Abstract
The free radical nitric oxide (NO), generated through the oxidation of l-arginine to l-citrulline by NO synthases (NOSs), has been shown to inhibit steroidogenic pathways. NOS isoforms are known to be present in rat and human testes. Our study examined the sensitivity of Leydig cells to NO and determined whether NOS activity resides in Leydig cells or in another cell type such as the testicular macrophage. The results showed a low level of l-[14C]arginine conversion in purified rat Leydig cell homogenates. Administration of the NOS inhibitor L-NG-nitro-arginine methyl ester (L-NAME), or the calcium chelator ethylenebis (oxyethylenenitrilo)tetraacetic acid (EGTA), had no effect on l-[14C]citrulline accumulation. Increased intracellular Ca2+ concentrations that were induced by a calcium ionophore, or the addition of luteinizing hormone (LH), failed to affect NO formation in intact cells that were cultured in vitro. Introduction of a high concentration of the NO precursor l-arginine did not decrease testosterone (T) production, and NOS inhibitors did not increase T biosynthesis. However, exposing Leydig cells to low concentrations of the NO donor S-nitrosoglutathione (GSNO) induced a dramatic blockade of T production under basal and LH-stimulated conditions. DNA array assays showed a low level of expression of endothelial NOS (eNOS), while the neuronal and inducible isoforms of NOS (nNOS and iNOS) were below detection levels. Reverse transcriptase-polymerase chain reaction (RT-PCR) analyses confirmed these findings and demonstrated the presence of high iNOS messenger RNA (mRNA) levels in activated testicular macrophages that produced large amounts of NO. These data suggest that, while T production in rat Leydig cells is highly sensitive to NO and an endogenous NO-generating system is not present in these cells, NOS activity is more likely to reside in activated testicular macrophages.
Keywords: Macrophage, testosterone, DNA gene array, nitric oxide synthase, S-nitrosoglutathione, L-NG-nitro-arginine methyl ester, L-NG-monomethyl-arginine
Leydig cells are the main site of testosterone (T) production and are thereby essential for sexual differentiation and fertility. While the major trophic stimulus for T biosynthesis is luteinizing hormone (LH), various locally produced factors have also been shown to modulate Leydig cell structure and function. Of these, nitric oxide (NO) has recently received attention as a possible mediator of testicular actions such as stress and inflammation. More specifically, the application of NO donors reduced testicular T production in vivo (Adams et al, 1994) as well as in vitro (Del Punta et al, 1996). Also, a short immobilization stress in rats produced a sharp decline of serum T levels and blocked the LH-induced T production in testicular cell cultures (Kostic et al, 1998). Further studies involving the inhibition of NO synthases (NOSs), the family of enzymes responsible for the generation of NO, prevented a similar inhibition of Leydig cell function (Kostic et al, 1998). Because stress and/or infection can result in infertility (Cutolo et al, 1988; Fenster et al, 1997; Diemer et al, 2000), we believe further studies concerning the specific mechanisms mediating this phenomenon are warranted.
Macrophages are potent secretory cells that release an array of mediators, including proinflammatory and cytotoxic cytokines (Hales, 1996; Hutson, 1998). When stimulated with agents such as lipopolysaccharide (LPS), these cells express inducible NOS (iNOS) and release NO (Di Rosa et al, 1990). Since testicular macrophages reside in close proximity to Leydig cells in the interstitial tissue (Hutson, 1992), they have been hypothesized to act as a source of NO, thereby modulating Leydig cell steroidogenesis under conditions of immune activation. In fact, testicular (Sun et al, 1993) or peritoneal macrophages (Pomerantz and Pitelka, 1998) depressed androgen biosynthesis when studied under in vitro conditions that mimic this immune-activated state. It is hypothesized that NO mediates this suppression, since l-nitro-l-arginine methyl ester (an NOS inhibitor) prevented this macrophage-mediated inhibition of steroidogenesis (Pomerantz and Pitelka, 1998).
In the present study, we examined whether rat Leydig cells have the capacity to generate NO and, if so, whether the amounts of NO generated locally in the testis are sufficient to inhibit T production. Data presented herein indicate that in purified rat Leydig cells, exogenously supplied NO potently abolishes T production. Nevertheless, the endogenous NOS substrate (ie, l-arginine) or NOS inhibitors (eg, L-NG-nitro-arginine methyl ester [L-NAME]) failed to modify NO generation or androgen biosynthesis. Moreover, DNA array assays clearly showed that adult rat Leydig cells do not express iNOS or neuronal NOS (nNOS), and only a marginal quantity of the endothelial isoform of NOS (eNOS) could be detected. Reverse transcriptase-polymerase chain reaction (RT-PCR) analyses confirmed these observations by demonstrating that messenger RNA (mRNA) expression of NOS isoforms is below the detection level. This technique, combined with measurements of NO generation, showed that LPS-stimulated testicular macrophages express iNOS and produce NO. Thus, to the extent that NO is locally produced in the testis, testicular macrophages are the best candidate to modulate Leydig cell T production through paracrine NO signaling.
Materials and Methods
Reagents
l-[14C]arginine (specific activity = 313 millicurie [mCi]/mmol) was purchased from NEN Life Science Products (Boston, Mass). The NOS detection kit (NOSdetect Assay Kit) and L-NAME were obtained from Stratagene (La Jolla, Calif). Ionomycin and L-NG-monomethyl-arginine (L-NMMA) were from Calbiochem (La Jolla, Calif). Ovine LH was provided by the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases (Rockville, Md), Rat Atlas I kits were purchased from Clontech Laboratories Inc (Palo Alto, Calif), and the total extract kit (Trireagent) was from Molecular Research Center Inc (Cincinnati, Ohio). Avian myeloblastosis virus RT was obtained from Promega (Madison, Wis). [32P]dATP [P32]deoxyadenosine 5′ triphosphate, 10 μCi/μL) was from Amersham Pharmacia Biotech (Baie d’Urfe, Canada). Ethylenebis (oxyethylenenitrilo)tetraacetic acid (EGTA), l-arginine, LPS (L-8274 from Escherichia coli), and Dulbecco modified Eagle medium (DMEM:F12 medium; DMEM-Ham media F-12, D-2906) were purchased from Sigma Chemical Co (St Louis, Mo).
Animals
Male Sprague-Dawley rats (250–300 g; Charles River Laboratories, Wilmington, Mass) were maintained under conditions of controlled lighting (lights on from 0700–1900 hours) and temperature (23°C) and were allowed free access to food. Testes were removed after asphyxiation with CO2 in a precharged chamber, in accordance with a procedure that was approved by Rockefeller University’s Animal Care and Use Committee (protocol 03-048).
Leydig Cell T Production
Purified Leydig cells were prepared as previously described (Klinefelter et al, 1993). Briefly, testes were subjected to collagenase digestion and, before loading onto a 55% continuous Percoll density gradient, the cell suspension was subjected to centrifugal elutriation to remove germ cell and sperm contaminants. The fraction of Leydig cells remaining in the elutriator chamber at a flow rate greater than 16 mL/min and a rotor speed of 800 × g was collected. Leydig cells were harvested from the Percoll gradient at densities of 1.070 g/mL and greater. The cells were counted using a hemacytometer, and purity was determined by histochemical staining for 3β-hydroxysteroid dehydrogenase (3β-HSD) (Payne et al, 1980) and was typically 95% to 97%. The amounts of T produced by Leydig cells were measured after incubation for 1 hour in 1 mL of 1:1 DMEM:F-12 culture medium containing 0.1% bovine serum albumin (BSA) and 0.5 mg of bovine lipoprotein per milliliter and were buffered with 14 mM NaHCO3. T production was expressed as nanograms of steroid per 106 cells/h.
Testicular Macrophages
Testicular macrophages were isolated from rats as previously described (Hutson et al, 1996; Nes et al, 2000). Briefly, testes were perfused through the gonadal vein with collagenase (100 U/mL) in medium (DME/F-12 plus 0.1% BSA, penicillin [100 U/mL], and streptomycin [100 μg/mL]). The testes were then decapsulated and further digested in collagenase in a shaking water bath. Undigested seminiferous tubules were removed, and the interstitial cells were recovered from the supernatant by centrifugation (350 × g, 6 minutes). Interstitial cells were suspended in the medium and plated into 35-mm culture dishes for 10 to 15 minutes to allow rapidly adhering macrophages to attach. Nonadherent cells were then removed by vigorous washing with the medium. Cells were maintained in 1 mL of medium at 34°C in a humidified atmosphere of 95% air and 5% CO2. Testicular macrophages isolated in this manner are approximately 95% positive for Fc receptor. For experiments involving NO production, testicular macrophages were separated without applying collagenase, as previously described (Moore and Hutson, 1994).
Testicular macrophages were plated into 35-mm dishes at a range of 45 000 to 300 000 cells/dish. After 1 to 2 hours in culture, cells were treated for 24 hours with LPS (0.1 or 10.0 μg/mL) in 1 or 2 mL of medium containing 0.2% BSA.
Tissue Preparation
Rat skeletal muscle (forelimb, ca 0.4 g) was frozen in liquid nitrogen, and the powder obtained was homogenized in 5 mL of homogenization buffer (1 mM EDTA, 1 mM EGTA, 25 mM Tris-HCl, pH 7.4) and centrifuged at 4°C for 60 minutes at 100 000 × g. NOS activity in cytosol and the membrane fractions was examined as described by Kobzik et al (1994).
Determination of l-[14C]Citrulline Formation in Cultured Leydig Cells
Purified Leydig cells from 3 to 6 rats (106 cells/tube) were washed with phosphate-buffered saline (PBS) and incubated for 30 minutes at 34°C in the presence of 5 × 105 cpm l-[14C]arginine as described by Wang et al (1997). Cells were then exposed for 20 minutes to ionomycin (5 μM) in the presence or absence of LH (100 ng/mL). Following an extensive wash with 4 mL of PBS containing 5 mM EDTA, cells were homogenized and centrifuged for 10 minutes at 12 000 × g, and l-[14C]citrulline in the supernatants was estimated by ion-exchange chromatography on Dowex AG 50X-8 columns (Na+ form) and liquid scintillation spectroscopy as described by Wang et al (1997). T biosynthesis in Leydig cells exposed to ionomycin was estimated by radioimmunoassay after 2 hours of incubation as described below.
NOS Activity
The activity of NOS in the homogenates of rat Leydig cells was determined by assessing the conversion of l-[14C]arginine to l-[14C]citrulline according to a previously reported procedure (Weissman and Gross, 1998). Leydig cells purified from 3 to 6 rats were centrifuged at 1000 × g for 10 minutes at 4°C, resuspended in PBS, and centrifuged again. The final pellet from 10 × 106 cells was homogenized in 100 μL of homogenization buffer by a 2 × 10-second sonication (20% maximum energy) using an ultrasonic homogenizer (Cole Parmer Instruments, Chicago, Ill), which was followed by 5 minutes of centrifugation at 1000 × g. Supernatants (ie, the cytosolic fraction) and resuspended pellets (ie, the plasma membrane) were then incubated in duplicate tubes at 34°C in reaction buffer (3 μM tetrahydrobiopterin [BH4], 1 μM flavin adenine dinucleotide, and 1 μM flavin mononucleotide) to which 0.1 μM of calmodulin and approximately 0.5 μCi of l-[14C]arginine were added. Reaction tubes contained samples with a protein content equivalent to 106 cells, and incubations were terminated by the addition of 1 mL of stop buffer (5 mM EDTA, 50 mM HEPES, pH 5.5). l-[14C]citrulline was separated by ion-exchange chromatography and estimated by liquid scintillation counting.
T Radioimmunoassay
T production by Leydig cells was measured with a tritium-based radioimmunoassay as described previously, with a 7% to 8% interassay variation (Cochran et al, 1981).
S-nitrosoglutathione Preparation
S-nitrosoglutathione (GSNO) was prepared as previously described by Mallis et al (2001) and was used immediately thereafter. Briefly, equal volumes of 220 mM of reduced glutathione (GSH, dissolved in 1 M HCl) and 220 mM of sodium nitrite were mixed and incubated in the dark at room temperature for 10 minutes. The solution was then neutralized with NaOH to give a final concentration of approximately 100 mM of GSNO. During these procedures, the mixture should be kept on ice and protected from light. The precise concentration was calculated from absorbance at 334 nm using an extinction coefficient of 767 M−1 cm−1.
DNA Array Assay
Preparation of RNA
Four pools of Leydig cells were used for RNA extraction and for the DNA analysis. For array analysis, RNA samples were processed according to the Altas gene protocol (Clontech). Total RNA was extracted from isolated Leydig cells by a single-step method, using phenol and guanidinium thiocyanate, according to the manufacturer’s instructions. The purity of isolated mRNAs was evaluated spectrophotometrically, using the A260/A280 ratio. To reduce contamination by genomic DNA, total RNA was treated with ribonuclease-free deoxyribonuclease I for 1 hour at 37°C, followed by phenol/chloroform extraction. The RNA was then purified by digestion with DNase I to eliminate DNA contamination. The Leydig cell total RNA (400 ng) was reverse transcribed with avian myeloblastosis virus RT in the presence of random hexamer plus deoxynucleotide triphosphates (dNTPs) at 42°C for 75 minutes, and the reaction was terminated by heating at 95°C for 5 minutes.
Probe Preparation and Hybridization
RNA samples of Leydig cells were used for hybridization to oligonucleotide arrays corresponding to approximately 1185 known genes. To generate reproducible gene expression data, 4 independent replicates of the Leydig cell samples were analyzed. To generate radiolabeled complementary DNA (cDNA) probes, total RNA was reverse transcribed with Moloney murine leukemia virus-reverse transcriptase (MMLV-RT) and radiolabeled with [32P]dATP (10 μCi/μL; Amersham Pharmacia Biotech). The radiolabeled cDNA probes were purified from unincorporated nucleotides by gel filtration on a Chroma Spin-200 column (Clontech) and hybridized overnight at 68°C to a rat microarray consisting of 1185 known rat genes, as described by the manufacturer (Clontech).
Phosphor-imaging
After a series of stringent washes (three 20-minute washes in 2× saline sodium citrate [SSC]/1% sodium dodecyl sulfate [SDS], followed by two 20-minute washes in 0.1× SSC/0.5% SDS) at 68°C, the membranes were sealed in plastic (Kapak Corp, Minneapolis, Minn) and exposed to phosphor-imager plates for 3 to 48 hours. Images of the hybridized filters were obtained after scanning the plates (Storm, Molecular Dynamics Inc, Sunnyvale, Calif).
Image Analysis
The image intensity of each cDNA was imported into Atlas-Image software (version 2.01, Clontech). The intensity of each spot, reflecting the relative abundance of mRNA in the sample, was analyzed in 12 different membranes. Individual gene intensities were normalized to an internal control (housekeeping genes), β-actin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). All data collected were exported into ASCII files and then imported into a relational database (Microsoft Access 2000), which was designated as a Leydig Cell Array Database. In the Leydig Cell Array Database, a bioinformatic listing for each gene was available via links from the accession numbers, Locus Link, and Swissprot Accession.
RT-PCR Analysis
Two hundred fifty nanograms of total RNA was reverse transcribed (MMLV-RT; Promega) in a total volume of 20 μL at 42°C for 75 minutes; then, heating at 95°C for 5 minutes terminated the reaction. PCR amplification was performed in a final volume of 25 μL to which 2 μL of cDNA had been added. The reaction was performed on a PTC-100 thermal controller (MJ Research, Boston, Mass), using the following sequences: TCAT-CA AGAAGG GAAAAG AA and TGAAGC AGATAG CA-CAGA TG (17α-hydroxylase), GACATTGAGATCAAAG-GACTGC and CGGCTCGTCACCTCCTGG (eNOS), AATA-GAGGAACATCTGGCCAGG and ACTTCCTCCAGGATG-TTGTA (iNOS), and GGTTGTCATCCCTCAGCCTGC and GGCAACAGCGACAATTTG (nNOS). The conditions for denaturation, annealing, and extension were 95°C for 15 minutes, 55°C for 30 minutes, and 72°C for 45 minutes, respectively. Assays were conducted at least 4 times for each gene. Densitometric signals from individual bands were divided by the respective density for rat ribosomal protein S16 to correct for differences in gel loading using an imaging system (Kodak Digital Science, Rochester, NY).
Nitrite Determination
The concentration of nitrite that had accumulated in Leydig cells and testicular macrophage culture media was measured by the Griess method (20). Briefly, 100 μL of cell-free supernatants was mixed with 100 μL of Griess reagent (1 part 1% sulfanilamide and 1 part 0.1% naphthyl-ethylenediamine in 5% phosphoric acid solution) in 96-well microtiter plates and incubated at room temperature for 5 minutes. After the incubation, the absorbency of the wells was determined using a microtiter plate reader (Bio-Rad Labs, Kaloon, Hong Kong) equipped with a 540-nm filter. Concentrations of nitrite were determined on the basis of standards of sodium nitrite in the range of 0 to 60 μM dissolved in H2O and expressed as micromolar concentrations of nitrite released per 3 × 105 cells per 24 hours.
Cell Viability
Leydig cell viability was estimated using the Trypan blue dye exclusion test. The stain (5 mg/mL) was added to the cell suspension for 5 minutes at a 1:5 dilution. Dead cells were stained blue. The number of cells excluding the Trypan blue dye was counted in a hemacytometer, and cell viability was the number of unstained divided by the total of stained plus unstained, expressed as a percentage.
Protein Determination
Protein concentrations were determined by the Bradford method (Bradford, 1976), using BSA as a standard.
Data Calculation and Statistical Analysis
T-production parameters were assessed by computer-assisted nonlinear least-square regression analysis using the Prism4 program (GraphPad Software Inc, San Diego, Calif). All data were analyzed by a 1-way analysis of variance, with multiple comparisons performed by the Duncan multiple range test to identify differences between groups. Differences were considered significant at P < .05.
Results
NOS Activity in Isolated Leydig Cells
The capacity of homogenates of purified rat Leydig cells to produce NO was assessed by monitoring the conversion of l-[14C]arginine to l-[14C]citrulline (Bredt and Snyder, 1990) as an index for the rate of NO formation. The levels of NOS activity in these preparations were remarkably low, at 413.7 ± 61.6 fmol/min/mg of protein/min in the particulate fraction (n = 7, Table 1). The ability of cytosolic fractions to convert l-arginine to l-citrulline was even lower, at 167.4 ± 48.9 fmol/min/mg of protein/min (n = 6). In contrast to these minute rates of l-citrulline accumulation, pellets prepared from rat skeletal muscles yielded markedly higher amounts of NO formation (3828 ± 934.9 fmol/mg of protein/min; n = 6, Table 1). It should be noted that most of the enzyme activity in the muscle was confined to the membrane fraction, with less than 15% of the total activity detected in the cytosol (441.5 ± 158.1 fmol/min/mg of protein/min; Table 1). Brain tissue had still higher NOS levels, with extracts prepared from rat cerebellum (the cytosolic fraction) measured at 142.43 ± 19.45 pmol/min/mg of protein/min (n = 5, Table 1). The results obtained using both muscle and cerebellum samples are in close agreement with data published by Kobzik et al (1994).
Table 1.
Determination of l-[14C]citrulline formation in rat Leydig cells, cerebellum, and skeletal muscles*†
| Tissue | Particulate Fraction (fmol/min/mg of Protein/min) | Cytosol (fmol/min/mg of Protein/min) |
|---|---|---|
| Leydig cells | 413.7 ± 61.6 (7) | 167.4 ± 48.9 (6) |
| Leydig cells + EGTA | 515.2 ± 140.5 (3) | ND |
| Leydig cells + L-NAME | 550.4 ± 115.6 (3) | ND |
| Cerebellum | ND | 142 430 ± 19 450 (5) |
| Cerebellum + L-NAME | ND | 1427 ± 495.1 (3) |
| Cerebellum + EGTA | ND | 5843 ± 232.1 (3) |
| Skeletal muscle | 3828 ± 934.9 (6) | 441.5 ± 158.1 (6) |
Rat Leydig cells and muscle samples were prepared as described in “Materials and Methods.” Rat cerebellum extracts were from Stratagene. l-[14C]citrulline formation was estimated in duplicates using the Stratagene kit and is expressed as fmol/min/mg protein/min (mean ± SEM) with the number of experiments in parentheses. EGTA and L-NAME were added at a final concentration of 5 and 1 mM, respectively.
ND indicates not determined; EGTA, ethylenebis (oxyethylenenitrilo)tetraacetic acid; and L-NAME, L-NQ-nitro-arginine methyl ester.
In another set of experiments, freshly isolated rat Leydig cells were incubated in an arginine-free medium and in the presence of l-[14C]arginine. Following the initial period in which radioactive arginine was taken up, stimulation of T production by LH (100 ng/mL) or a robust increase in intracellular Ca2+ concentration due to the action of the calcium ionophore ionomycin (5 μM) did not affect l-[14C]citrulline levels. Under these conditions, rat Leydig cells were less sensitive to LH, as registered by their rates of T production: 6.56 ± 0.18, basal, vs 12.31 ± 0.47 ng/106 cells/h, LH-stimulated (n = 3). Additionally, this concentration of ionomycin had no effect on T production (data not shown). In a separate experiment, a comparison of the capacity of testis homogenates and Leydig cells to support the conversion of l-arginine to l-citrulline yielded rates of 356 and 95 fmol/min/mg of protein/min, respectively. These results indicate that most of the (low) NOS enzymatic activity in testes does not co-purify with Leydig cells.
The commonly used calcium chelator EGTA (5 mM, Table 1) and the NOS inhibitor L-NAME (1 mM) failed to significantly affect the rate of l-citrulline formation in Leydig cell homogenates (data not shown). Under the same conditions, these agents potently inhibited NOS activity in homogenates prepared from rat cerebellum. EGTA and L-NAME produced 96% (n = 3) and 99% (n = 3, Table 1), respectively, inhibition of l-citrulline production in samples of cerebellum.
Modulation of T Production in Leydig Cells
LH induced a concentration-dependent elevation of T production by purified rat Leydig cells with a median effective concentration (EC50) value of 0.67 ng/mL (Figure 1). The fivefold stimulation observed at this LH concentration is in the same range as that reported by Payne et al (1982) for Leydig cells isolated from Sprague-Dawley rats. On the basis of this observation, a concentration of 0.5 ng/mL was used throughout this study.
Figure 1.
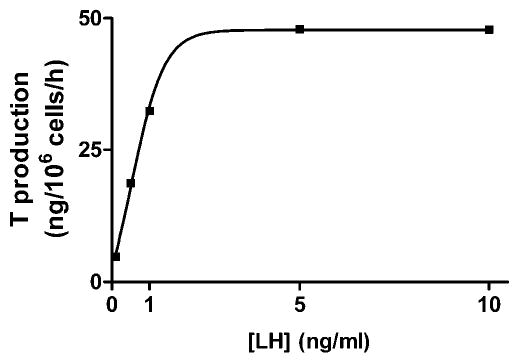
A representative concentration-response curve for luteinizing hormone (LH) in incubated rat Leydig cells. Purified rat Leydig cells were prepared as described in “Materials and Methods” and incubated in the presence of increasing concentrations of LH. Results are presented as nanograms of testosterone formed per 106 cells during a 1-hour incubation. This experiment was repeated 3 times with similar results.
The addition of the NO donor GSNO (0.01 mM) to the incubation media of Leydig cells resulted in a potent inhibition of basal T production by approximately 70% (from 11.49 ± 4.2 to 3.02 ± 0.9 ng/106 cells/h, n = 9). This blockade of LH-stimulated steroidogenesis by GSNO was concentration dependent, could be detected at concentrations below 1 μM, and exhibited a concentration that inhibits 50% (IC50) value of 4.3 ± 1.5 μM (Figure 2). To exclude the possibility that changes in T production result from cell injury caused by NO, we examined the effects of GSNO on Leydig cell survival. As noted by others (eg, (Eizirik et al, 1996), the Trypan blue test showed an 86.05% ± 1.47% viability in control incubations (n = 6) compared to an 82.87% ± 1.34% viability in the presence of 0.1 mM of GSNO, a concentration that induced a complete inhibition of LH-evoked T production (n = 3). Under similar conditions, the survival rate of Leydig cells exposed to 0.01 mM of GSNO was 87.03% ± 1.92% (n = 5). Therefore, the inhibitory effects of NO on T production were not attributable to cytotoxicity.
Figure 2.
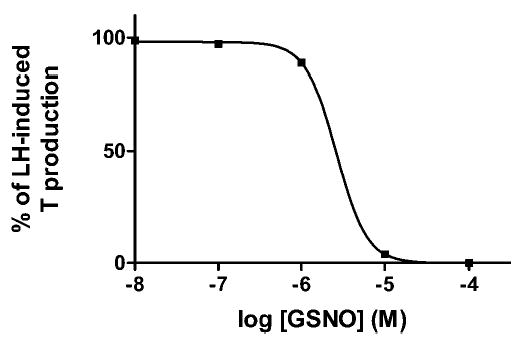
A concentration-response curve for S-nitrosoglutathione (GSNO) in incubated rat Leydig cells. Purified rat Leydig cells were prepared as described in “Materials and Methods” and incubated in the presence of luteinizing hormone (LH) (0.5 ng/mL) and various concentrations of freshly prepared GSNO. Results are presented as in Figure 1 and are a representative experiment repeated 3 times with similar results.
In a representative experiment repeated with similar results 3 times, basal T production in l-arginine–free Leydig cell medium (LCMA) was 8.98 ± 2.9 ng/106 cells/h (n = 3). The addition of 0.5 ng of LH per milliliter to this medium induced an approximately fourfold stimulation (31.81 ± 13.27), whereas further supplementing the medium with 5 mM of l-arginine did not cause a significant change (30.64 ± 7.81 ng/106 cells/h). While LH produced a statistically significant increase of T formation (P < .05), there was no such difference between its effects on the 2 media types. Adding the same amount of l-arginine to resting Leydig cells in LCMA failed to affect T production (10.14 ± 2.56 ng/106 cells/h, n = 3; Figure 3). Finally, the addition of 5 mM of l-arginine to normal LCM containing 0.1 mM of this amino acid also failed to cause a significant decrease in T production (data not shown). This indicated that basal NOS activity in Leydig cells is insufficient to negatively modulate T production.
Figure 3.
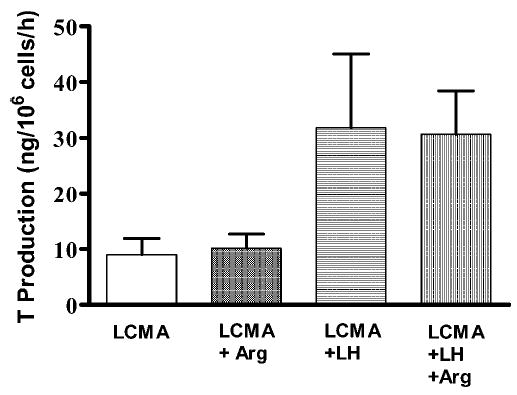
The effects of l-arginine and luteinizing hormone (LH) on testosterone production by rat Leydig cells. Purified rat Leydig cells were prepared as described in “Materials and Methods” and were incubated in l-arginine–free Leydig cell medium (LCMA), LCMA with added l-arginine (5 mM, LCMA + Arg), LCMA in the presence of LH (0.5 ng/mL, LCMA + LH), and LCMA in the presence of LH and l-arginine (LCMA + LH + Arg). Each bar represents the mean ± SEM for testosterone production and is the outcome of 3 separate determinations repeated with similar results.
Two NOS inhibitors, L-NAME (displaying eNOS preference) and L-NMMA (a nonselective blocker), had no discernible effect on the T production of resting rat Leydig cells. Moreover, these agents at a concentration of 1 mM did not show any considerable effect on the LH-induced elevation of T accumulation (Figure 4). Notably, while LH produced statistically significant increases of T formation (P < .05), there was no difference between T levels in incubations carried out in the absence or presence of the 2 blockers.
Figure 4.
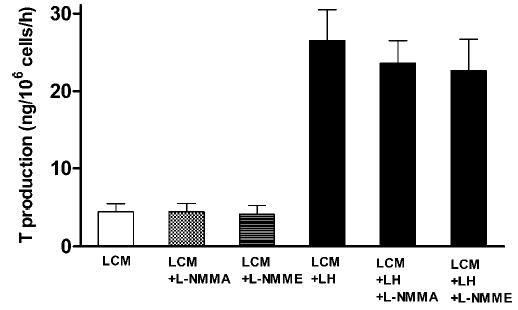
The effects of nitric oxide synthase (NOS) inhibitors L-Ng-monomethyl-arginine (L-NMMA) (1 mM; n = 12) and L-NG-nitro-arginine methyl ester (L-NAME) (1 mM; n = 9) on testosterone production by rat purified Leydig cells prepared as described in Figure 3 and incubated in Leydig cell medium (LCM). Luteinizing hormone (LH) was present at a concentration of 0.5 ng/mL. Results are presented as the mean ± SEM of testosterone formed by 106 cells during a 1-hour incubation in the absence or presence of the NOS inhibitor.
DNA Gene Array Assays
Table 2 illustrates the expression level of several genes encoding enzymes relevant for steroidogenesis in adult rat Leydig cells. The housekeeping gene β-actin was assigned a value of 1000, and all other genes are noted relative to this value. The detection level is set at twice the standard deviation of the noise, namely 2 × 2.7 = 5.4. Of the 3 NOS isoforms, only the endothelial isoform exhibited a significant level of expression (10.38), while the other two did not reach the detection level. Arginase 1, a member of the urea cycle that metabolizes l-arginine to urea and l-ornithine, was expressed in rat Leydig cells at a level slightly lower than that of eNOS (ie, 7.59, Table 2). In contrast to these data, genes that are related to steroid biosynthesis were abundantly expressed, ranging from 380.51 for 11-βHSD-1 to 2493.21 and 8654.86 for 3β-HSD and 17α-hydroxylase, respectively.
Table 2.
| Gene Code | Protein Name | Expression Level (Arbitrary Units) |
|---|---|---|
| A08f | iNOS | 4.08 ± 1.31 |
| C10n | eNOS | 10.38 ± 1.25 |
| F06h | nNOS | 3.02 ± 0.21 |
| C09a | Arginase 1 | 7.59 ± 1.33 |
| CYP17 | 17α-hydroxylase | 8654.86 ± 760.12 |
| C07a | 3β-HSD | 2493.21 ± 599.51 |
| C051 | 11-β-HSD1 | 380.51 ± 37.93 |
| G27 | GAPDH | 179.60 ± 25.29 |
| G43 | β-Actin | 1000 |
Gene expression levels were estimated as described in “Materials and Methods” and are presented as the ratio to the housekeeping gene β-actin, which was set at 1000. The detection level is set at twice the standard deviation of the noise at 5.4, and values represent the mean ± SEM of 4 separate determinations.
NO indicates nitric oxide; iNOS, inducible NO synthase; eNOS, endothelial NO synthase; nNOS, neuronal NO synthase; HSD, hydroxysteroid dehydrogenase; and GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
RT-PCR Analyses
The results shown in Figure 5 clearly demonstrate that the levels of mRNA expression of eNOS and nNOS in adult Leydig cells, as determined by RT-PCR analyses, are below detection levels following a 20-cycle paradigm (panel A). Under the same conditions, the message level for 17α-hydroxylase in these cells, and that of nNOS in the cerebellum, was high. In addition, there were detectable levels of eNOS mRNA in the cerebellum (panel A).
Figure 5.
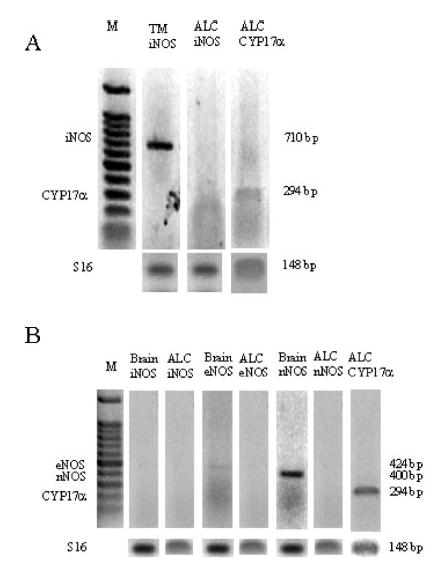
Representative reverse transcriptase-polymerase chain reaction (RT-PCR) analyses of 17α-hydroxylase (CYP17α), endothelial (eNOS), neuronal (nNOS), and inducible (iNOS) messenger RNA (mRNA) in Leydig cells (ALC), testicular macrophages (TMs), and cerebellum (brain) of the rat. (A) Testicular macrophages were stimulated for 24 hours with lipopolysaccharide (LPS) (0.1 μg/mL) and then processed as described in “Materials and Methods.” (B) Samples of cerebellum and Leydig cells were examined to detect the presence of the specific mRNAs. Molecular markers are depicted under M. These experiments were repeated 4 times on separate samples with similar results.
RT-PCR and Griess Analyses of Testicular Macrophages
A high level of iNOS expression was noted in LPS-activated testicular macrophages, whereas it was absent in Leydig cells (Figure 5, panel B). Treatment of testicular macrophages with LPS (10.0 μg/mL) for 24 hours resulted in a significant (P < .01) nitrite accumulation in the medium (5.94 ± 0.38 and 44.3 ± 7.3 [n = 3] nmol/105 cells/24 h, for the control vs the LPS-stimulated samples, respectively). The population of testicular macrophages in the adult rat testis is 7.5 × 106, compared to 26 × 106 Leydig cells (Hardy et al, 1989). Using these numbers, it can be calculated that 138 nmol of NO would be released by activated macrophages per hour. Given an average volume of a rat testis of 1.66 cm3 and an interstitial volume of 0.35026 cm3 (Christensen and Peacock, 1980), the cumulative concentration of NO in the interstitial milieu may reach 394 mM/h. Even allowing for just 1 second of NO accumulation yields a concentration higher than 0.1 mM, which was observed to block T production completely (Figure 2). Under the conditions used to activate macrophages, resting or LPS-treated freshly isolated rat Leydig cells did not release nitrite into the medium. Moreover, LPS had no effect on T production (32.36 ± 1.50 and 36.40 ± 2.04 [n = 3] ng/106 cells/24 h, for the basal vs the LPS-stimulated samples, respectively).
Discussion
Our results show 3 novel and important aspects of rat Leydig cell function: 1) unlike other steroidogenic tissues, they are devoid of the l-arginine/NO pathway; 2) their T production is highly sensitive to exogenous NO; and 3) under pathologic conditions, testicular macrophages are the most likely source of this reactive free radical.
Studies localizing NOS proteins (Davidoff et al, 1995) and NOS activity (Burnett et al, 1995) to human and rat testis, respectively, were followed by reports on the effects of NO on T production. When purified Leydig cells were exposed to NO donors, a reduction in the production of T was observed (Del Punta et al, 1996). Subsequent analysis of NOS inhibition in testicular preparations provided further evidence that NO may act as an interstitial regulator of steroidogenesis (Welch et al, 1995). Robust support for the putative involvement of the l-arginine/NO pathway in steroid biosynthesis was provided by investigation of other endocrine organs. For example, NO donors reduce corticosterone production in rat adrenal zona fasciculata cells in vitro (Cymeryng et al, 1998). In addition, the NOS inhibitor L-NG-nitro-arginine brought about an increase in the synthesis of steroids in these cells. Moreover, the NOS substrate and NO precursor l-arginine decreased corticosterone levels (Cymeryng et al, 1999), adding more support to the hypothesis that endogenous formation of NO attenuates steroidogenesis. Indeed, our data demonstrate that the exogenous NO that was produced by GSNO inhibited the biosynthesis of T either by resting or LH-stimulated Leydig cells at concentrations in the low micromolar range (Figure 2). It is notable that previous studies (eg, Del Punta et al, 1996) using agents such as a diethylamine/NO complex (DEA/NO) required 3 orders of magnitude higher concentrations (ie, 0.5–1.0 mM) to obtain partial inhibition of T production.
We show that the omission of l-arginine from the incubation medium did not induce an increase in basal T production (Figure 3). In fact, an attempt to influence NO production in rat Leydig cells by elevating the extracellular concentration of l-arginine to 5 mM did not affect T production. Moreover, the rise in T production invoked by LH (0.5 ng/mL) was only slightly reduced by the addition of this high concentration of l-arginine (Figure 3). While this observation is in contrast with reports on the effect of l-arginine on estrogen (Vega et al, 1998) or corticosterone (Cymeryng et al, 1999) synthesis, it corroborates our results showing that the rate of l-[14C]arginine conversion in purified rat Leydig cell preparations is noticeably low (Table 1). The putative NOS activity measured in the membrane and cytosolic fractions of these cells amounts to less than one hundredth of that detected in the rat cerebellum and is significantly lower than that found in muscle tissue (Table 1) (Kobzik et al, 1994). Moreover, the calcium chelator EGTA failed to alter the minute quantity of l-citrulline formed by Leydig cell homogenates. Since the iNOS isoform is calcium independent (Xie et al, 1992), the failure of EGTA to decrease arginine conversion could be the result of the presence of a trace amount of this enzyme in rat Leydig cells. Nevertheless, the fact that a supramaximal concentration of the NOS inhibitor L-NAME was ineffective in blocking l-[14C]arginine conversion, and, in addition, failed to alter (ie, increase) T production, strongly indicates the absence of any NOS isoform in normal rat Leydig cells. This hypothesis received added support from other studies in which L-NAME did not increase T production in untreated and LPS-inflamed rats (O’Bryan et al, 2000a). In addition, unlike cells expressing either eNOS (Murray et al, 1986) or nNOS (Payne et al, 1982; Reiser, 1990; Wang et al, 1997) that exhibit a conspicuous Ca2+-evoked NO production, the NOS activity or T production in intact rat Leydig cells was not affected by the presence of 5 μM ionomycin.
According to Burnett et al (1995), the activity of NOS in the rat testis is remarkably low and is mainly confined to vascular endothelial cells, whereas Leydig cells do not exhibit any NADPH-diaphorase staining or l-citruline formation. These findings substantiate an earlier account showing no significant NOS activity in the human urogenital tract and Leydig cells (Ehren et al, 1994). While L-NAME potently blocked l-[14C]citrulline accumulation in various male rat reproductive tissues, this activity in the testis could be reduced by only 50% (Burnett et al, 1995). Using immunohistochemical techniques, eNOS has been detected in rat Sertoli, Leydig, and germ cells (Zini et al, 1996), nNOS was identified in Leydig cells by immunocytochemistry (Lissbrant et al, 1997), and constitutively expressed iNOS was proposed as a modulator of T production in these cells (O’Bryan et al, 2000a; Koksal et al, 2003). Nevertheless, these studies did not demonstrate enzyme activity in Leydig cells, and, in fact, it has been argued that the absence of NADPH-diaphorase activity in Leydig cells indicates the existence of an inactive form of nNOS (Lissbrant et al, 1997).
It has been proposed that iNOS is present in many cell types of the rat testis (O’Bryan et al, 2000a) but that it is absent from both normal and LPS-treated testicular macrophages. However, this conflicts with other results (Ehren et al, 1994; Gerdprasert et al, 2002), demonstrating the absence of iNOS activity in human and rat testes, respectively, and contrasts with our results (Figure 5). While iNOS was detected in numerous cell types in the testis, the application of L-NAME resulted in a decrease in T production in normal animals and had no effect when administered to LPS-treated rats (O’Bryan et al, 2000b). These results clearly rule out the involvement of endogenously generated NO in T production. As with other cell types exhibiting a barely detectable level of iNOS mRNA, immature rat Leydig cells have been shown to express this enzyme when challenged with proinflammatory cytokines (Tatsumi et al, 1997). Together, the reports that place NOS proteins in Leydig cells cast the putative functional role of NOS in these cells in doubt.
Our results suggest that the minor conversion of l-[14C]arginine observed is a product(s) of enzyme(s) activities other than NOS. A likely candidate for the transformation of l-arginine to a less basic amino acid in rat Leydig cells is arginase 1. The l-[14C]ornithine formed by this enzyme would then be monitored as l-[14C]citrulline by the assay used in this study. Indeed, the arginase 1 gene is expressed in Leydig cells at a level slightly lower than that of eNOS (Table 2). The combined activity of these 2 enzymes could account for the low l-[14C]arginine conversion products detected in this and other studies (Ehren et al, 1994; Burnett et al, 1995). Robust support for the biochemical data that imply the absence of NOS isoforms in rat Leydig cells is derived from DNA gene array and RT-PCR analyses (Table 2; Figure 5). In fact, while the former method showed that rat Leydig cells express the complete arsenal of steroidogenic enzymes and only a residual level of eNOS, the latter strengthened and emphasized these findings.
In summary, using several different experimental approaches, we present data leading to the conclusion that under normal in vitro conditions, rat Leydig cells are devoid of an l-arginine/NO pathway. It remains possible that some of the differences between the results of the present work and those previously indicating that Leydig cells are a source of NO are a result of differences observed in vitro and in vivo. However, most other steroid-producing tissues that express NOS do so even in vitro, suggesting that the in vitro conditions are not restrictive to this parameter. Figure 6 depicts the settings for the involvement of NO in Leydig cell function under basal and pathologic conditions. Leydig cells reside in the interstitial tissue that contains blood vessels, lymphatics, and macrophages. LH from testicular circulation stimulates Leydig cell T production, while LPS derived from testicular or systemic bacterial infection affects the adjacent testicular macrophages. As a result, NO is generated by the testicular macrophages and inhibits the neighboring Leydig cells. This model is based on the foundation that 1) T production is sensitive to NO (Adams et al, 1994; Del Punta et al, 1996; and results reported herein), and 2) Leydig cell T production was dramatically reduced when these cells were cocultured with either testicular or peritoneal macrophages (Sun et al, 1993; Pomerantz and Pitelka, 1998). By showing that an NO scavenger and an NOS inhibitor block the reduction in T production, the latter study provided evidence to support the notion that NO is the agent mediating the inhibitory action of activated macrophages. Thus, after we demonstrated that Leydig cells are essentially devoid of NOS activity, we showed that they are highly sensitive to exogenous NO. In purified Leydig cells, submicromolar concentrations of GSNO, an endogenous NO donor, decreased T secretion. In addition, testicular macrophages reside in close proximity to Leydig cells in the interstitial tissue and are known to secrete NO when activated. Close cellular proximity is crucial, since NO is an unstable free radical. Therefore, we infer that testicular macrophages are a likely source of NO under pathologic conditions.
Figure 6.
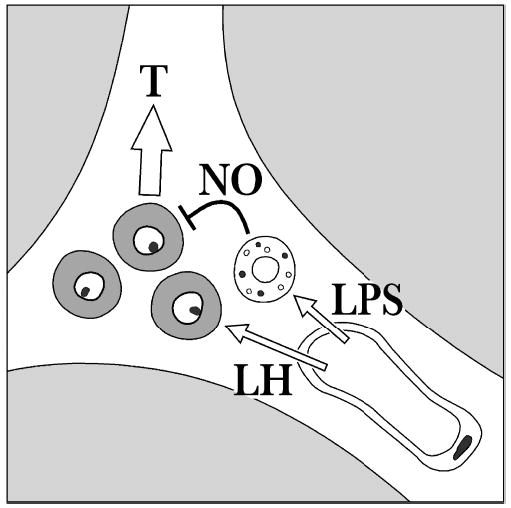
A schematic representation of the rat interstitial tissue with an emphasis on blood vessels, testicular macrophages (depicted with vacuoles), and Leydig cells (shaded gray). Luteinizing hormone (LH) from the blood circulation interacts with its receptors on Leydig cells to stimulate testosterone (T) production. Lipopolysaccharide (LPS) stimulates testicular macrophages, which, in turn, release cytokines and nitric oxide (NO) that block T formation by Leydig cells.
Footnotes
This study was supported in part by NATO Expert Visit grant LST/EV 979507 (B.A.W.); NIH HD33000 (M.P.H.); and an NIH Minority Supplement HD33000 (M.H.).
References
- Adams ML, Meyer ER, Sewing BN, Cicero TJ. Effects of nitric oxide–related agents on rat testicular function. J Pharmacol Exp Ther. 1994;269:230–237. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett AL, Ricker DD, Chamness SL, Maguire MP, Crone JK, Bredt DS, Snyder SH, Chang TS. Localization of nitric oxide synthase in the reproductive organs of the male rat. Biol Reprod. 1995;52:1–7. doi: 10.1095/biolreprod52.1.1. [DOI] [PubMed] [Google Scholar]
- Christensen AK, Peacock KC. Increase in Leydig cell number in testes of adult rats treated chronically with an excess of human chorionic gonadotropin. Biol Reprod. 1980;22:383–391. doi: 10.1093/biolreprod/22.2.383. [DOI] [PubMed] [Google Scholar]
- Cochran RC, Ewing LL, Niswender GD. Serum levels of follicle-stimulating hormone, prolactin, testosterone, 5α-dihydrotestosterone, 5α-androstane-3α,17β-diol, 5α-androstane-3β,17β-diol and 17β-estradiol from male beagles with spontaneous or induced benign prostatic hyperplasia. Invest Urol. 1981;19:142–147. [PubMed] [Google Scholar]
- Cutolo M, Balleari E, Giusti M, Monachesi M, Accardo S. Sex hormone status of male patients with rheumatoid arthritis: evidence of low serum concentrations of testosterone at baseline and after human chorionic gonadotropin stimulation. Arthritis Rheum. 1988;31:1314–1317. doi: 10.1002/art.1780311015. [DOI] [PubMed] [Google Scholar]
- Cymeryng CB, Dada LA, Colonna C, Mendez CF, Podesta EJ. Effects of L-arginine in rat adrenal cells: involvement of nitric oxide synthase. Endocrinology. 1999;140:2962–2967. doi: 10.1210/endo.140.7.6848. [DOI] [PubMed] [Google Scholar]
- Cymeryng CB, Dada LA, Podesta EJ. Effect of nitric oxide on rat adrenal zona fasciculata steroidogenesis. J Endocrinol. 1998;158:197–203. doi: 10.1677/joe.0.1580197. [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Mayer B, Holstein AF. Nitric oxide synthase (NOS-I) in Leydig cells of the human testis. Arch Histol Cytol. 1995;58:17–30. doi: 10.1679/aohc.58.17. [DOI] [PubMed] [Google Scholar]
- Del Punta K, Charreau EH, Pignataro OP. Nitric oxide inhibits Leydig cell steroidogenesis. Endocrinology. 1996;137:5337–5343. doi: 10.1210/endo.137.12.8940355. [DOI] [PubMed] [Google Scholar]
- Di Rosa M, Radomski M, Carnuccio R, Moncada S. Glucocorticoids inhibit the induction of nitric oxide synthase in macrophages. Biochem Biophys Res Commun. 1990;172:1246–1252. doi: 10.1016/0006-291x(90)91583-e. [DOI] [PubMed] [Google Scholar]
- Diemer T, Ludwig M, Huwe P, Hales DB, Weidner W. Influence of urogenital infection on sperm function. Curr Opin Urol. 2000;10:39–44. doi: 10.1097/00042307-200001000-00010. [DOI] [PubMed] [Google Scholar]
- Ehren I, Adolfsson J, Wiklund NP. Nitric oxide synthase activity in the human urogenital tract. Urol Res. 1994;22:287–290. doi: 10.1007/BF00297196. [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Delaney CA, Green MH, Cunningham JM, Thorpe JR, Pipeleers DG, Hellerstrom C, Green IC. Nitric oxide donors decrease the function and survival of human pancreatic islets. Mol Cell Endocrinol. 1996;118:71–83. doi: 10.1016/0303-7207(96)03768-9. [DOI] [PubMed] [Google Scholar]
- Fenster L, Katz DF, Wyrobek AJ, Pieper C, Rempel DM, Oman D, Swan SH. Effects of psychological stress on human semen quality. J Androl. 1997;18:194–202. [PubMed] [Google Scholar]
- Gerdprasert O, O’Bryan MK, Muir JA, Caldwell AM, Schlatt S, de Kretser DM, Hedger MP. The response of testicular leukocytes to lipopolysaccharide-induced inflammation: further evidence for heterogeneity of the testicular macrophage population. Cell Tissue Res. 2002;308:277–285. doi: 10.1007/s00441-002-0547-6. [DOI] [PubMed] [Google Scholar]
- Hales DB. Leydig cell–macrophage interactions: an overview. In: Payne AH, Hardy MP, Russell LD, eds. The Leydig Cell. Vienna, Ill: Cache River Press; 1996:451–465.
- Hardy MP, Zirkin BR, Ewing LL. Kinetic studies on the development of the adult population of Leydig cells in testes of the pubertal rat. Endocrinology. 1989;124:762–770. doi: 10.1210/endo-124-2-762. [DOI] [PubMed] [Google Scholar]
- Hutson JC. Development of cytoplasmic digitations between Leydig cells and testicular macrophages of the rat. Cell Tissue Res. 1992;267:385–389. doi: 10.1007/BF00302977. [DOI] [PubMed] [Google Scholar]
- Hutson JC. Interactions between testicular macrophages and Leydig cells. J Androl. 1998;19:394–398. [PubMed] [Google Scholar]
- Hutson JC, Garner CW, Doris PA. Purification and characterization of a lipophilic factor from testicular macrophages that stimulates testosterone production by Leydig cells. J Androl. 1996;17:502–508. [PubMed] [Google Scholar]
- Klinefelter GR, Kelce WR, Hardy MP. Isolation and culture of Leydig cells from adult rats. Methods Toxicol. 1993;3A:166–181. [Google Scholar]
- Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Koksal IT, Usta MF, Akkoyunlu G, et al. The potential role of advanced glycation end product and iNOS in chronic renal failure–related testicular dysfunction. An experimental study. Am J Nephrol. 2003;23:361–368. doi: 10.1159/000072971. [DOI] [PubMed] [Google Scholar]
- Kostic T, Andric S, Kovacevic R, Maric D. The involvement of nitric oxide in stress-impaired testicular steroidogenesis. Eur J Pharmacol. 1998;346:267–273. doi: 10.1016/s0014-2999(98)00057-0. [DOI] [PubMed] [Google Scholar]
- Lissbrant E, Lofmark U, Collin O, Bergh A. Is nitric oxide involved in the regulation of the rat testicular vasculature? Biol Reprod. 1997;56:1221–1227. doi: 10.1095/biolreprod56.5.1221. [DOI] [PubMed] [Google Scholar]
- Mallis RJ, Buss JE, Thomas JA. Oxidative modification of H-ras: S-thiolation and S-nitrosylation of reactive cysteines. Biochem J. 2001;355:145–153. doi: 10.1042/0264-6021:3550145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Hutson JC. Physiological relevance of tumor necrosis factor in mediating macrophage–Leydig cell interactions. Endocrinology. 1994;134:63–69. doi: 10.1210/endo.134.1.8275970. [DOI] [PubMed] [Google Scholar]
- Murray JJ, Fridovich I, Makhoul RG, Hagen PO. Stabilization and partial characterization of endothelium-derived relaxing factor from cultured bovine aortic endothelial cells. Biochem Biophys Res Commun. 1986;141:689–696. doi: 10.1016/s0006-291x(86)80227-3. [DOI] [PubMed] [Google Scholar]
- Nes WD, Lukyanenko YO, Jia ZH, Quideau S, Howald WN, Pratum TK, West RR, Hutson JC. Identification of the lipophilic factor produced by macrophages that stimulates steroidogenesis. Endocrinology. 2000;141:953–958. doi: 10.1210/endo.141.3.7350. [DOI] [PubMed] [Google Scholar]
- O’Bryan MK, Schlatt S, Gerdprasert O, Phillips DJ, de Kretser DM, Hedger MP. Inducible nitric oxide synthase in the rat testis: evidence for potential roles in both normal function and inflammation-mediated infertility. Biol Reprod. 2000a;63:1285–1293. doi: 10.1095/biolreprod63.5.1285. [DOI] [PubMed] [Google Scholar]
- O’Bryan MK, Schlatt S, Phillips DJ, de Kretser DM, Hedger MP. Bacterial lipopolysaccharide-induced inflammation compromises testicular function at multiple levels in vivo. Endocrinology. 2000b;141:238–246. doi: 10.1210/endo.141.1.7240. [DOI] [PubMed] [Google Scholar]
- Payne AH, Downing JR, Wong KL. Luteinizing hormone receptor and testosterone synthesis in two distinct populations of Leydig cells. Endocrinology. 1980;106:1424–1429. doi: 10.1210/endo-106-5-1424. [DOI] [PubMed] [Google Scholar]
- Payne AH, O’Shaughnessy PJ, Chase DJ, Dixon GE, Christensen AK. LH receptors and steroidogenesis in distinct populations of Leydig cells. [Review] [23 refs] Ann N Y Acad Sci. 1982;383:174–203. doi: 10.1111/j.1749-6632.1982.tb23168.x. [DOI] [PubMed] [Google Scholar]
- Pomerantz DK, Pitelka V. Nitric oxide is a mediator of the inhibitory effect of activated macrophages on production of androgen by the Leydig cell of the mouse. Endocrinology. 1998;139:922–931. doi: 10.1210/endo.139.3.5773. [DOI] [PubMed] [Google Scholar]
- Reiser G. Endothelin and a Ca2+ ionophore raise cyclic GMP levels in a neuronal cell line via formation of nitric oxide. Br J Pharmacol. 1990;101:722–726. doi: 10.1111/j.1476-5381.1990.tb14147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XR, Hedger MP, Risbridger GP. The effect of testicular macrophages and interleukin-1 on testosterone production by purified adult rat Leydig cells cultured under in vitro maintenance conditions. Endocrinology. 1993;132:186–192. doi: 10.1210/endo.132.1.8419122. [DOI] [PubMed] [Google Scholar]
- Tatsumi N, Fujisawa M, Kanzaki M, Okuda Y, Okada H, Arakawa S, Kamidono S. Nitric oxide production by cultured rat Leydig cells. Endocrinology. 1997;138:994–998. doi: 10.1210/endo.138.3.4961. [DOI] [PubMed] [Google Scholar]
- Vega M, Johnson MC, Diaz HA, Urrutia LR, Troncoso JL, Devoto L. Regulation of human luteal steroidogenesis in vitro by nitric oxide. Endocrine. 1998;8:185–191. doi: 10.1385/ENDO:8:2:185. [DOI] [PubMed] [Google Scholar]
- Wang Y, Goligorsky MS, Lin M, Wilcox JN, Marsden PA. A novel, testis-specific mRNA transcript encoding an NH2-terminal truncated nitric-oxide synthase. J Biol Chem. 1997;272:11392–11401. [PubMed] [Google Scholar]
- Weissman BA, Gross SS. Measurement of NO and NO synthase. In: Crawley J, Gerfen C, Rogawski M, Sibley D, Skolnick P, eds. Current Protocols in Neuroscience. New York, NY: John Wiley & Sons Inc; 1998:7.13.1–7.13.22.
- Welch C, Watson ME, Poth M, Hong T, Francis GL. Evidence to suggest nitric oxide is an interstitial regulator of Leydig cell steroidogenesis. Metabolism. 1995;44:234–238. doi: 10.1016/0026-0495(95)90271-6. [DOI] [PubMed] [Google Scholar]
- Xie QW, Cho HJ, Calaycay J, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Zini A, O’Bryan MK, Magid MS, Schlegel PN. Immunohistochemical localization of endothelial nitric oxide synthase in human testis, epididymis, and vas deferens suggests a possible role for nitric oxide in spermatogenesis, sperm maturation, and programmed cell death. Biol Reprod. 1996;55:935–941. doi: 10.1095/biolreprod55.5.935. [DOI] [PubMed] [Google Scholar]


