity for either muscle, indicating that the central pattern generator for hyolaryngeal elevation is immutable with short term stimulation that augments laryngeal elevation during the reflexive, pharyngeal phase of swallowing.
INTRODUCTION
Anterior and superior hyolaryngeal movements are important for protecting the airway during the pharyngeal phase of swallowing. This phase begins with submental muscle activity, which raises the hyoid bone followed by pharyngeal and infrahyoid muscle activity to raise the larynx (Doty and Bosma 1956). Hyolaryngeal elevation shapes the pyriform sinuses and contributes to epiglottal inversion, which helps close the laryngeal vestibule and divert the bolus around the larynx (Logemann et al. 1992). The patterns of muscle activity during the pharyngeal phase of swallowing are bilaterally programmed in the medulla (Jean 1972) and can be reflexively triggered by sensory stimulation of the glossopharyngeal and superior laryngeal nerves when a bolus enters the pharynx (Ertekin et al. 2001). The medullary swallowing center also receives both direct and indirect signals from the cortex (Car et al. 1975; Jean et al. 1975; Martin et al. 1999).
After a cerebrovascular accident, many patients have a swallowing disorder and are at risk of developing aspiration pneumonia, although 87% regain functional swallowing within 6 mo (Huckabee and Cannito 1999; Mann et al. 1999). Reduced and delayed hyolaryngeal elevation are the most common causes of laryngeal penetration or aspiration during swallowing (Lundy et al. 1999; Sundgren et al. 1993). Treatment for such patients includes strengthening exercises and diet or posture modifications to prevent aspiration. Recently, electrical neuromuscular stimulation has received attention, although its efficacy has not yet been determined. Some have applied electrical stimulation to the mucosa on the palate to increase afferent input to the medullary swallowing center (Park et al. 1997), whereas others propose applying electrical stimulation to the neck surface in the hope of aiding laryngeal elevation (Freed et al. 2001; Leelamanit et al. 2002).
Once the early recovery period has ended, there are no proven treatments for patients who continue to be unable to swallow safely, and such patients must rely on non-oral feeding methods for adequate nutrition and hydration (Ha and Hauge 2003; Langmore et al. 1998). To develop a treatment alternative for these patients, we aim to determine the feasibility of using intramuscular electrical stimulation as an assistive device. By placing electrodes intramuscularly, individual muscles can be stimulated to achieve specific movements. Intramuscular stimulation of the mylo- and thyrohyoid muscles at rest can raise the larynx ~50% of the distance it elevates during 2-ml water swallows (Burnett et al. 2003). If applied at the appropriate moment during swallowing, neuromuscular stimulation could potentially augment a patient’s reflexively produced laryngeal elevation.
The mylo- and thyrohyoid muscles are of particular interest for hyolaryngeal elevation. The mylohyoid suspends the hyoid bone from the mandible, and its activation pulls the hyoid bone superiorly. The thyrohyoid inserts into the hyoid and thyroid cartilages and on activation will reduce the distance between these structures to either lower the hyoid bone or raise the larynx. Co-contraction of the mylohyoid and thyrohyoid muscles helps produce laryngeal elevation in humans; however, the specific patterns of muscle activity may vary across individuals (Gay et al. 1994; Holzer and Ludlow 1996; Schultz et al. 1994).
A crucial prerequisite of a functional electrical stimulation (FES) system for swallowing is a means of triggering hyolaryngeal muscle stimulation that is reliably active just before laryngeal elevation is needed but is otherwise silent. Although Leelamanit et al. (2002) demonstrated that surface electrodes in the submandibular region could detect the initiation of swallowing with ~80% accuracy, these muscles are also active during the oral-preparatory phase of deglutition, making selective activation to trigger FES unlikely. Furthermore, if a patient’s own submandibular muscle activity is delayed because of impaired central control, then FES will also be delayed, and the airway will remain unprotected. A voluntary, manual FES control system could bypass this problem by allowing the user to identify the most appropriate time during swallowing to stimulate hyolaryngeal elevation.
It is unknown whether or not individuals can volitionally synchronize FES with swallowing. Although swallowing may briefly stretch some of the submental and infrahyoid muscles, sensations of swallowing most likely come from afferents in the oropharyngeal mucosa that sense bolus movement and increased pressures due to tongue retraction and pharyngeal constriction. Individuals could either predict or react to such sensations when trying to synchronize FES with swallowing.
Assuming that synchronization of FES and swallowing is possible, it needs to be determined whether or not there is motor adaptation to its facilitative effects. In swallowing, muscle activity and movement timing can be modified by bolus viscosity and volume (Dantas and Dodds 1990; Dantas et al. 1990; Dodds et al. 1988; Kendall et al. 2001; Reimers-Neils et al. 1994), indicating that oropharyngeal sensation affects the central motor control of swallowing. Swallowing muscle activity is also affected by voluntary control (Ertekin and Aydogdu 2003; Ertekin et al. 2001; Kahrilas et al. 1991; Pouderoux and Kahrilas 1995), suggesting that oropharyngeal control during swallowing is adaptive rather than immutable. In voluntary limb movement, sudden unanticipated load removal can cause an acceleration error. If load removal is self-regulated, however, the acceleration error is nearly eliminated because of a decreased antagonist latency and a reduced agonist burst (Diedrichsen et al. 2003; Latash 1994). Such a predictive motor adaptation enables a moving limb to reach a target position smoothly and accurately despite the load perturbation. If intramuscular stimulation during swallowing produced an “unloading” of the muscles active for laryngeal elevation, subjects might decrease activity to prevent unnecessary movement.
In this study, we tested the hypothesis that healthy adults could trigger intramuscular mylo- and thyrohyoid stimulation in synchrony with volitional swallows of 2 ml water. Taking advantage of the symmetric bilateral control exerted at the level of the medulla (Doty et al. 1967), we also evaluated whether or not volitionally triggered hyolaryngeal stimulation in the short-term altered the amplitude, duration, and relative timing of mylohyoid and thyrohyoid activity during swallowing. Because both muscles are hyolaryngeal elevation agonists, we predicted that any central adaptation to FES would decrease either the duration or amplitude of activity. Finally, the measures obtained in this study provided an opportunity to describe the timing of mylohyoid and thyrohyoid activity relative to one another and whether or not this temporal relation was altered by FES during swallowing.
METHODS
Nine healthy adults (1 female, 8 males) with an average age of 43 yr (range: 28–71) participated in this study. Inclusion criteria were blood pressure and electrocardiogram readings within normal limits, no history of injury or surgery to the head or neck, and normal laryngeal structure and function as determined on flexible nasoendoscopy by a laryngologist (E. A. Mann). All participants provided informed consent, and the National Institute of Neurological Disorders and Stroke Institutional Review Board approved the experimental protocol. All procedures were performed in accordance with the ethical standards of the1964 Declaration of Helsinki.
Participants sat in a reclined position with the head supported. For safety, an electrical grounding pad was adhered to the back. Both respiration and heart rate were monitored; plethysmograph bands were placed around the abdomen and chest (NonInvasive Monitoring Systems, Miami Beach, FL), and a three-lead electrocardiogram system was used.
Small subcutaneous injections of 2% lidocaine HCl anesthetized the skin in the regions of the thyro- and mylohyoid muscles bilaterally prior to electrode insertion. A 27-gauge monopolar stimulating electrode (TECA, Oxford Instruments, Hawthorn, NY) was inserted in the desired muscle region, and a reference electrode was placed on the neck. A train of biphasic stimulating pulses (200-μs pulse width, 0.5–to 6.0-mA amplitude, 30-Hz frequency) was delivered using a Nicolet Viking IV system under experimenter control to induce a muscle contraction.
The optimal location for stimulating each muscle was confirmed physiologically (Burnett et al. 2003). Specifically, mylohyoid placement was confirmed when stimulation retracted the submental tissue and moved the prominence of the thyroid cartilage rostrally. Thyrohyoid placement was identified when stimulation caused the thyroid prominence to move rostrally and twist slightly contralaterally. Once an insertion site was located, the monopolar needle was removed, and 0.0002-in-diam bipolar hooked-wire electrodes were inserted in its place using a 27-gauge hypodermic needle as a carrier. Because hooked wire electrodes were inserted within the muscle being stimulated, current was delivered to nerve endings closest to the electrodes. If there was current spread, it could have activated nerve endings in underlying or overlying muscles on the same side but not muscles on the opposite side of the neck. We were able to observe the effects of unilateral muscle stimulation from the neck and movement occurred only on the side being stimulated (Burnett et al. 2003).
Placement of each hooked-wire electrode pair was verified using the same physiological criteria described in the preceding text, and stimulation amplitude was gradually increased in 0.5-mA steps to the highest level comfortably tolerated (maximum = 6 mA). Once the hooked wire electrodes were in place, the carrier needle was removed.
A piezoelectric movement sensor (Siemens) was taped to the skin overlying the thyroid prominence, providing a voltage change as a result of movement of the thyroid prominence. This piezoelectric signal was amplified through one channel of the Nicolet Viking system and was used during data analysis to identify when laryngeal movement began during swallowing (Ertekin et al. 2001; Holzer and Ludlow 1996). External video recordings were collected from seven of the males for an adjunct investigation of the effect of stimulation on thyroid cartilage position (Burnett et al. 2003).
Because thyrohyoid muscle stimulation alone could lower the hyoid and possibly reduce airway protection during swallowing, it was deemed important to ensure that some suprahyoid muscles were also active during thyrohyoid stimulation. Therefore FES was always delivered to a mylohyoid muscle in unison with a thyrohyoid muscle. The muscles selected were those that produced the greatest observable movement or, if no difference was observed, to an ipsilateral muscle pair. Ipsilateral pairs were selected in eight volunteers. For neuromuscular stimulation, the bipolar wires in the muscle of interest were both connected to the positively charged stimulator anode, and the surface reference electrode was connected to the cathode. Bipolar hooked wire electrodes in the mylo- and thyrohyoid muscles not selected for stimulation were used to record swallow-related EMG activity. EMG recordings were acquired by connecting each wire to separate poles of an amplifier channel of the Nicolet Viking system. Volunteers controlled the onset of stimulation by pressing a thumb switch held in their dominant hand. When pressed, the thumb switch produced a 5-V transistor-transistor logic (TTL) pulse that was recorded along with the EMG and piezoelectric sensor signals on a Powerlab A to D system (AD Instruments). A locally constructed control box was used to set muscle stimulation duration to either 1 or 2 s and to enable the circuitry of the thumb switch. This provided a safeguard from unintentional stimulation and a means to covertly disable the switch for a foil stimulation condition. Stimulation duration was at 1 s for all but volunteer M4, for whom it was 2 s. When triggered, the Nicolet system delivered a pulse train at the predetermined amplitude and duration to the electrodes and a TTL pulse to the recording devices. A circuit delay between the thumb switch press onset and the first stimulating pulse to the muscles averaged 47 ± 8 ms. The variability was due to the time interval between pulses when stimulating at 30 Hz.
Adaptation paradigm
All EMG recordings were made during discrete volitional swallows of 2 ml water delivered via a syringe into one corner of the volunteer’s mouth. Seating position remained semi-reclined. The first three trials were baseline swallows without any FES. After three baseline swallows, volunteers were handed the thumb switch and instructed to press it at whatever moment best coordinated the stimulation with their swallow onset (practice swallows). Movements were self-paced, with no external “go” cue given other than to occasionally tell volunteers to “go ahead” after water was placed in the mouth. No external biofeedback or error correction was provided. Recall that the volunteer was already familiar with the sensation of simultaneous mylohyoid and thyrohyoid stimulation delivered at rest by the experimenter. From 9 to 13 practice swallows with FES were attempted. Only rarely did a volunteer interrupt this sequence with a spontaneous dry swallow and no button press. The final trial in the practice sequence was a foil, in which the volunteer pressed the thumb switch but did not receive FES because the experimenter did not enable the switch, unknown to the volunteer.
Analysis
The Nicolet amplifier band-pass filtered the EMG and piezoelectric signals between 30 and 5,000 Hz. The resulting EMG signals and a 500-μV square wave calibration signal from the Nicolet Viking V were conditioned with a 1-kHz low-pass filter and digitized at 2 kHz using a Powerlab A–D system (AD Instruments). The piezoelectric signal was subsequently low-pass filtered at 200 Hz.
The onset times of TTL pulses representing the thumb switch press and the stimulation pulse train were automatically marked using analysis software (Chart, AD Instruments). Laryngeal elevation onset associated with swallowing was identified by a prominent rapid variation in the piezoelectric signal (Fig. 1). Because muscle contraction due to FES could have affected the piezoelectric signal, the onset of laryngeal elevation was not measured during FES.
FIG. 1.
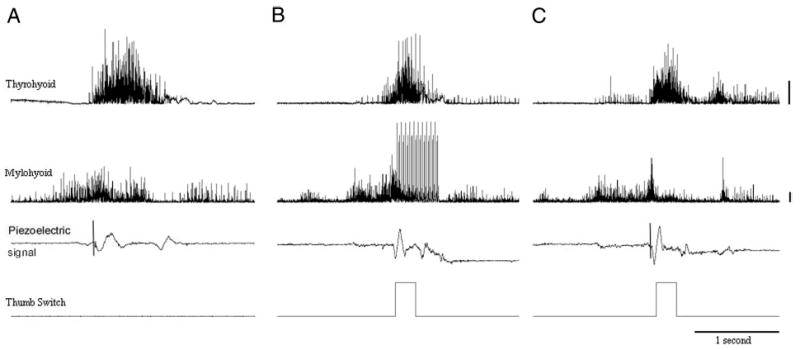
An example of electromyographic (EMG) recordings of the thyrohyoid and mylohyoid muscles contralateral to the side of stimulation, piezoelectric laryngeal motion signals, and TTL pulses showing the time of stimulation onset from 1 volunteer during a baseline swallow (no stimulation, A), synchronization swallow during which the volunteer attempted to trigger functional electrical stimulation onset in time with a discrete swallow (B), and foil trial during which the volunteer anticipated stimulation but it was surreptitiously withheld by the experimenter (C). Additional bursts in trial iii after offset are not typical and may represent the volunteer’s reaction to a lack of stimulation during the trial. Horizontal lines represent 500 μV on the electromyographic signals. The horizontal lines on the piezoelectric signal (i and iii) shows the onset of laryngeal movement.
Swallow-related EMG recordings were screened for movement artifact, then full-wave rectified. The activity onset times were marked using a threshold-crossing algorithm (criterion of 100% over baseline) with visual verification by the experimenter. In baseline and foil swallows, end times were similarly marked and durations calculated. The durations for the three baseline swallows were averaged to compute the mean baseline duration for each muscle in each volunteer.
Mylo- and thyrohyoid EMG signals from baseline and foil swallows were then smoothed using a 15-ms triangular (Bartlett) window. To calibrate the EMG signals recorded in Powerlab, the amplitude of the 500-μV square wave was measured and divided by 0.0005 to compute the gain of the recording system amplifiers for each muscle in each subject. The amplitude measures of each muscle were then divided by the gain for that muscle and the result multiplied by 106 to determine the absolute amplitude in microvolts. The mean amplitudes of mylohyoid and thyrohyoid activation (in microvolts) were calculated by computing the area under the curve minus the minimum value to remove baseline activity levels and then dividing by the duration. Artifact from the stimulation pulses prevented measurements of EMG amplitude or peak time during the FES practice swallows. Within each volunteer, amplitude measures in the three baseline swallows were averaged to compute the mean baseline amplitude for each muscle.
Statistical analysis
Our first hypothesis was that healthy adults could accurately synchronize FES with reflexively triggered muscle activity during discrete volitional swallows of 2 ml water. To test this hypothesis, we first subtracted FES onset time from muscle activation onset time to calculate the mean muscle-to-FES onset interval for each muscle in each volunteer. Using these mean interval values, we conducted a two-tailed, one-sample t-test for both the mylo- and thyrohyoid to test whether or not the muscle-to-FES onset intervals differed from zero. A Bonferroni corrected P value of 0.025 (to correct for multiple comparisons, P = 0.05/2 = 0.025) was required for significance. Repeated ANOVAs were used to compare the intervals between muscle onset and FES onset over the seven practice trials for the mylo- and thyrohyoid. These analyses tested for possible linear and quadratic trends in the intervals over the practice trials for each muscle. A two-way ANOVA was done to test for both muscle and subject effects as well as their interactions on the intervals between FES and muscle onsets. Scheffé post hoc testing determined if there were subject differences in the intervals.
Our second hypothesis was that the central control of laryngeal elevation during swallowing would rapidly adapt to the facilitative contribution of FES on mylo- and thyrohyoid muscle activity during swallowing. Amplitude, duration, and mylohyoid-to-thyrohyoid onset interval measures from the three baseline swallows were averaged for each volunteer. A paired t-test was performed comparing the muscle onset interval durations between the average baseline and foil swallows. To determine if the average mylohyoid-to-thyrohyoid muscle onset interval for each volunteer during the practice trials differed from zero, a one-sample t-test was performed. Two repeated-measures ANOVAs (baseline vs. foil trials) were conducted (1 for duration, the other for amplitude) with muscle treated as an independent factor to test for an interaction between muscle type and FES effects. The hypothesized effect in each test was unidirectional; however, the performance of two comparisons necessitated a Bonferroni correction yielding an alpha level of 0.05 to correct for multiple comparisons. An ANOVA tested for subject and subject-by-muscle interaction effects on the muscle to FES onset interval. Finally, an ANOVA compared the SDs of volunteer’s FES-muscle intervals to determine if some volunteers were more consistent than others at synchronization.
To determine how the onsets of mylo- and thyrohyoid muscles related to one another we conducted paired t-test contrasting volunteers’ mean onset times for mylo- and thyrohyoid muscle activity during the baseline swallows. We also used t-test to determine whether the intervals between muscle activation onset and laryngeal movement onset differed from zero for the mylo- and thyrohyoid muscles.
RESULTS
We aimed to record data from three prestimulation swallows, nine stimulated swallows, and one foil swallow for each of the nine volunteers. Figure 1 shows sample data of a baseline (i), practice (ii), and foil (iii) trial in one volunteer. Complete EMG data were obtained from seven of the nine volunteers. For volunteer M7, mylohyoid onset was twice obscured by movement artifact, and for volunteer M5, only seven stimulated swallows could be recorded. The piezoelectric signal was unavailable for measurement in one of the nine volunteers because of technical difficulties.
Power analyses
We used Systat 11 (SYSTAT Software, Richmond, CA) to determine whether a paired t-test with a sample size of nine subjects was adequate for detecting an effect size of between 0.8 and 1.0, which represents about a 30% change in the mean of the baseline EMG amplitudes and durations for both the thyro- and mylohyoid muscles. For a change of 155 ms in the duration of thyrohyoid muscle activity (1 S.D of all 3 baseline trials and an effect size of 0.923), the power estimate was 0.81. For thyrohyoid amplitude changes of 33 μV (an effect size of 0.994), the power was 0.858. For a change of 380 ms in the duration of mylohyoid activity (an effect size of 0.860), the expected power was 0.761, and for an amplitude change of 38 μV (an effect size of 1.0), the power was 0.885. Therefore for each of these analyses the sample size was adequate to test whether or not muscle activation changed with FES.
Muscle activation and laryngeal elevation
In baseline swallows, mylohyoid activation onset occurred earlier than thyrohyoid activation [312 ± 243 (SD) ms; n = 9], a difference that was statistically significant on a paired t-test (t = −3.283, df = 7, P = 0.013). Laryngeal elevation, as determined by piezoelectric signal deflection, began an average of 345 ± 267 ms after the onset of mylohyoid activity (n = 8), and 52 ± 73 ms after the onset of thyrohyoid activity (n = 8). The interval between muscle activation and laryngeal movement onset differed from zero for the mylohyoid muscle (t = 3.661, df = 7, P = 0.008) but not for the thyrohyoid muscle (t = 2.003, df = 7, P = 0.085).
Synchronization of FES with muscle activity
The mean interval between thyrohyoid muscle activity onset and self-triggered FES did not differ from zero (t = −0.623, P = 0.54). Thyrohyoid activity occurred on average 50 ± 237 ms after FES onset (n = 9). In contrast, mylohyoid activity occurred 223 ± 302 ms before FES (n = 9), an interval that approached a significant difference from zero (t = −2.218; P = 0.057). When a single extreme outlier produced by volunteer M6 on synchronization practice trial 7 (when stimulation onset preceded swallowing by 2.5 s) was removed from the data set, thyrohyoid onset followed FES by an average of 17 ± 156 ms (n = 9), and mylohyoid activity onset preceded FES onset by 256 ± 269 ms (n = 9), which brought the difference to statistical significance (t = −2.858; P < 0.025).
Synchronization between muscle activity and FES onset differed significantly by muscle (F = 13.932, df = 1, P < 0.0005) and subject (F = 5.5024.73, df = 8, P < 0.0005) with no muscle-by-subject interaction (F = 0.794 df = 8, P = 0.608). Post hoc Scheffé tests revealed that volunteer M6 triggered FES significantly earlier than did volunteers M2 and M4 (P < 0.05), an average of 608 ms before the onset of thyrohyoid activity or 306 ms before thyrohyoid activity onset if the previously mentioned extreme outlier was omitted (Fig. 2, i and ii).
FIG. 2.
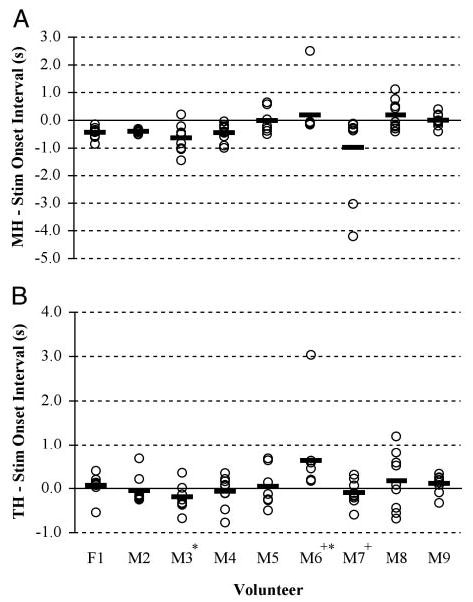
Dot plot of muscle activity onsets relative to FES onset for each volunteer. —, the average onset time for each volunteer. A: synchronization of FES with mylohyoid. B: synchronization with thyrohyoid. Positive values represent muscle activity onsets that occur after FES onset, and negative values represent muscle onsets that occur before FES onset. + and *, volunteers who differed significantly from 1 another.
There was no significant difference between volunteers in the consistency with which they synchronized FES and muscle onset (F = 1.61, df = 8, P = 0.246). We used repeated-measures ANOVAs to examine for linear effects of practice on the intervals between muscle activation and FES onset for the mylohyoid and thyrohyoid muscles. Compound symmetry of the data were demonstrated based on the Huynh-Felt and Greenhouse-Geissner probabilities. Synchronization with muscle onset times did not significantly improve with practice for either the thyrohyoid (F = 1.715, df = 1,8; P = 0.227) or mylohyoid (F = 0.1.455, df = 1, 7; P = 0.267; Fig. 3, i and ii).
FIG. 3.
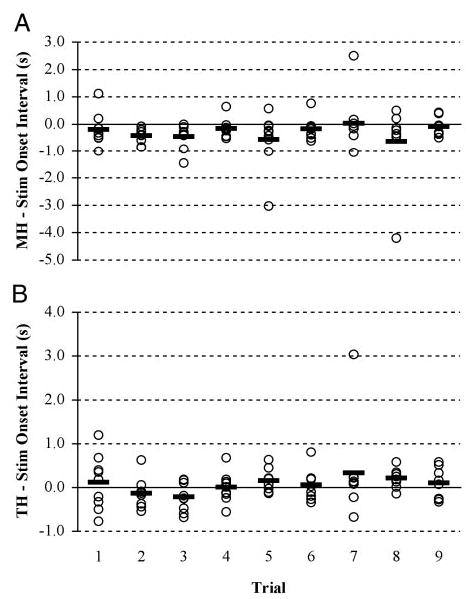
Dot plot of muscle activity onsets relative to FES onset for each trial. —, the volunteers’ average onset time for each trial. A: synchronization of FES with mylohyoid. B: synchronization with thyrohyoid. Positive values represent muscle activity onsets that occur after FES onset, and negative values represent muscle onsets that occur before FES onset.
Effect of FES on muscle activity
The data used for the repeated-measures ANOVA comparing muscle EMG amplitudes and durations between the baseline and foil trials (condition) had compound symmetry based on Huynh-Felt and Greenhouse-Geissner probabilities for condition, muscle, and the interaction of the muscle by condition.
Practice self-triggering paired mylo- and thyrohyoid stimulation during swallowing did not significantly reduce the mean amplitude of volunteers’ muscle activation on the foil trial when compared with the mean amplitude of baseline swallows (F = 0.464; df = 1; P = 0.506), and no significant muscle-by-practice interaction was found (F = 0.568; df = 1; P = 0.452; Fig. 4i). Similarly, practice using FES did not change the duration of muscle activation between the baseline and foil trials (F = 0.402; df = 1; P = 0.535), with no significant muscle-by-practice interaction (F = 0.268; df = 1; P = 0.612; Fig. 4ii). During the foil swallow, the intervals between mylo-and thyrohyoid activity onset did not differ from those during baseline swallows (paired t = 0.636; P = 0.271). In addition, the mylohyoid-to-thyrohyoid onset intervals in the foil trial continued to differ from zero (t = 3.906, P = 0.0095), indicating that repeatedly swallowing with self-triggered synchronous mylo- and thyrohyoid stimulation did not alter the timing of mylo- and thyrohyoid activity onsets (Fig. 5).
FIG. 4.
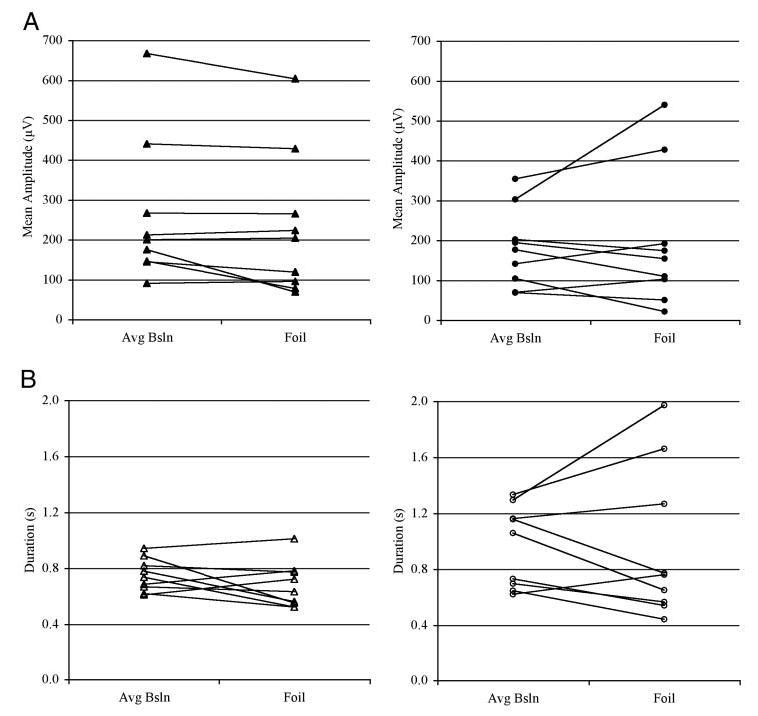
Line plots showing the mean amplitude (A) and duration (B) across average baseline and foil trials for mylohyoid (•) and thyrohyoid (▴) muscles in all volunteers.
FIG. 5.
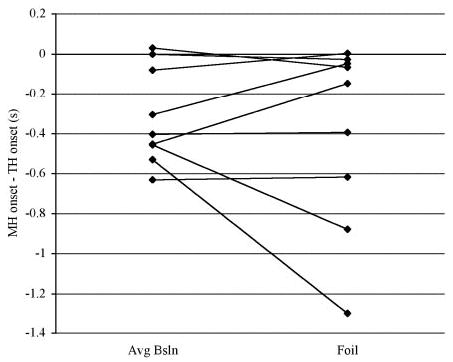
Line plots displaying the interval in seconds between the onset of mylohyoid activity and the onset of thyrohyoid activity for average baseline and foil swallows. Negative values represent trials in which mylohyoid onset preceded thyrohyoid onset.
DISCUSSION
In baseline swallows, mylohyoid activity onset preceded laryngeal elevation by an average of 345 ms, whereas thyro-hyoid onset preceded laryngeal elevation by an average of 52 ms. Further, the onset and offset of thyrohyoid activity mirrored the rise and fall of the larynx (see Fig. 1). These baseline measures indicate that laryngeal movement during swallowing is more closely associated in time with thyrohyoid than with mylohyoid activity.
The first purpose of this investigation was to test the hypothesis that healthy adults could manually trigger FES of the mylo- and thyrohyoid muscles at an appropriate moment during the pharyngeal phase of swallowing. Although volunteers had experienced the kinematic effects of FES during electrode placement and when the stimulation amplitudes were set, they had no prior knowledge of the 47-ms interval between the thumb press and the first stimulation pulse. Nevertheless, the volunteers were immediately able to press the thumb switch so that there was no significant difference between their onset of thyrohyoid activity for swallowing and the onset of FES stimulation of the mylo- and thyrohyoid muscles on the opposite side of the neck.
These healthy adults performed consistently; the interval between FES onset and contralateral mylohyoid or thyrohyoid onsets for swallowing did not decrease over the practice trials. All but one volunteer began the trials with mylohyoid activity followed by the thumb press, FES, and thyrohyoid activity in rapid succession. By triggering FES of a unilateral mylo- and thyrohyoid pair in time with the onset of contralateral thyro-hyoid activity, these volunteers maintained the normal pattern of muscle activation with suprahyoid muscle activity preceding infrahyoid muscle activity. Had they triggered FES in concert with mylohyoid activity, thyrohyoid contraction on the stimulated side of the larynx would have occurred prematurely.
Volunteer M6 differed from the others in that he did not synchronize FES with the onset of thyrohyoid activity. This is the same volunteer who, on practice trial 7, triggered FES ~2.5 s before initiating his swallow. Except during trial 7, he performed consistently, pressing the thumb switch on average 306 ms before thyrohyoid onset, and 126 ms after the onset of mylohyoid activity. Possibly this volunteer was attempting to synchronize the thumb press with an unmeasured swallow event.
It is not clear what strategy was used by the other volunteers to synchronize FES and thyrohyoid activity onset. Typically, self-paced attempts at synchronous voluntary movements feature onsets that are staggered, correcting for differences in central to spinal conduction time (Paillard 1948) as cited by (Devanne and Maton 1998). In this way, sensory feedback signals from each limb reach the cortex at the same moment and movements are perceived as simultaneous. The difference in conduction time between proximal (cranial) and distal (hand) muscles is ~10 ms (Meyer et al. 1990). Therefore if volunteers had synchronized their thumb press with their voluntary transport of the bolus into the oropharynx, then the thumb press would have preceded mylohyoid activity by ~10 ms. Actually the thumb press occurred 239 ms after the onset of mylohyoid activity indicating that volunteers used either predictive or reactive control strategies to initiate FES onset.
Predictive control would have the volunteers attempting to press the button at a time when they expected laryngeal elevation to occur during the reflexive phase of swallowing. The volunteers had extensive experiential knowledge regarding the relation between sensations of oral transport and reflexive laryngeal elevation during swallowing. Because they could sense and control the bolus, they could have predicted the time of onset of laryngeal elevation during the pharyngeal stage of swallowing. Given this, the volunteers could have timed the button press so that FES occurred simultaneous with the predictable timing of laryngeal elevation that occurs simultaneous with thyrohyoid activity.
Another strategy to consider is a reactive one. Rapid manual reaction times are usually ~150 ms on trained reaction-time tasks (Reich et al. 1981) and between 200 and 300 ms on tasks without a time constraint. Volunteers using an oropharyngeal sensation as a go cue for the thumb press could have been delayed in triggering FES by ~200–350 ms. The latency between bolus arrival at the vallecula and the onset of elevation of the aryepiglottic folds (probably co-occurring with the onset of thyrohyoid activity) is 250 ± 290 ms in healthy young individuals and 150 ± 470 ms in healthy individuals over age 65 (Kendall and Leonard 2001). Possibly volunteers used the sensation of the bolus entering the pharynx as a go cue for their onset of volitional thumb press and synchronization resulted because of the delay in their manual reaction times.
However synchronization was accomplished, having manual control allowed users to select the optimal moment to stimulate laryngeal elevation. We predict that a manually triggered hyolaryngeal FES system will be of greatest benefit to chronically dysphagic individuals with inadequate hyolaryngeal elevation but with adequate oral sensory, limb motor, and cognitive function.
The second purpose of this study was to determine if the self-application of stimulated laryngeal elevation during swallowing would lead to an adaptive reduction in the activity of muscle agonists for laryngeal elevation. By comparing muscle activity in the foil swallow to activity in the average of the baseline swallows for each volunteer, we found that repeatedly swallowing with self-triggered stimulated laryngeal elevation did not result in a significant reduction in the amplitude or duration of activity for either the mylo- or thyrohyoid muscle. These findings suggest that the central control of reflexive laryngeal elevation does not limit muscle contraction force, even when it exceeds what is required. Increased muscle contraction due to FES may be permissible because the target of hyolaryngeal elevation during swallowing is airway protection, not a specific endpoint. No “error” occurred by increasing the activity of laryngeal elevation agonist muscles. This absence of any decrease in muscle activity is consistent with previous studies in which the pharyngeal phase of swallowing has been found resistant to adaptation (Barkmeier et al. 2002; Borgstrom and Ekberg 1989).
In conclusion, our results demonstrate that normal volunteers can quickly synchronize a manual trigger with the pharyngeal phase of swallowing and that their muscle activation patterns do not quickly adapt to the augmentation effects of muscle stimulation. It remains to be determined whether such findings pertain to the long-term use of FES in patients with chronic swallowing disorders.
Acknowledgments
The authors thank Dr. Christopher Poletto for technical support of the study. Present addresses: T. Burnett: Department of Speech and Hearing Sciences, 200 S. Jordan Ave., Rm. C133, Indiana University, Bloomington, IN 47405; J. Stoklosa, 5615 Hempstead #202, Pittsburgh, PA 15217; E. A. Mann, Division of Opthalmic and Ear, Nose, and Throat Devices, 9200 Corporate Blvd., Rockville, MD 20850.
Footnotes
GRANTS
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke Grant Z01 NS-02980.
References
- Barkmeier JM, Bielamowicz S, Takeda N, Ludlow CL. Laryngeal activity during upright vs. supine swallowing. J Appl Physiol. 2002;93:740–745. doi: 10.1152/japplphysiol.00380.2001. [DOI] [PubMed] [Google Scholar]
- Borgstrom PS, Ekberg O. Peristalsis in pharyngeal constrictor musculature in relation to positioning and gravity. Acta Radiol. 1989;30:183–185. [PubMed] [Google Scholar]
- Burnett TA, Mann EA, Cornell SA, Ludlow CL. Laryngeal elevation achieved by neuromuscular stimulation at rest. J Appl Physiol. 2003;94:128–134. doi: 10.1152/japplphysiol.00406.2002. [DOI] [PubMed] [Google Scholar]
- Car A, Jean A, Roman C. A pontine primary relay for ascending projections of the superior laryngeal nerve. Exp Brain Res. 1975;22:197–210. doi: 10.1007/BF00237689. [DOI] [PubMed] [Google Scholar]
- Dantas RO, Dodds WJ. Effect of bolus volume and consistency on swallow-induced submental and infrahyoid electromyographic activity. Braz J Med Biol Res. 1990;23:37–44. [PubMed] [Google Scholar]
- Dantas RO, Kern MK, Massey BT, Dodds WJ, Kahrilas PJ, Brasseur JG, Cook IJ, Lang IM. Effect of swallowed bolus variables on oral and pharyngeal phases of swallowing. Am J Physiol Gastrointest Liver Physiol. 1990;258:G675–681. doi: 10.1152/ajpgi.1990.258.5.G675. [DOI] [PubMed] [Google Scholar]
- Devanne H, Maton B. Role of proprioceptive information in the temporal coordination between joints. Exp Brain Res. 1998;119:58–64. doi: 10.1007/s002210050319. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Verstynen T, Hon A, Lehman SL, Ivry RB. Anticipatory adjustments in the unloading task: is an efference copy necessary for learning? Exp Brain Res. 2003;148:272–276. doi: 10.1007/s00221-002-1318-z. [DOI] [PubMed] [Google Scholar]
- Dodds WJ, Man KM, Cook IJ, Kahrilas PJ, Stewart ET, Kern MK. Influence of bolus volume on swallow-induced hyoid movement in normal subjects. Am J Roentgenol. 1988;150:1307–1309. doi: 10.2214/ajr.150.6.1307. [DOI] [PubMed] [Google Scholar]
- Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956;19:44–60. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- Doty RW, Richmond WH, Storey AT. Effect of medullary lesions on coordination of deglutition. Exp Neurol. 1967;17:91–106. doi: 10.1016/0014-4886(67)90125-2. [DOI] [PubMed] [Google Scholar]
- Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol. 2003;114:2226–2244. doi: 10.1016/s1388-2457(03)00237-2. [DOI] [PubMed] [Google Scholar]
- Ertekin C, Kiylioglu N, Tarlaci S, Turman AB, Secil Y, Aydogdu I. Voluntary and reflex influences on the initiation of swallowing reflex in man. Dysphagia. 2001;16:40–47. doi: 10.1007/s004550000041. [DOI] [PubMed] [Google Scholar]
- Freed ML, Freed L, Chatburn RL, Christian M. Electrical stimulation for swallowing disorders caused by stroke. Respir Care. 2001;46:466–474. [PubMed] [Google Scholar]
- Gay T, Rendell JK, Spiro J. Oral and laryngeal muscle coordination during swallowing. Laryngoscope. 1994;104:341–349. doi: 10.1288/00005537-199403000-00017. [DOI] [PubMed] [Google Scholar]
- Ha L, Hauge T. Percutaneous endoscopic gastrostomy (PEG) for enteral nutrition in patients with stroke. Scand J Gastroenterol. 2003;38:962–966. doi: 10.1080/00365520310005190. [DOI] [PubMed] [Google Scholar]
- Holzer SE, Ludlow CL. The swallowing side effects of botulinum toxin type A injection in spasmodic dysphonia. Laryngoscope. 1996;106:86–92. doi: 10.1097/00005537-199601000-00017. [DOI] [PubMed] [Google Scholar]
- Huckabee ML, Cannito MP. Outcomes of swallowing rehabilitation in chronic brainstem dysphagia: A retrospective evaluation. Dysphagia. 1999;14:93–109. doi: 10.1007/PL00009593. [DOI] [PubMed] [Google Scholar]
- Jean A. Localization and activity of medullary swallowing neurons. J Physiol. 1972;64:227–268. [PubMed] [Google Scholar]
- Jean A, Car A, Roman C. Comparison of activity in pontine versus medullary neurones during swallowing. Exp Brain Res. 1975;22:211–220. doi: 10.1007/BF00237690. [DOI] [PubMed] [Google Scholar]
- Kahrilas PJ, Logemann JA, Krugler C, Flanagan E. Volitional augmentation of upper esophageal sphincter opening during swallowing. Am J Physiol Gastrointest Liver Physiol. 1991;260:G450–456. doi: 10.1152/ajpgi.1991.260.3.G450. [DOI] [PubMed] [Google Scholar]
- Kendall KA, Leonard RJ. Bolus transit and airway protection coordination in older dysphagic patients. Laryngoscope. 2001;111:2017–2021. doi: 10.1097/00005537-200111000-00028. [DOI] [PubMed] [Google Scholar]
- Kendall KA, Leonard RJ, McKenzie SW. Accommodation to changes in bolus viscosity in normal deglutition: a videofluoroscopic study. Ann Otol Rhinol Laryngol. 2001;110:1059–1065. doi: 10.1177/000348940111001113. [DOI] [PubMed] [Google Scholar]
- Langmore SE, Terpenning MS, Schork A, Chen Y, Murray JT, Lopatin D, Loesche WJ. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia. 1998;13:69–81. doi: 10.1007/PL00009559. [DOI] [PubMed] [Google Scholar]
- Latash ML. Control of fast elbow movement: a study of electromyographic patterns during movements against unexpectedly decreased inertial load. Exp Brain Res. 1994;98:145–152. doi: 10.1007/BF00229119. [DOI] [PubMed] [Google Scholar]
- Leelamanit V, Limsakul C, Geater A. Synchronized electrical stimulation in treating pharyngeal dysphagia. Laryngoscope. 2002;112:2204–2210. doi: 10.1097/00005537-200212000-00015. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Kahrilas PJ, Cheng J, Pauloski BR, Gibbons PJ, Rademaker AW, Lin S. Closure mechanisms of laryngeal vestibule during swallow. Am J Physiol Gastrointest Liver Physiol. 1992;262:G338–344. doi: 10.1152/ajpgi.1992.262.2.G338. [DOI] [PubMed] [Google Scholar]
- Lundy DS, Smith C, Colangelo L, Sullivan PA, Logemann JA, Lazarus CL, Newman LA, Murry T, Lombard L, Gaziano J. Aspiration: cause and implications. Otolaryngol Head Neck Surg. 1999;120:474–478. doi: 10.1053/hn.1999.v120.a91765. [DOI] [PubMed] [Google Scholar]
- Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke. 1999;30:744 –748. doi: 10.1161/01.str.30.4.744. [DOI] [PubMed] [Google Scholar]
- Martin RE, Kemppainen P, Masuda Y, Yao D, Murray GM, Sessle BJ. Features of cortically evoked swallowing in the awake primate (Macaca fascicularis) J Neurophysiol. 1999;82:1529–1541. doi: 10.1152/jn.1999.82.3.1529. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Britton TC, and Benecke R. Magnetic stimulation of the corticonuclear system and of proximal cranial nerve in humans. In: Motor Disturbances II., edited by Berardelli A, Benecke R, Manfredi M, and Marsden CD. London: Academic, 1990, p. 235–248.
- Paillard J. Quelques données psychophysiologiques relatives au déclenchement de la commande motrice. Année Psychol. 1948;48:28–47. [Google Scholar]
- Park CL, O’Neill PA, Martin DF. A pilot exploratory study of oral electrical stimulation on swallow function following stroke: an innovative technique. Dysphagia. 1997;12:161–166. doi: 10.1007/PL00009531. [DOI] [PubMed] [Google Scholar]
- Pouderoux P, Kahrilas PJ. Deglutitive tongue force modulation by volition, volume, and viscosity in humans. Gastroenterology. 1995;108:1418–1426. doi: 10.1016/0016-5085(95)90690-8. [DOI] [PubMed] [Google Scholar]
- Prosiegel M, Heintze M, Sonntag EW, Schenk T, Yassouridis A. Kinematic analysis of laryngeal movements in patients with neurogenic dysphagia before and after swallowing rehabilitation. Dysphagia. 2000;15:173–179. doi: 10.1007/s004550000024. [DOI] [PubMed] [Google Scholar]
- Reich A, Till J, Goldsmith H. Laryngeal and manual reaction times of stuttering and nonstuttering adults. J Speech Hear Res. 1981;24:192–196. doi: 10.1044/jshr.2402.192. [DOI] [PubMed] [Google Scholar]
- Reimers-Neils L, Logemann J, Larson C. Viscosity effects on EMG activity in normal swallow. Dysphagia. 1994;9:101–106. doi: 10.1007/BF00714596. [DOI] [PubMed] [Google Scholar]
- Schultz JL, Perlman AL, VanDaele DJ. Laryngeal movement, oropharyngeal pressure, and submental muscle contraction during swallowing. Arch Phys Med Rehabil. 1994;75:183–188. [PubMed] [Google Scholar]
- Sundgren P, Maly P, Gullberg B. Elevation of the larynx on normal and abnormal cineradiogram. Br J Radiol. 1993;66:768–772. doi: 10.1259/0007-1285-66-789-768. [DOI] [PubMed] [Google Scholar]


