Abstract
Background. Flavors from the mother’s diet during pregnancy are transmitted to amniotic fluid and swallowed by the fetus. Consequently, the types of food eaten by women during pregnancy and, hence, the flavor principles of their culture may be experienced by the infants before their first exposure to solid foods. Some of these same flavors will later be experienced by infants in breast milk, a liquid that, like amniotic fluid, comprises flavors that directly reflect the foods, spices, and beverages eaten by the mother. The present study tested the hypothesis that experience with a flavor in amniotic fluid or breast milk modifies the infants’ acceptance and enjoyment of similarly flavored foods at weaning.
Methods. Pregnant women who planned on breast-feeding their infants were randomly assigned to 1 of 3 groups. The women consumed either 300 mL of carrot juice or water for 4 days per week for 3 consecutive weeks during the last trimester of pregnancy and then again during the first 2 months of lactation. The mothers in 1 group drank carrot juice during pregnancy and water during lactation; mothers in a second group drank water during pregnancy and carrot juice during lactation, whereas those in the control group drank water during both pregnancy and lactation. Approximately 4 weeks after the mothers began complementing their infants’ diet with cereal and before the infants had ever been fed foods or juices containing the flavor of carrots, the infants were videotaped as they fed, in counterbalanced order, cereal prepared with water during 1 test session and cereal prepared with carrot juice during another. Immediately after each session, the mothers rated their infants’ enjoyment of the food on a 9-point scale.
Results. The results demonstrated that the infants who had exposure to the flavor of carrots in either amniotic fluid or breast milk behaved differently in response to that flavor in a food base than did nonexposed control infants. Specifically, previously exposed infants exhibited fewer negative facial expressions while feeding the carrot-flavored cereal compared with the plain cereal, whereas control infants whose mothers drank water during pregnancy and lactation exhibited no such difference. Moreover, those infants who were exposed to carrots prenatally were perceived by their mothers as enjoying the carrot-flavored cereal more compared with the plain cereal. Although these same tendencies were observed for the amount of cereal consumed and the length of the feeds, these findings were not statistically significant.
Conclusions. Prenatal and early postnatal exposure to a flavor enhanced the infants’ enjoyment of that flavor in solid foods during weaning. These very early flavor experiences may provide the foundation for cultural and ethnic differences in cuisine. Pediatrics 2001;107(6). URL: http://www.pediatrics.org/cgi/content/full/107/6/e88; infant nutrition, prenatal, lactation, weaning, flavor, development, preferences.
Throughout human history, what a woman experiences during pregnancy and lactation has been believed to somehow influence her child’s character for a lifetime.1 For example, stresses or shocks experienced by the pregnant mother, as well as faulty diets, were assumed to cause mental imbalances in the child.1,2 That this notion extends to early postnatal life is suggested by the rich folklore that surrounded the choice of wet nurses.3 Before the 1800s, many believed that the lactating mother or wet nurse, through her milk, provided the infant with not only nourishment but characteristics of her personality, such as her ideas, intelligence, speech, and emotional qualities.4,5
During the past few decades, a series of experiments demonstrated that fetal learning does indeed occur.1,6 The fetus not only learns the speech characteristics of the mother prenatally,7 but shortly after birth, infants prefer their mother’s voice,8 a passage recited to them prenatally,7 and the theme music of a soap opera watched by their mothers during pregnancy.9 The ability to detect other sensory stimuli, such as tastes and smells, also seems to be developed before birth.10
Within days of birth, human infants will orient toward the odor of their own amniotic fluid, which suggests that prenatal sensory experiences can bias the newborn’s behaviors and preferences.11,12 Moreover, the environment from which the newborn came, the amnion, contains compounds derived from flavors of foods eaten by the pregnant mother.13,14 Such exposure to dietary transmitted flavors (eg, garlic, anise) in amniotic fluid has been shown to influence the newborn’s facial, mouthing, and orienting responses to the flavor in the short-term.15,16 Because some of these same flavors will later be experienced in breast milk,17–20 the fetus and breast-fed infant experience the flavors of their mother’s diet before their first exposure to these flavors insolid foods. The present study expands on these findings and provides the first experimental evidence in humans that prenatal flavor experiences enhance the acceptance and enjoyment of similarly flavored foods during weaning. It also shows that postnatal exposure has similar consequences. Thus, very early flavor experiences may provide the foundation for cultural and ethnic differences in cuisine.
METHODS
Participants
Forty-six pregnant mothers (39 white, 4 black, 2 Hispanic, 1 Asian) who were in their last trimester of pregnancy and who planned to breastfeed their infants were recruited from advertisements in local newspapers; 65% were multiparous. All but 1 delivered a healthy singleton at term and successfully breastfed their infants during the exposure periods; the remaining mother had fraternal twins. Six additional mothers participated in the study but were disqualified either because they did not breastfeed long enough (n = 2), the infant refused to eat during both testing days (n = 3), or the mother did not comply with the experimental procedures (n = 1). All procedures were approved by the Committee on Studies Involving Human Beings at the University of Pennsylvania.
Exposure Procedures
The pregnant women were randomly assigned to 1 of 3 groups. During the last trimester of pregnancy (33.2 ± 0.3 weeks of gestation), the women were given either bottles of water (Naya, St-André Est, Québec, Canada) or cartons of frozen organic carrot juice (Ferraro, Glendora, CA) and instructions regarding the time of day (10 am to 2 pm) to drink the beverage. Carrot flavor was chosen as the stimulus for several reasons. First, it is a flavor that is transmitted to human milk and is quite distinctive.20 Second, it was easy to ensure that the nursing mothers’ only source of this flavor was under experimental control.20 Finally, carrot flavor has been added to the infants’ pap during the time of weaning for centuries.21
The women consumed either 300 mL of carrot juice or water daily for 4 days per week for 3 consecutive weeks during the last trimester of pregnancy and then again during the first 2 months of lactation (1.3 ± 0.1 months). As shown in Fig 1, the mothers in 1 group drank carrot juice during pregnancy and water during lactation (CW; n = 16); mothers in another (WC) group did the opposite (n = 17); whereas those in the control group drank water during both exposure periods (WW; n = 14). There were no significant differences among the groups in the timing of the exposure periods during pregnancy or lactation (F[2,43 df] = 1.42; P = .25). Mothers refrained from eating carrots or drinking carrot juice during and between the 2 exposure periods. To encourage and verify compliance, mothers maintained diet logs of all the foods and beverages that they ate during the exposure period and daily phone or e-mail contact was made with each woman.
Fig 1.
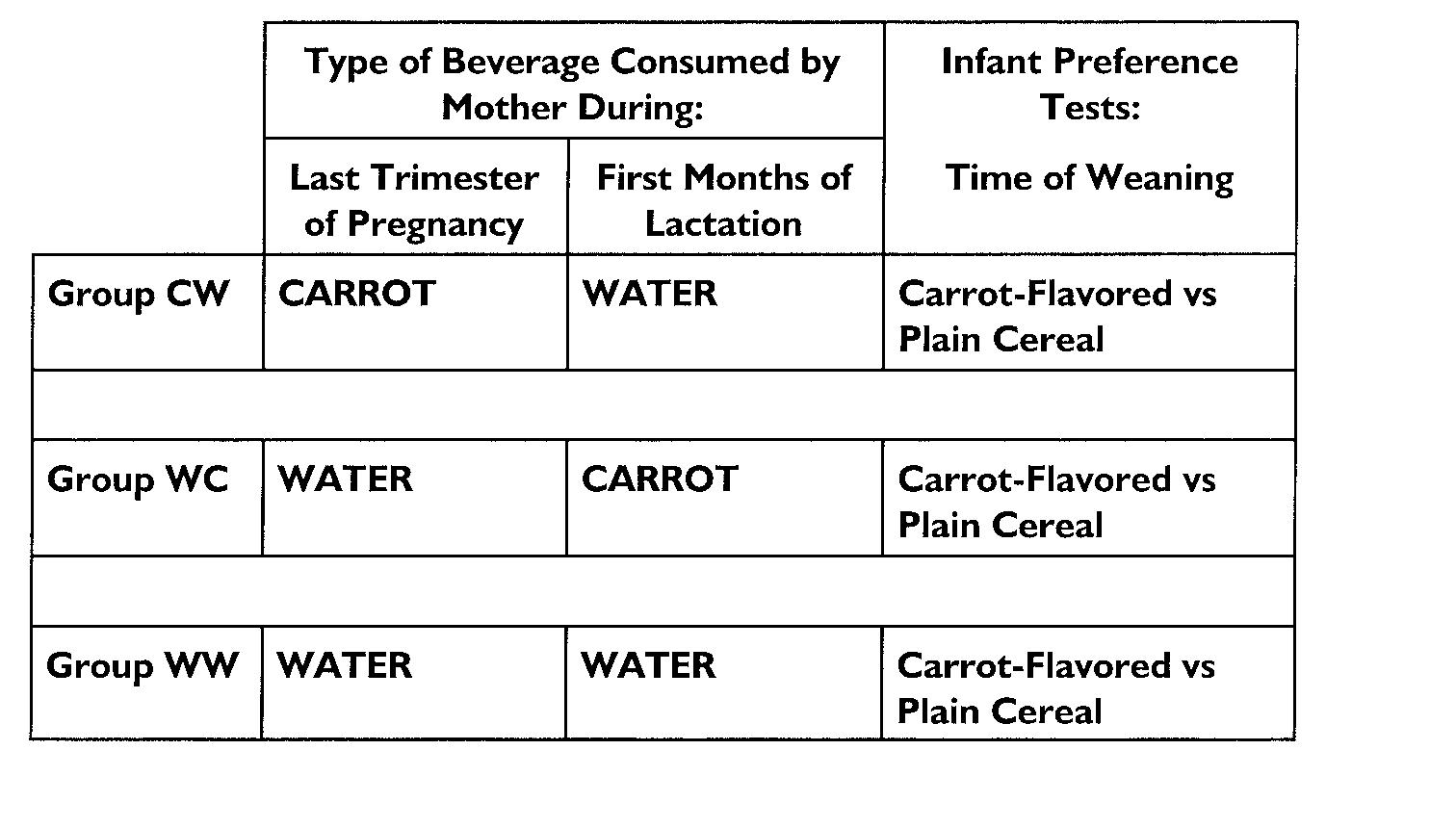
The design of the experimental protocol for the 3 groups of participants. The women consumed either 300 mL of carrot juice or water daily for 4 days per week for 3 consecutive weeks during the last trimester of pregnancy and then again during the first 2 months of lactation. The mothers in 1 group (CW) drank carrot juice during pregnancy and water during lactation (n = 16); mothers in another (WC) drank water during pregnancy and carrot juice during lactation (n = 17); whereas those in the control group (WW) drank water during both exposure periods (n = 14). Approximately 4 weeks after the mothers began complementing their infants’ diet with cereal but before the introduction of carrot-flavored foods or beverages, the infants, who were ∼6 months of age, were videotaped at the Monell Center as they fed, in counterbalanced order, cereal prepared with water (1 part cereal, 1 part water) during 1 test day and cereal prepared with carrot juice (1 part cereal, 1 part carrot juice) during another.
The characteristics of each group are listed in Table 1. There were no significant differences between the groups in the ages of the mothers (F[2,43 df] = 2.21; P = .12) or infants (F[2,43 df] = 0.54; P = .95), the infants’ body mass index (BMI; F[2,43 df] = 2.31; P = .11), or the sex ratio of the infants (χ2 [2 df] = 1.70; P = .43). There was not a significant interaction between group and sex on the infants’ BMI (F[2,40 df] = 1.66; P = .20). However, there was a significant effect of sex (F[1,40 df] = 4.60; P = .04); posthoc analysis revealed that overall boys tended to have a larger BMI than did girl infants of this age (F[1,44 df] = 3.67; P = .07).
TABLE 1.
Participant Characteristics
| Type of Beverage Consumed by the Mother During | Group CW |
Group WC |
Group WW |
|---|---|---|---|
| Pregnancy: | Carrot | Water | Water |
| Lactation: | Water | Carrot | Water |
| Mothers′ age in y | |||
| Mean ± SEM | 29.0 ± 1.6 | 31.9 ± 1.0 | 32.6 ± 1.2 |
| Infants′ age in mo at cereal test | |||
| Mean ± SEM | 5.6 ± 0.2 | 5.7 ± 0.4 | 5.7 ± 0.4 |
| BMI | |||
| Mean ± SEM | 17.3 ± 0.5 | 16.2 ± 0.4 | 17.3 ± 0.4 |
| Sex of infants | |||
| girls:boys | 4:11 | 7:10 | 7:7 |
| Number of mother–child pairs | 15 | 17 | 14 |
Testing Procedures
A within-participants design that controlled for time of day was implemented. Approximately 4 (± 0.5) weeks after the mothers began complementing their infants’ diet with cereal and before the introduction of carrot foods to their diet, the infants, who were 5.7 ± 0.2 months old, were videotaped at the Monell Center as they fed, in counterbalanced order, cereal prepared with water (1 part cereal, 1 part water) during 1 test day and cereal prepared with carrot juice (1 part cereal, 1 part carrot juice) during another. These test sessions were separated by, on average, 4 (± 0.5) days. All liquids were room temperature before cereal preparation. None of the infants had ever been fed carrot juice or any foods containing carrots. There were no significant differences among the groups in the number of weeks the infants had been fed cereal (F[2,43 df] = 0.32; P = .73). The vast majority of the mothers (n = 39) were still nursing at the time of testing; the remaining 7 women had weaned their infants from the breast, on average, 3.5 ± 0.6 weeks before the first test session.
To minimize possible effects attributable to different levels of satiation and familiarization, each test session began at approximately the same time of day that the infants were usually fed cereal and the mothers drank the juice or water during the exposure periods (10 am to 2 pm). The infants were last fed ∼2.5 ± 0.2 hours before testing and there was no significant difference in this duration of time between the 2 test sessions (paired t [45 df] = 0.35; P = .73). In addition, the type of cereal offered was the brand that the infant was currently being fed and, thus, was familiar.
The infants sat in a chair that had a tray attached to its arms and their mothers fed them with utensils and the infants wore bibs that were identical to those used at home.22 The mothers refrained from talking or making faces during the feeding sessions to eliminate any potential influence of the mother’s verbal or facial responses on her infant’s behaviors; replays of the videotapes verified that this was indeed the case. Mothers were instructed to feed the infants at their customary pace until they refused the cereal 3 consecutive times, using the criterion that the infant exhibited such behaviors as turning his or her head away, pushing the spoon away, crying, or becoming playful. The experimenter, who was unaware of the experimental conditions of the participants, then signaled to the mother to stop feeding. The experimenter sat behind the video camera, which was placed at the far corner of the testing room ∼10 to 12 feet from the mother–infant dyad and was out of view of the mother and her infant.
Immediately after each feeding session, the mothers, who were unaware of the hypothesis of the study, then rated, how much that they thought their infant liked the cereal on a 9-point scale. Intermediate ratings were to be marked at the appropriate locations between the extremes such that ratings could range from 1 (did not like at all) to 9 (liked very much). The infants’ behaviors were monitored by videotape and the amount of cereal consumed by the infant was assessed by weighing the bowl immediately before and after each feed on a Mettler pm 15 top-loading balance (Greifensee, Switzerland). All food that spilled onto the tray or bib was placed in the bowl before weighing. Mothers were not told how much their infants had ingested.
Trained raters, who were unaware of the experimental conditions, scored the videotaped records of the first 2 minutes of each feed in real time.22 Because previous research15,23,24 has shown that negative facial configurations are more discriminating than positive configurations in gauging the infants’ hedonic responsiveness, the videotape analyses focused on frequency of negative facial responses (eg, nose wrinkling, brow lowering, upper lip raising, gaping, head turning) made in response to each spoonful of cereal proffered. During scoring, the sound was turned off so that the raters would not be influenced by the infants’ vocalizations. Reliabilities for each measure were determined by correlating the scoring of at least 2 raters. The mean Pearson product–moment coefficients for the scoring of all behaviors were above 0.80.
Questionnaires on Mother’s Eating Habits
All but 1 mother completed an 8-item scale that measured their variety-seeking tendency with respect to foods,25 during both pregnancy and lactation. Each mother was also queried about the frequency with which they consumed carrots and other foods and rated how much they liked eating carrots.
Statistical Analyses
For each infant, we determined the frequency of negative facial expressions, the mothers’ ratings of their infants’ enjoyment of the food, total cereal intake, and the length of feedings on each of the 2 test sessions. These data were then analyzed in separate 2 × 2 analyses of variance with flavor (plain, carrot) as the within-participant factors and treatment group (groups CW, WC, and WW) as the grouping factor. Significant effects in the analysis of variance were probed by posthoc tests. All summary statistics are expressed as mean ± standard error of the mean (SEM).
Because this is a within-participants design study, proportional difference scores are more informative than are mean group data. Therefore, for each infant, we calculated proportional responses by dividing each infant’s response to the carrot-flavored cereal by his or her response to the carrot cereal plus plain cereal (carrot/[carrot + plain]; Table 2 and Fig 2). Scores above 0.50 indicate increased display of negative facial expressions, maternal ratings of infants’ enjoyment, or increased intake when feeding the carrot-flavored cereal relative to the plain cereal.
TABLE 2.
Effect of Exposure on Infants′ Acceptance and Enjoyment of Plain and Carrot-Flavored Cereal
| Type of Beverage Consumed by the Mother During | Group CW |
Group WC |
Group WW |
|---|---|---|---|
| Pregnancy: | Carrot | Water | Water |
| Lactation: | Water | Carrot | Water |
| Frequency of negative facial responses during initial min of feed | |||
| Plain cereal | 5.4 ± 0.8 | 4.7 ± 0.8 | 3.6 ± 0.8 |
| Carrot-flavored cereal | 4.0 ± 0.8* | 3.4 ± 0.6* | 4.4 ± 0.6 |
| Proportional difference | 0.39 ± 0.05 | 0.41 ± 0.04 | 0.56 ± 0.05 |
| Mothers′ perception of infants′ enjoyment of cereal (Rating: 1 = did not like at all; 9 = liked very much) | |||
| Plain cereal | 5.3 ± 0.6 | 5.5 ± 0.7 | 5.6 ± 0.7 |
| Carrot-flavored cereal | 7.5 ± 0.5* | 6.7 ± 0.5 | 5.2 ± 0.8 |
| Proportional difference | 0.60 ± 0.03 | 0.56 ± 0.04 | 0.46 ± 0.06 |
| Intake of cereal (g) | |||
| Plain cereal | 55.2 ± 18.2 | 63.4 ± 13.2 | 34.5 ± 9.7 |
| Carrot-flavored cereal | 80.5 ± 18.2 | 81.4 ± 14.6 | 43.9 ± 12.3 |
| Proportional difference (carrot/[carrot + plain]) | 0.62 ± 0.05 | 0.57 ± 0.04 | 0.51 ± 0.04 |
| Length of feeding (min) | |||
| Plain cereal | 13.4 ± 1.7 | 10.0 ± 1.1 | 12.1 ± 2.6 |
| Carrot-flavored cereal | 18.2 ± 2.2 | 13.7 ± 1.6 | 13.2 ± 2.4 |
| Proportional difference | 0.57 ± 0.04 | 0.58 ± 0.03 | 0.52 ± 0.03 |
| Number of mother–child pairs | 15 | 17 | 14 |
P < .05 compared with plain cereal.
Fig 2.
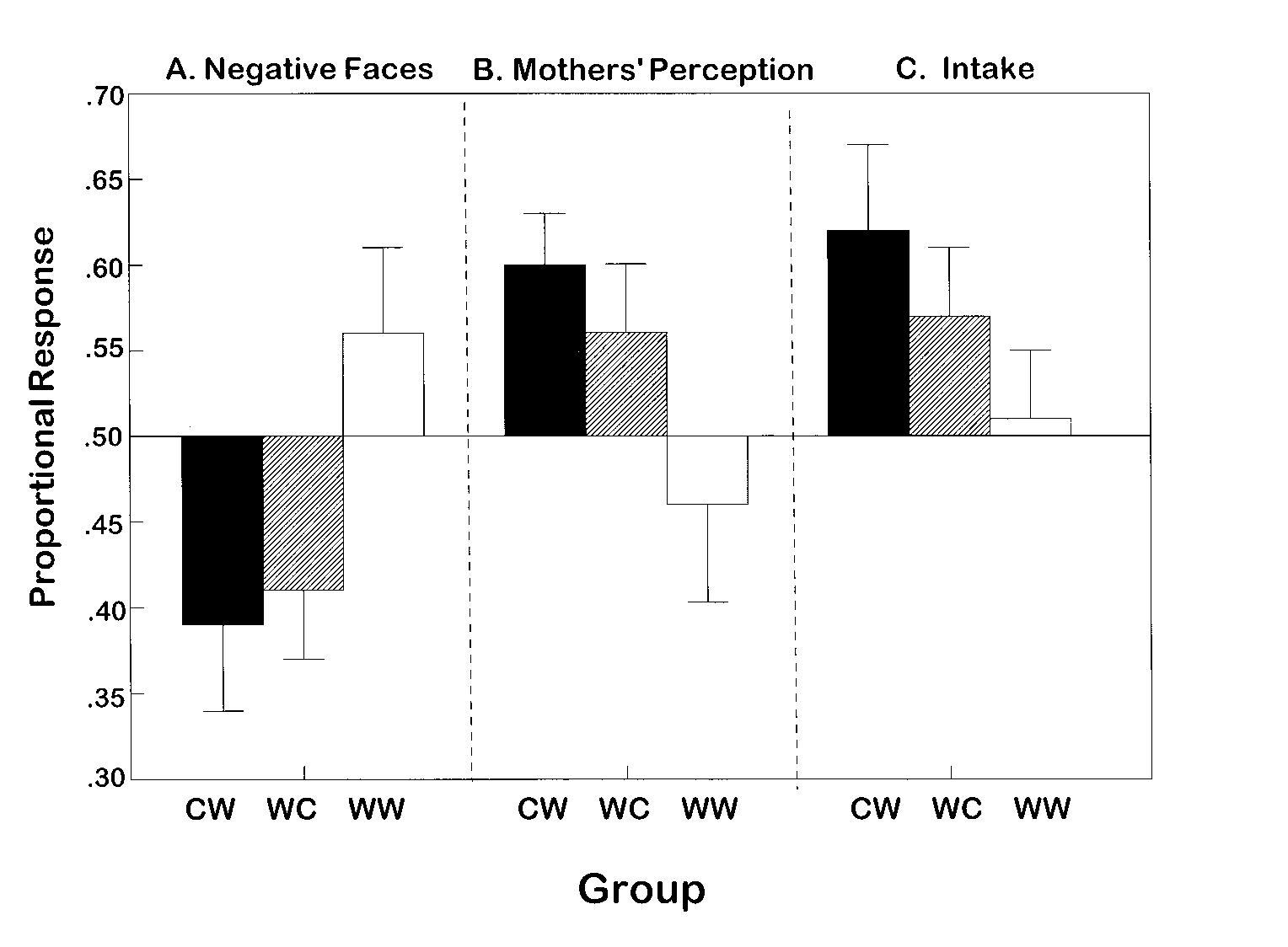
The infants’ relative acceptance of the carrot-flavored cereal as indicated by display of negative facial expressions (left panel), mothers’ ratings of their infants’ enjoyment of the cereals (middle panel), and intake (right panel). Proportional responses were calculated by dividing each infant’s response to the carrot-flavored cereal by his or her response to the carrot cereal plus plain cereal (carrot/[carrot + plain]). For example, scores above 0.50 indicate increased display of negative facial expressions, maternal ratings of infants’ enjoyment, or increased intake when feeding the carrot-flavored cereal relative to the plain cereal. Mothers of infants in the CW group drank carrot juice during pregnancy and water during lactation; those in the WC group did the opposite; whereas those in the control group (WW) drank water during both exposure periods.
RESULTS
Facial Responses During Feeding
Videotape analyses revealed that although there were no significant differences among the groups in the number of spoonfuls of the carrot or plain cereal offered to the child during the initial 2 minutes of the feed (F[2,43 df] = 0.86; P = .43), there was a significant interaction between group and flavor on the frequency of negative facial responses made during the initial minutes of the feed (F[2,43 df] = 3.31; P = .05; Table 2). Those infants who were exposed to the carrot flavor either prenatally (paired t [14 df] = 2.88; P = .01) or postnatally (paired t [16 df] = 2.41; P = .03) exhibited less negative facial responses while feeding the carrot-flavored relative to the plain cereal, whereas the control group exhibited the opposite tendency, although this was not significant (paired t [13 df] -0.79; P = .44; Fig 2).
Mothers’ Perceptions of Infants’ Enjoyment of Cereal
That the mothers perceived differences in their infants’ enjoyment of these cereals as a function of its flavoring and the infants’ previous experience is indicated by the significant interaction between group and flavor (F[2,43 df] = 3.37; P = .04). Those who were exposed to carrots during pregnancy were perceived by their mothers as enjoying the carrot-flavored cereal more (paired t [14 df] = 3.32; P = .005); the difference for those who were exposed to carrot flavor during breastfeeding was in the same direction but did not reach significance (paired t [16 df] = 1.63; P = .12). There was no significant difference in the mothers’ ratings of the control (WW) infants’ enjoyment of the cereals (paired t [13 df] = 0.40; P = .70). That the mothers’ rating reflected, in part, how much the infants ate and how long they fed is suggested by the significant correlations between maternal ratings and these 2 behaviors (intake: r [45 df] = 0.54; P = .000; length of feed: r [45 df] = 0.45; P = .002).
Acceptance of Cereals
There was a significant effect of flavor on the amount of cereal consumed (F[1,43 df] = 16.73; P < .0002) and the length of the feed (F[1,43 df] =7.47; P = .009). Overall, more carrot-flavored cereal was consumed than plain cereal by the 3 groups of infants combined (F[1,45 df] = 17.15; P = .0001). Similarly, infants spent a longer time feeding the carrot flavored cereal relative to the plain cereal for the 3 groups combined (F[1,45 df] = 7.91; P = .007). The interaction effects between group and intake (F[2,43 df] = 1.09; P = .34) or length of feed (F[2,43 df] = 0.88; P = .42) were not statistically significant. Thus, although the experimental groups consumed more carrot-flavored cereal relative to plain cereal (these 2 groups of infants consumed, on average, 3 times [3.4 ± 1.3] more [carrot/plain]) and the control group generally did not, there was not a significant effect of experimental treatment.
Maternal Eating Habits
There were no significant differences among the groups in the mothers’ variety-seeking scores during pregnancy (F[2,43 df] = 0.12; P = .89) and lactation (F[2,42 df] = 0.37; P = .69), how much they liked carrots (F[2,42 df] = 0.27; P = .77), or their frequency of eating carrots (excluding, of course, the experimental periods) during either pregnancy (F[2,42 df] = 0.09; P = .91) or lactation (F[2,39 df] = 0.65; P = .53). The mothers’ reported variety-seeking scores during pregnancy were highly correlated with those obtained during lactation (r [44 df] = 0.73; P < .000).
DISCUSSION
This is the first experimental evidence in humans that prenatal flavor experiences influence postnatal responses to that flavor in solid foods. The data also provide additional evidence that early postnatal experience has similar effects and, thus, highlight the importance of a varied diet for both pregnant and lactating women. The differences observed in the infants’ relative enjoyment, as inferred from facial expressions made during feeding carrot-flavored cereal, seem not to be attributable to the mothers’ eating habits or attitudes toward foods because there were no significant differences among the 3 groups of mothers on any of these measures.
Two related lines of evidence support the conclusion that prenatal and postnatal sensory experiences influenced the acceptance of carrot flavor in solid foods. First, only those infants who were exposed to carrot flavor either prenatally (CW) or postnatally (WC) displayed fewer negative facial responses while eating the carrot-flavored cereal relative to the plain; this was not the case for the control (WW) infants. Second, only the mothers of the 2 experimental groups (although this was significant only for the CW group) rated their infants’ enjoyment of the carrot-flavored cereal as exceeding that of the plain cereal. In contrast, control mothers rated their infant’s enjoyment of the plain and carrot-flavored cereal almost identically. These ratings by the mothers reflected, in part, how much and how long their infants ate the carrot-flavored cereal.
The present study aimed to experimentally control for a number of factors that could bias the infants’ behaviors. For example, testing took place at the same time of day that the infants were usually fed this familiar brand of cereal and their mothers drank the juice or water during the exposure periods, and the duration of time since the infants were last fed was controlled for to minimize effects attributable to different levels of satiation and familiarization. In addition, the infants determined when the feeding ended because the test session terminated when the infants rejected the cereal on 3 consecutive occasions. Mothers, who were unaware of the hypothesis being tested, were not told how much their infants consumed during each of the 2 test sessions that were separated by an interval of ∼4 days. Their ratings of how much their infants enjoyed the cereal reflected, in part, how much the infants ate and how long they fed, as suggested by the significant correlations between maternal ratings and these 2 behaviors. Therefore, it is unlikely that group differences maternal ratings and the infants’ facial responses were attributable to changes in the infants’ interaction with their mothers or to experimental bias. Rather, it seems that our findings are attributable to experiential effect of carrot exposure in amniotic fluid or mothers’ milk.
Although we have not demonstrated experimentally that carrot flavor is transmitted from the pregnant mother into her amniotic fluid, this is highly likely for several reasons. First, we demonstrated earlier that at least 1 flavor consumed by pregnant women, garlic, does enter amniotic fluid to levels detectable by the adult human nose.14 Second, we have shown that carrot flavor,20 like garlic17 and several other flavors,18,19 is transmitted from the nursing mother’s diet to her milk. Thus, the basis for the effects of prenatal carrot feeding is most likely via the influence of fetal exposure to carrot flavor.
The findings that early flavor experience impacts on later food enjoyment by infants are consistent with animal model studies that demonstrate that early exposure to flavors serves to heighten the acceptability of those flavors in foods and even facilitates weaning, as has been shown in swine.26 Young animals prefer flavors that were experienced during either gestation or lactation.27–30 Such redundancy of dietary information has importance biologically because it provides complementary routes of transferring information on the types of foods available in the environment, should the mothers’ diet change.27 Although it is likely that such experience influences acceptance at older ages in humans, this hypothesis remains to be tested.
The flavor of a food includes, among other sensory stimuli, the oral sensation of taste and the retronasal sensation of smell. It has been suggested that relative to taste, where hedonic tone and liking are more hard-wired, liking for olfactory components of flavor is largely determined by individual experience.31 Although the importance of early experience in setting likes and dislikes in omnivores, such as humans, has been disputed,32 we suggest that prenatal and early postnatal exposure, at the least, predisposes the young infant to favorably respond to the now familiar flavor, which, in turn, facilitates the transition from fetal life through the breastfeeding period to the initiation of a varied solid food diet.33 In this way, culture-specific flavor preferences are likely initiated early in life.33,34 Significant traces of this may remain as children become adults and pass on their food habits to the next generation, often via amniotic fluid and breast milk-associated cues.
ACKNOWLEDGMENTS
This work was supported by Grant HD37119 from the National Institutes of Child Health and Human Development and a grant from the Gerber Companies Foundation.
We appreciate the expert technical assistance of Corinne Simon and Eboni Woodard.
Footnotes
Portions of this work were presented at the Presidential Symposium, 12th Annual Convention of the American Psychological Society; June 9, 2000; Miami, FL.
- CW
- carrot juice during pregnancy and water during lactation
- WC
- water during pregnancy and carrot juice during lactation
- WW
- water during both exposure periods
- BMI
- body mass index
- SEM
- standard error of the mean
REFERENCES
- 1.Fedor-Freybergh P, Vogel MLV, editors. Prenatal and Perinatal Psychology and Medicine: Encounter With the Unborn: A Comprehensive Survey of Research and Practice. Parthenon Publishing Group; Park Ridge, NJ: 1988. [Google Scholar]
- 2.Kutumbiah P. Pediatrics in ancient India. Indian J Hist Med. 1964;9:22–31. [Google Scholar]
- 3.Fildes V. Breasts, Bottles and Babies: A History of Infant Feeding. Edinburgh University Press; Edinburgh, Scotland: 1986. [Google Scholar]
- 4.SoranusSoranus’ Gynecology 1956The Johns Hopkins University Press; Baltimore, MD: (First/Second Century AD)Temkin O, Eastman NJ, Edelstein L, Guttmacher AF, trans. [Google Scholar]
- 5.Susruta Samhita.An English Translation of the Susruta Samhita 1911Telford Press, Inc; Caldwell, NJ: (Fourth-Second Centuries BC)Bishagratna KL, trans. [Google Scholar]
- 6.Smotherman WP, Robinson SR, editors. Behavior of the Fetus. The Telford Press; Caldwell, NJ: 1988. [Google Scholar]
- 7.DeCasper AJ, Spence MJ. Prenatal maternal speech influences newborns’ perception of speech sound. Infant Behav Dev. 1986;9:133–150. [Google Scholar]
- 8.DeCasper AJ, Fifer WP. Of human bonding: newborns prefer their mother’s voices. Science. 1980;208:1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- 9.Hepper PG. Foetal “soap” addiction. Lancet. 1988;1:1247–1248. doi: 10.1016/s0140-6736(88)92170-8. [DOI] [PubMed] [Google Scholar]
- 10.Mennella JA. Taste and smell. In: Swaiman KF, Ashwall S, editors. Pediatric Neurology: Principles and Practice. 3rd ed. CV Mosby Company; Philadelphia, PA: 1999. pp. 104–113. [Google Scholar]
- 11.Schaal B, Marlier L, Soussignan R. Olfactory function in the human fetus: evidence from selective neonatal responsiveness to the odor of amniotic fluid. Behav Neurosci. 1998;112:1438–1439. doi: 10.1037//0735-7044.112.6.1438. [DOI] [PubMed] [Google Scholar]
- 12.Marlier L, Schaal B, Soussignan R. Neonatal responsiveness to the odor of amniotic and lacteal fluids: a test of perinatal chemosensory continuity. Child Dev. 1998;69:611–623. [PubMed] [Google Scholar]
- 13.Hauser GJ, Chitayat D, Berbs L, Braver D, Mulbauer B. Peculiar odors in newborns and maternal pre-natal ingestion of spicy foods. Eur J Pediatr. 1985;144:403. doi: 10.1007/BF00441788. [DOI] [PubMed] [Google Scholar]
- 14.Mennella JA, Johnson A, Beauchamp GK. Garlic ingestion by pregnant women alters the odor of amniotic fluid. Chem Senses. 1995;20:207–209. doi: 10.1093/chemse/20.2.207. [DOI] [PubMed] [Google Scholar]
- 15.Hepper P. Human fetal “olfactory” learning. Int J Prenatal Perinatal Psychol. 1995;2:147–151. [Google Scholar]
- 16.Schaal B, Marlier L, Soussignan R. Human fetuses learn odors from their pregnant mother’s diet. Chem Senses. 2000;25:729–737. doi: 10.1093/chemse/25.6.729. [DOI] [PubMed] [Google Scholar]
- 17.Mennella JA, Beauchamp GK. Maternal diet alters the sensory qualities of human milk and the nursling’s behavior. Pediatrics. 1991;88:737–744. [PubMed] [Google Scholar]
- 18.Mennella JA, Beauchamp GK. The human infants’ responses to vanilla flavors in human milk and formula. Inf Behav Dev. 1996;19:13–19. [Google Scholar]
- 19.Desage M, Schaal B, Soubeyrand J, Orgeur P, Brazier JL. Gas chromatographic-mass spectrometric method to characterize the transfer of dietary odorous compounds into plasma and milk. J Chromatogr B Biomed Appl. 1996;678:205–210. doi: 10.1016/0378-4347(95)00527-7. [DOI] [PubMed] [Google Scholar]
- 20.Mennella JA, Beauchamp GK. Experience with a flavor in mother’s milk modifies the infant’s acceptance of flavored cereal. Dev Psychobiol. 1999;35:197–203. doi: 10.1002/(sici)1098-2302(199911)35:3<197::aid-dev4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Bouchet M. Practical Treatise on the Diseases of Children and Infants at the Breast; Including the Hygiene and Physical Education of Young Children. John Churchill; London, England: 1855. pp. 48–51. [Google Scholar]
- 22.Mennella JA, Beauchamp GK. Mothers’ milk enhances the acceptance of cereal during weaning. Pediatr Res. 1997;41:188–192. doi: 10.1203/00006450-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Soussignan R, Schaal B, Marlier L. Olfactory alluiesthesia in human neonates: prandial state and stimulus familiarity modulate facial and autonomic responses to milk odors. Dev Psychobiol. 1999;35:3–14. doi: 10.1002/(sici)1098-2302(199907)35:1<3::aid-dev2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 24.Rosenstein D, Oster H. Differential facial responses to four basic tastes in newborns. Child Dev. 1988;59:1555–1566. [PubMed] [Google Scholar]
- 25.Van Trijp HCM, Steenkamp JEM. Consumers’ variety seeking tendency with respect to foods: measurement and managerial implications. Euro R Agr Eco. 1992;19:181–195. [Google Scholar]
- 26.Campbell RG. A note on the use of feed flavour to stimulate the feed intake of weaner pigs. Anim Prod. 1976;23:417–419. [Google Scholar]
- 27.Bilkó A, Altbacker V, Hudson R. Transmission of food preference in the rabbit: the means of information transfer. Physiol Behav. 1994;56:907–912. doi: 10.1016/0031-9384(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 28.Domínquez HD, López MF, Molina JC. Interactions between perinatal and neonatal association learning defined by contiguous olfactory and tactile stimulation. Neurobiol Learn Mem. 1999;71:272–288. doi: 10.1006/nlme.1998.3882. [DOI] [PubMed] [Google Scholar]
- 29.Hepper PG. Adaptive fetal learning: prenatal exposure to garlic affects postnatal preferences. Anim Behav. 1988;36:935–936. [Google Scholar]
- 30.Semke E, Distel H, Hudson R. Specific enhancement of olfactory receptor sensitivity association with foetal learning of odors in the rabbit. Naturwisshenschaften. 1995;82:148–149. doi: 10.1007/BF01177279. [DOI] [PubMed] [Google Scholar]
- 31.Bartoshuk LM. In: Taste, smell and pleasure. The Hedonics of Taste. Bolles RC, editor. Lawrence Erlbaum Associates; Mahwah, NJ: 1991. pp. 15–28. [Google Scholar]
- 32.Rozin P. Psychological aspects of the use of low-calorie foods: changing beliefs and preferences. In: Altschul AM, editor. Low-Calorie Food Handbook. Marcel Dekker, Inc; New York, NY: 1993. pp. 535–550. [Google Scholar]
- 33.Mennella JA. The flavor world of infants: a cross-cultural perspective. Nutr Today. 1997;32:142–151. [Google Scholar]
- 34.Haller R, Rummel C, Hennenberg S, Pollmer U, Koester EP. The influence of early experience with vanillin on food preference later in like. Chem Senses. 1999;24:465–467. doi: 10.1093/chemse/24.4.465. [DOI] [PubMed] [Google Scholar]


