Abstract
Bone morphogenetic proteins (BMPs) play important roles in reproduction including primordial germ cell (PGC) formation, follicular development, spermatogenesis and FSH secretion. Dragon, a recently identified glycosylphosphatidylinositol (GPI)-anchored member of the repulsive guidance molecule (RGM) family, is also a BMP co-receptor. In the present study, we determined the tissue and cellular localization of Dragon in reproductive organs using immunohistochemistry and in situ hybridization. Among reproductive organs, Dragon was expressed in testis, epididymis, ovary, uterus, and pituitary. In the testis of early postnatal mice, Dragon was found in gonocytes and spermatogonia while in immature testes, Dragon was only weakly expressed in spermatogonia. Interestingly, PMSG treatment of immature mice robustly induced Dragon production in spermatocytes. In adult testis, Dragon was found in spermatocytes and round spermatids. In the ovary, Dragon was detected exclusively within oocytes and primarily those within secondary follicles. In the pituitary, Dragon expressing cells overlapped FSH expressing cells. Dragon was also expressed in a number of cell lines originating from reproductive tissues including Ishikawa, Hela, LβT2, MCF-7 and JEG3 cells. Immunocytochemistry and gradient sucrose ultracentrifugation studies showed Dragon was localized in lipid rafts within the plasma membrane. In reproductive cell lines, Dragon expression enhanced signaling of exogenous BMP2 or BMP4. The present studies demonstrate that Dragon expression is dynamically regulated throughout the reproductive tract and that Dragon protein modulates BMP signaling in cells from reproductive tissues. The overlap between Dragon expression and the functional BMP signaling system suggests that Dragon may play a role in mammalian reproduction.
Keywords: Dragon, RGM, BMP signaling, testis, ovary, epididymis, uterus, pituitary, localization, lipid raft
Introduction
Mammalian reproduction is regulated by endocrine hormones such as pituitary FSH and LH, as well as by locally produced growth factors, including TGF-β superfamily members activin, inhibin and bone morphogenetic proteins (BMPs) (reviewed in (1)). BMPs were originally identified by their ability to induce bone and cartilage formation (2). However, numerous studies have revealed that BMPs have a wide variety of effects on many cell types, including monocytes, and epithelial, mesenchymal, and neuronal cells, and play pivotal roles in cytodifferentiation, morphogenesis and organogenesis (3). Members of the TGF-β superfamily, including BMP’s, transduce their signals through binding to type I and II serine/threonine kinase receptors. BMP signaling is mediated intracellularly by the phosphorylation of receptor-activated Smads (R-Smad) 1, 5, and 8. Activated R-Smads complex with the common partner Smad 4 and translocate to the nucleus where they initiate BMP-stimulated alterations in target gene expression. Signaling of TGFβ superfamily members including BMPs is also modulated by soluble extracellular proteins such as noggin, chordin and gremlin. In addition, membrane-associated proteins, including betaglycan (TGFβ type III receptor), endoglin and crypto are also critical for assisting with ligand binding to receptor, or for altering receptor specificity (Reviewed in (4–6)).
The mRNAs encoding BMP 2, 3, 3b, 4, 6, 7 and 15 have been identified in mammalian ovaries. Moreover, BMPR-IA, -IB, and –II are widely expressed in the ovary, with the strongest expression in the granulosa cells and oocytes of developing follicles in normally cycling rats (7;8). BMPs and their receptors are also expressed in uterine stroma and glandular epithelium (9). In males, BMP2, BMP4, BMP 8A and 8B are expressed in germ cells and BMP4, BMP7 and BMP8A are expressed in the epididymis (reviewed in: (8)). Moreover, mice deficient in BMP4, BMP8A or BMP8B show germ cell degeneration in the testis and/or epithelial cell degeneration in the epididymis (10–12). Together, these results suggest that BMPs may play important roles in regulating reproduction.
Dragon was identified through a genomic screening strategy for genes regulated by DRG11, a homeobox transcription factor that is expressed in embryonic dorsal root ganglion (DRG) (13). Independently, this gene was also cloned as RGMb, one of three mouse homologues of the chicken repulsive guidance molecule (RGM) (14). The Dragon gene encodes a 436 amino acid glycosylphosphatidylinositol (GPI)-anchored protein, suggesting it may be associated with lipid rafts within the plasma membrane. Indeed, adhesion of DRG neurons to HEK293 cells was increased following transfection of HEK293 cells with Dragon cDNA (13). Dragon is expressed in a number of neural tissues including embryonic and adult mouse DRGs, spinal cord and brain (13;15;16). Interestingly, Dragon is also involved in BMP signaling since: 1) injection of Dragon mRNA into Xenopus embryos induced expression of a number of BMP regulated genes; 2) Dragon binds directly to BMP2, BMP4 and BMP receptors; and 3) transfection of Dragon cDNA into BMP-responsive cells enhances transcription of a BMP-responsive reporter (17). These observations indicate that Dragon acts as a BMP co-receptor which regulates cellular response to BMP signals.
To understand the potential role of Dragon in BMP signaling within the reproductive tissues, we examined Dragon expression in murine reproductive tissues and cell lines. We found that Dragon is expressed and dynamically regulated in gonadal germ cells, as well as in epithelial cells of the reproductive tract including epididymis and uterus. Dragon is also expressed in the pituitary. As predicted from its being anchored to the cell membrane by a GPI-anchor, Dragon is indeed localized in lipid rafts where it enhances BMP2 and 4 signaling. Taken together with the overlapping expression and function of BMPs in the reproductive system, our results indicate that Dragon may play an important role in reproduction through enhancement of BMP signaling.
Materials and methods
Animals
All animal studies were conducted in accordance with an animal use protocol approved by the Massachusetts General Hospital animal use committee in accordance with USDA guidelines. Mice [B6C3F1 (C57BI/6 × C3H)] were maintained in the animal barrier facility and sacrificed at different ages to collect tissues for immunohistochemistry and RNA extraction. In addition, mice at 19 days of age were injected with PMSG (ip, 5 IU/mouse; Sigma, St. Louis, MO), and sacrificed 48 hr later to collect gonads for immunohistochemistry.
RT-PCR
Total RNA was extracted from tissues stored in RNAlater (Ambion, Austin, TX) or cells stored in Trizol (Life Technologies, Inc., Carlsbad, CA) according to manufacturer’s protocol. Total RNA (0.5–1.0 ug) was reverse transcribed as previously described (18). Aliquots (2 ml) of first strand cDNA mix were used in PCR reactions (35 cycles) to amplify Dragon and β-actin. The primers, which amplify both human and mouse Dragon cDNA, were TGT TCC AAG GAT GGA CCC ACA TC (forward) and GCA GGT CAT CTG TCA CAG CTT GG (reverse).
Immunohistochemistry and immunocytochemistry
A rabbit polyclonal antibody was raised against a peptide corresponding to the C-terminus of Dragon upstream of its GPI-anchor. This antibody has been shown to specifically recognize Dragon protein (13).
Immunohistochemistry on paraffin sections were performed as previously described (19). Briefly, ovaries were fixed in Bouin’s solution and other organs were fixed in 4% paraformaldehyde. Antigen retrieval was performed on paraffin sections in 0.01 M citrate buffer (pH 6.0). Tissue sections were incubated overnight with anti-Dragon (1:4,000), washed, incubated for 1hr with biotinylated goat anti-rabbit IgG, then 30 minutes with Vectastain Elite ABC (Vector Laboratories, Inc., Burlingame, CA), and developed with DAB for detection (ICN Biomedicaia, Inc., Aurora, OH). Sections were then counterstained with Harris’ hematoxylin.
To co-localize Dragon and FSH in the pituitary, mouse pituitaries were fixed in 4% paraformaldehyde at 4 oC overnight, cryoprotected in 30% sucrose overnight, and frozen in Tissue-Tek OCT embedding compound (Elextron Microscopy Sciences, Washington, PA). Sections (12 μm) were incubated with a mixture of rabbit anti-Dragon serum (1:2000) and guinea pig anti-mouse FSH (1:1600, AFP-3080, NIDDK) for 1 hour, washed, and then with a mixture of FITC-conjugated donkey antirabbit IgG and TRITC-conjugated donkey anti-guinea pig IgG (diluted 1:200; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA).
For immunocytochemistry, Ishikawa cells were grown on glass coverslips. Live cells were incubated with anti-Dragon serum (1:2000) for 1 hr on ice. Cells were then fixed in 2 % paraformaldehyde for 20 minutes. The bound antibodies were detected by incubating with FITC-conjugated donkey antirabbit IgG (diluted 1:200 in PBS) for 1 hr. To demonstrate specificity, the Dragon antibody was pre-incubated overnight with 10 mM of immunization peptide before being applied to sections or cells.
In situ hybridization
Air-dried frozen sections (14–18 μm) were fixed in 4% paraformaldehyde-PBS, digested with proteinase K, acetylated, washed and dehydrated. Anti-sense and sense cRNA probes were prepared by means of in vitro transcription in the presence of [α-35S]UTP, which were then hybridized in 50% deionized formamide, 10 mM Tris/HCl (pH 7.6), 600 mM NaCl, 0.25% SDS, 200 μg/ml yeast tRNA, 50 mM dithiothreitol, 1 × Denhardt’s solution and 10% dextran sulfate overnight at 55 oC in a humidified chamber. After hybridization, the sections were incubated with ribonuclease A and washed in 0.1 × SSC containing β-mercaptoethanol (875 μl in 300 ml) and 0.5 mM EDTA at 65 oC for 1 hr. After developing, the slides were counterstained with hematoxylin and mounted for photography.
Lipid raft protein extraction
Lipid raft proteins were prepared following protocols previously described (20;21). Briefly, Ishikawa cells were scraped and pelleted in ice-old PBS, resuspended in 2 ml of ice cold lysis buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 2mM EDTA, proteinase inhibitors) and allowed to stand on ice for 30 min. The lysate was centrifuged for 5 min at 1300 g to remove nuclei and large cellular debris. The supernatant was mixed with equal volume of 85 % sucrose in TBS (10 mM Tris-HCl, pH 7.5, 150 mM NaCl), placed at the bottom of a 10-ml ultracentrifuge tube, and then overlaid with 5 ml of 35% sucrose and 1.4 ml of 5 % sucrose. The sample was then centrifuged for 14 hr at 150,000 x g in a SW41Ti rotor so that rafts could float to the tip while cytoskeletal and cytoplasmic proteins would remain at the bottom. Five fractions of 1 ml and four fractions of 1.35 ml were collected from the top of the tube. The light-scattering band, an indicator of the location of lipid rafts (20) was located primarily in fraction 2. These fractions were analyzed by trichloroacetic acid precipitation of proteins from 100 ul aliquots followed by SDS-PAGE and Western blotting.
Western blotting
Western blotting analyses were performed as previously described (19). Briefly, samples from sucrose gradient fractions were subjected to SDS-PAGE under reducing conditions, transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA), blocked in 10% non-fat dry milk and incubated overnight at 4oC with rabbit anti-Dragon (1:4000) or anti Caveolin-1 (1:2000; BD Biosciences, San Jose, CA) antibodies. The membranes were washed 3 times before being incubated for 2 hours at room temperature with second antibody, which was detected with enhanced chemiluminescence (ECL) Reagent Plus (PerkinElmer Life Sciences, Boston, MA). After exposure, membranes were stripped for 30 min at 50 oC and reprobed with a monoclonal β-actin antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA).
Transfection and luciferase assay
Ishikawa and KGN cells were maintained in TT medium [1:1 mixture of Dulbecco’s modified Eagle medium and F-12, supplemented with 1% l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin sulfate and 10% FBS (Life Technologies, Inc., Rockville, MD)]. To examine the effect of Dragon on BMP signaling, transfections were performed in 24-well trays using Lipofectamine 2000 (Invitrogen) with a total of 400 ng DNA (180 ng BRE-Luc, a BMP response element kindly provided by Dr. ten Dijke (22), 10 ng pRL-TK, and the indicated doses of Dragon cDNA and pcDNA3). Approximately 24 h after transfection, the media was replaced with serum-free TT medium supplemented with 0.1% BSA, with or without BMP ligands (R&D Systems, Minneapolis, MN). After treating for 16 h, the cells were lysed and assayed for luciferase activity using the dual luciferase reporter assay kit (Promega, Madison, WI, USA).
To obtain noggin protein for neutralization of endogenous BMP’s, the human noggin cDNA (IMAGE clone # 4737725 from ATCC) was subcloned into pcDNA3 (Invitrogen, Carlsbad, CA) and transfected into HEK-293-F suspension cultures in “Freestyle” serum-free medium (Invitrogen) as previously described (23). Concentrated conditioned medium was calibrated by two independent methods including: (a) biological assay and (b) Western blotting. In the biologic assay, increasing amounts of noggin conditioned medium were mixed with 10ng/ml BMP2 and used to treat HepG2 cells that were previously transfected with the BRE-Luc reporter. At the effective concentrations for half-maximum response (EC50), the amount of noggin in the culture medium was equal to that of the BMP2 concentration, assuming molar equivalent antagonism activity (24). For the Western blotting analysis, serial dilutions of noggin condition medium were resolved by 12% PAGE under reducing conditions and visualized by staining with an anti-mouse noggin polyclonal antibody (R&D Systems). Noggin concentrations were estimated at the detection limit dose according to the manufacturers information. Both methods gave similar results. As a control, concentrated conditioned medium from mock transfected 293 cells was tested and determined to have no BMP-inhibitory activity.
To demonstrate that Ishikawa cells transfected with Dragon cDNA actually produce Dragon protein, transfected cells were extracted in RIPA buffer (150 mM NaCl, 50 mM Tris pH 7.5, 1 mM EDTA, 50 mM NaF, 0.5% NP40, 0.5% DOC and 0.1% SDS) and the lysates were then analyzed by Western blotting for Dragon as described above.
Data analysis
Figure 6A, C and D depict the mean ± SE of triplicates from representative experiments. Data in Fig 6C and D were analyzed by t-test. In vitro bioassay experiments in the presence or absence of transfected Dragon (Figure 6E) represents the mean ± SE of six determinations from three independent experiments and were analyzed by two-way ANOVA. Differences between ligand doses or between presence and absence of Dragon were identified by SNK post hoc test. Differences of p < 0.05 were considered significant.
Figure 6.
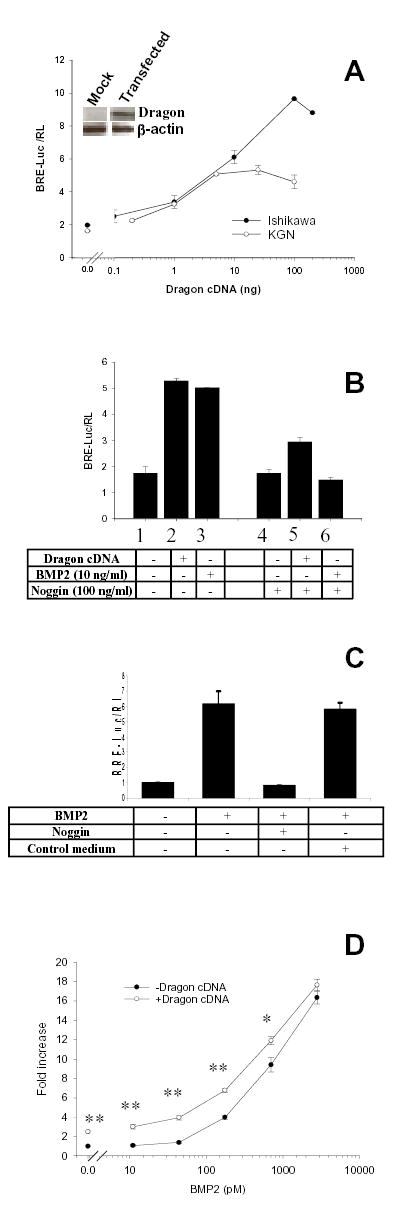
Dragon enhances cellular response to BMP’s in cell lines derived from reproductive tissues. A) Ishikawa or KGN cells were transiently transfected with BRE-Luc reporter in combination with increasing doses of Dragon cDNA and assayed for luciferase activity. Transfection with Dragon increases BRE-Luc response in the absence of exogenous ligand. Values are ratios of BRE-Luc to pRL-TK and are mean ± SE of triplicates from representative experiments. Dragon protein is detectable by Western blot in Ishikawa cells after transfection with 100 ng cDNA. The membrane was stripped and re-probed with β-actin antibody as a control for loading (inset). B) Ishikawa cells were transfected with BRE-Luc reporter and Dragon cDNA and treated with BMP2 alone, or together with noggin. Values are ratios of BRE-Luc to pRL-TK and are mean ± SE of triplicates from representative experiments. C) Control medium was used in parallel with noggin conditioned medium to demonstrate the specificity of the inhibitory activity of noggin conditioned medium in BMP2 signaling. While noggin inhibited BMP2 signaling, control conditioned medium had no inhibitory effect. D) Ishikawa cells transiently transfected with BRE-Luc reporter and Dragon (0 or 10 ng) in 24-well plates were incubated with increasing doses (0–2800 pM) of BMP2. Values are fold increases of luciferase activity in treated cells relative to untreated cells, and are the means ± SE of six determinations from three independent experiments in duplicate. Asterisks indicate significant differences between transfected and untransfected cells at each BMP2 dose (**, P < 0.01; *, P < 0.05).
Results
Expression of Dragon mRNA in reproductive organs
Dragon expression in mouse reproductive tract tissues was examined by RT-PCR (Figure 1). Dragon mRNA was detected in the testis, epididymis and seminal vesicles in males and in the ovary, uterus and pituitary in females.
Figure 1.
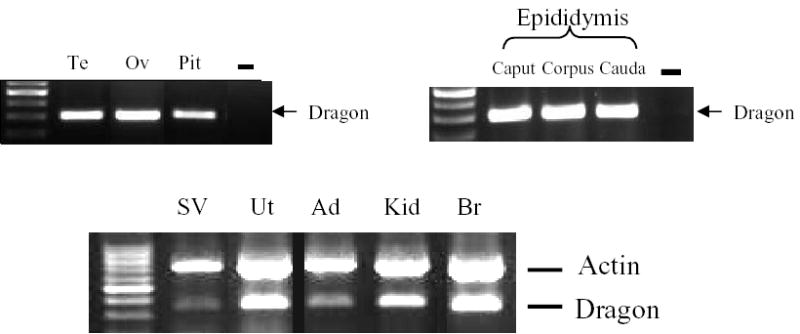
Dragon mRNA expression in mouse tissues. RNA extracted from various mouse tissues was examined for Dragon mRNA expression by RT-PCR. In tissues where Dragon expression was low, β-actin was used as a control for cDNA quality. Dragon expression was strongly detected in testis (Te), ovary (Ov), pituitary (Pit), epididymis, uterus (Ut), kidney (Kid), and brain (Br). Weaker Dragon signals were detected in seminal vesicles (SV) and adrenal (Ad).
Cellular localization of Dragon in the testis and epididymis
Immunohistochemical analyses of day 1 and 3 mouse testes showed that Dragon was localized to gonocytes both in the center and at the basement membrane of seminiferous tubules (Figure 2A, a and b). In testes from day 9 mice, spermatogonia at the basement membrane were positive for Dragon expression (Figure 2A, c). However, Dragon staining in spermatogonia became much weaker in testes from 21-day-old mice (Figure 2A, d). Interestingly, a few gonocytes, which remain in the central region of the tubules from day 21 testes, were strongly stained with Dragon (Figure 2A, d). Some interstitial cells showed weaker staining in day 1 testes (Figure 2A, a), but no staining was observed in interstitial cells in older testes. In adult (day 60) testis, Dragon was expressed in spermatocytes and round spermatids, while spermatogonia and Sertoli cells did not appear to express Dragon (Figure 2A, e and f). The staining in gonocytes, spermatogonia and spermatocytes was completely abolished when the antiserum was preincubated with the competing immunizing peptide, demonstrating the specificity of the antiserum (Figure 2A, g and h). Dragon expression in spermatocytes and round spermatids from adult testes was confirmed by in situ hybridization (Figure 2C).
Figure 2.
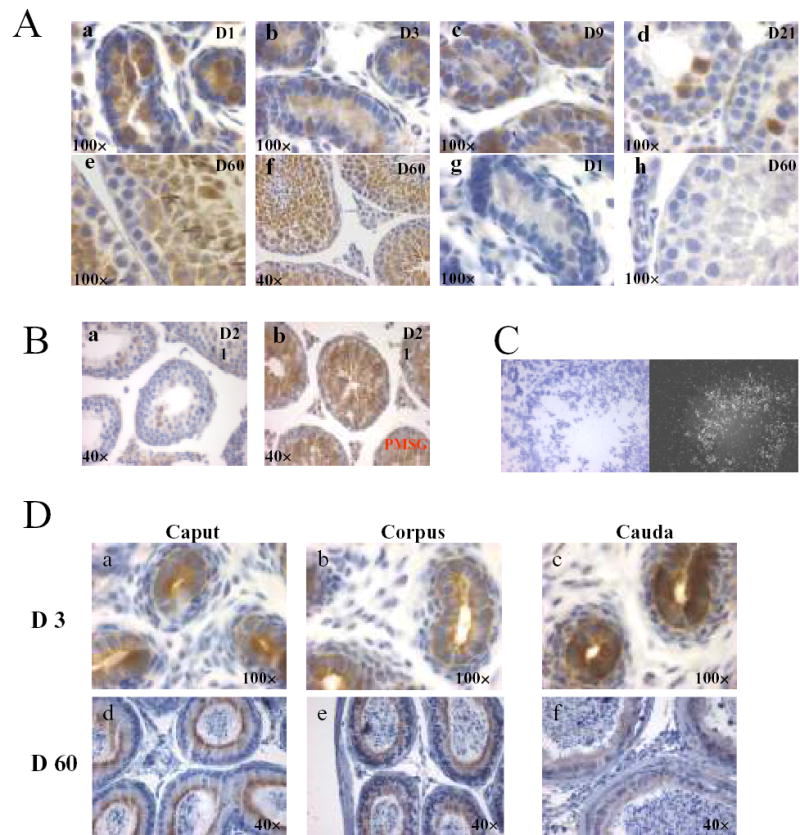
Cellular localization of Dragon in the mouse testis and epididymis during postnatal development by immunohistochemistry and in situ hybridization. For immunohistochemistry, all sections were stained with DAB (brown) and counterstained with hematoxylin (blue). Images are shown at lower (40×) or higher (100×) magnification. (A) Immunolocalization of Dragon in testes at day 1 (a, D1), day 3 (b, D3), day 9 (c, D9), day 21 (d, D21) and day 60 (e and f, D60). For negative control, sections were incubated with Dragon antibody pre-incubated with competing immunizing peptide (g and h). Dragon is highly expressed in gonocytes and spermatogonia in testes of newborn mice and spermatocytes and round spermatids in testes of adult mice. Dragon is not expressed in spermatocytes of day 21 testes. Spermatogonia are weakly stained in day 21 testes but not stained in adult testes (B) Immunostaining of day 21 testes with (b) and without (a) PMSG (5 IU) injection two days earlier. Immunostaining in spermatocytes is turned on by PMSG administration. (C) Localization of Dragon mRNA in mouse testes by in situ hybridization. Bright (left panel) and dark (right panel) field images are shown. (D) Immunolocalization of Dragon in Day 3 (D3/a, b and c) and Day 60 (D60/d, e and f) epididymis. Caput (a and d), corpus (b and e) and cauda (c and f) were dissected.
Spermatocytes of day 21 testes were not stained with Dragon antibody while those cells of adult testes were strongly stained (Figure 2A, d&e). Interestingly, Dragon was highly expressed in spermatocytes of testes collected after two days of PMSG administration to 19-day-old mice (Figure 2B) suggesting that Dragon expression levels are hormonally regulated.
In day 3 epididymis, Dragon protein was found on both the apical and basal sides of epithelial cells with staining in the apical side stronger compared to the basal side. Dragon staining was stronger in caudal than in caput or corpus epididymis of 3-day-old mice (Figure 2D, a, b and c). In contrast, Dragon expression was primarily localized to the apical side of epithelial cells of adult epididymis and it appeared that Dragon was more highly expressed in caput or corpus, compared to caudal epididymis (Figure 2D, d, e, and f). These results suggest that Dragon may be involved in regulation of spermatogenesis and epididymal epithelial function.
Cellular localization of Dragon in the ovary and uterus
Within the adult mouse ovary, Dragon protein was detected exclusively within oocytes (Figure 3A, a, b and d) and was more intense in oocytes from secondary follicles compared to antral follicles (Figure 3A, a). In contrast, no Dragon staining was found in oocytes of primordial (Figure 3A, b) or primary (Figure 3A, c) follicles, nor in somatic cells of any follicles (Figure 3A). In atretic follicles, oocytes showed weak Dragon staining (Figure 3A, d). There was no staining of the ovarian surface epithelium (Figure 3A, b, c and d). Oocyte staining was completely blocked by preincubating antiserum with competing immunizing peptide (Figure 3A, e). In day 9 ovaries, Dragon staining was only detected in the oocytes of secondary follicles, but not in the primordial or primary follicles (Figure 3A, f). PMSG treatment had no effect on Dragon staining in oocytes (data not shown).
Figure 3.
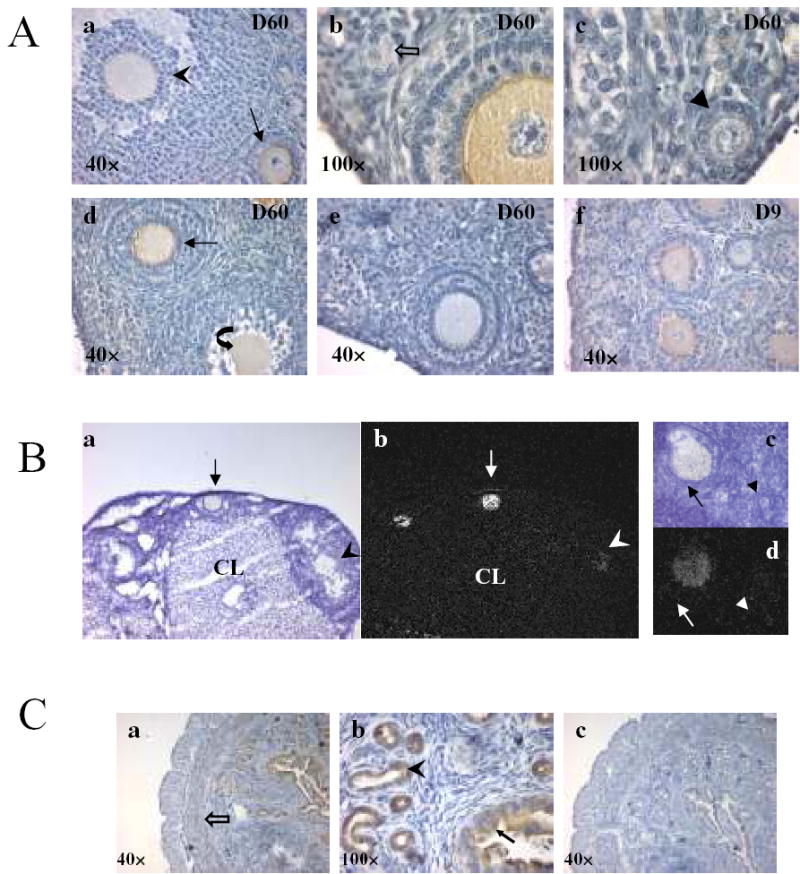
Cellular localization of Dragon in the mouse ovary and uterus by immunohistochemistry and in situ hybridization. For immunohistochemistry, all sections were stained with DAB (brown) and counterstained with hematoxylin (blue). Images are shown at lower (40×) or higher (100×) magnification. (A) Dragon immunostaining in ovaries at day 60 (a, b, c, d/D60) and day 9 (f/D9). The staining in oocytes of secondary follicle (arrow) is stronger than that in oocytes of antral (arrowhead) and atretic (curved arrow) follicles (a and d). Dragon is not expressed in oocytes of primordial (open arrow) and primary (triangle) follicles (b and c). For negative control, sections were incubated with Dragon antibody pre-incubated with competing immunizing peptide (e). (B) Localization of Dragon mRNA in mouse adult ovaries by in situ hybridization. Dark (b and d) and bright (a and c) field images are shown. Signals are confined to oocytes, and signals are stronger in the secondary (arrow) than in antral (arrowhead) follicles (a and b). No signals are seen in primary follicles (triangle, c and d) and in corpus luteum (CL). (C) immunohistochemistry of the mouse uterus showing protein expression of Dragon (a and b), and negative control (c), incubation with Dragon antibody pre-incubated with competing immunizing peptide. Filled arrow: luminal epithelial cells of endometrium; Arrowhead: glandular epithelial cells of endometrium; Open arrow: circular muscle.
Consistent with immunostaining, Dragon mRNA, as detected by in situ hybridization was stronger in oocytes from secondary follicles (Figure 3B, arrows) compared to antral (Figure 3B, arrowheads) follicles, and was undetectable in oocytes from primary follicles (Figure 3B, triangle). Dragon mRNA was not detectable in ovarian somatic cells. These results suggest that Dragon may regulate the development of oocytes and follicles by influencing the interaction between the oocyte and granulosa cells.
In the uterus, Dragon protein was expressed in luminal and glandular epithelial cells of the endometrium (Figure 3C, a and b). Weak staining was also found in circular muscle (Figure 3C, c). Localization of Dragon in the luminal and glandular epithelial cells suggest Dragon may also be required for normal endometrial function.
Cellular localization of Dragon in the pituitary
In the pituitary, sporadic staining was observed in both the anterior and posterior lobes, while no staining was detected in the intermediate lobe (Figure 4A). Since BMPs and their receptors are expressed in mouse pituitary gonadotropes, as well as in the LβT2 gonadotrope cell line (25;26), and BMP’s can stimulate FSH biosynthesis (27), we examined whether FSH expressing gonadotropes also express Dragon. To this end, frozen pituitary sections were dual-labeled with Dragon and FSH antibodies. FSH expressing cells indeed overlapped extensively, albeit not completely, with Dragon expressing cells (Figure 4B). Interestingly, LβT2 cells also express Dragon (Figure 5A). These results suggest that Dragon may influence BMP-mediated FSH biosynthesis in vivo and in vitro.
Figure 4.
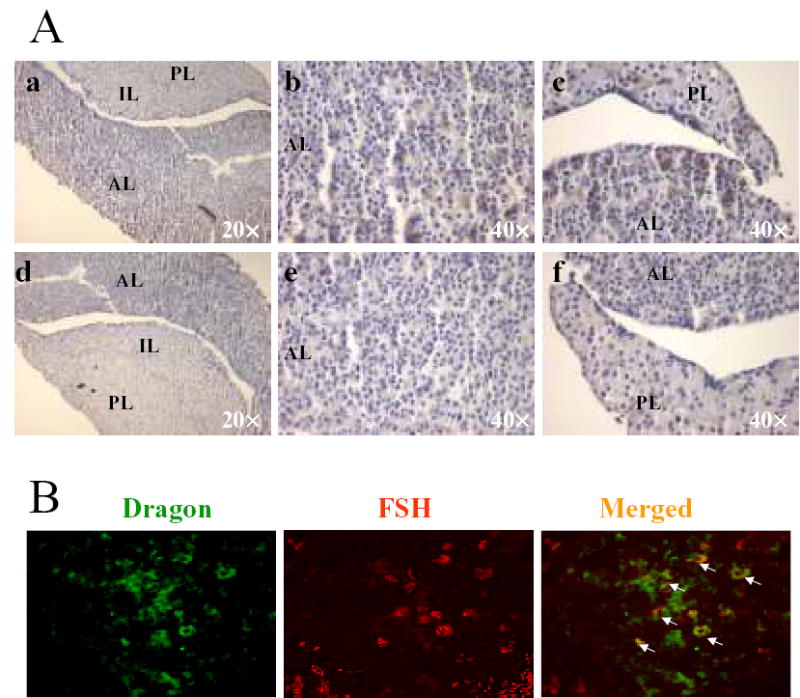
Immunolocalization of Dragon in the pituitary and colocalization of Dragon and FSH. (A) immunostaining for Dragon (in brown color) in the mouse pituitary (paraffin sections). Sections were incubated with Dragon antibody (a, b and c) or with Dragon antibody pre-incubated with competing immunizing peptide (d, e, and f). AL, anterior lobe; IL, intermediate lobe; PL, posterior lobe. (B) colocalization of FSHβ and Dragon in the mouse anterior pituitary (frozen sections). Dragon (green) and FSHβ (red) were detected in the anterior pituitary. Double-labeled cells (yellow, arrows) indicate Dragon expression in the mouse pituitary gonadotrope. Note that some FSHβ-positive cells are negative for Dragon staining and some Dragon-positive cells are negative for FSH staining.
Figure 5.
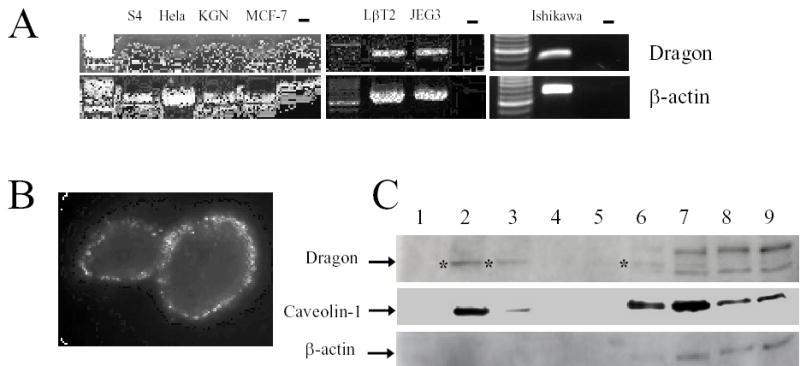
Expression of Dragon in cell lines derived from reproductive tissues and localization of Dragon into lipid rafts. (A) RT-PCR analyses of Dragon mRNA in cell lines. β-actin was used as a control for cDNA quality. (B) Immunochemical localization of Dragon in Ishikawa cells. The live unfixed cells were incubated with rabbit anti-Dragon serum on ice and then fixed in 2 % paraformaldehyde. The bound antibodies were detected by incubating with FITC-conjugated donkey antirabbit IgG. (C) Localization of Dragon in raft-enriched fractions prepared from Ishikawa cells. Cells were extracted using a buffer containing 1% Triton X-100. The lysate was mixed with 85% sucrose, sequentially layered with 35% and 5% sucrose, and centrifuged for 14 hr at 150,000 g. Nine fractions were collected and analyzed for dragon, caveolin-1 and β-actin by Western blot. The bands specific for Dragon are marked with asterisks.
Dragon expression in cell lines of the reproductive axis
In screening cell lines originating from reproductive organs (Figure 5A), we found that Dragon was expressed in Hela (cervical carcinoma), MCF-7 (breast carcinoma), LβT2 (pituitary carcinoma), JEG3 (placenta carcinoma) and Ishikawa cells (endometrium adenocarcinoma). In contrast, Dragon mRNA was undetectable in S4 spermatogonial cells (28) or KGN granulosa tumor cells (29).
Lipid raft localization of Dragon
We explored the location of Dragon on the cell surface using Ishikawa cells as a model. Live cells were incubated with Dragon antibody at 4 oC, then fixed and processed for immunocytochemistry. Dragon appeared to have a punctate pattern on the cell membrane, which is typical of lipid raft proteins (Figure 5B). To further characterize Dragon localization within membrane sub-domains, cells were extracted on ice in the presence of 1% Triton X-100 and then subjected to sucrose gradient ultracentrifugation. As expected, Dragon was detectable primarily within the low-density fractions (Fig 5C; fractions 2 and 3), along with Caveolin-1 which is typically associated with lipid rafts. The high density fractions which include cellular and cytoskeletal proteins (fractions 6–9) contained β-actin, some Caveolin-1, and a small amount of Dragon. These results indicate that Dragon is indeed located within lipid rafts in Ishikawa cells.
Dragon enhances signaling of BMP2 and BMP4
Our results demonstrate that Dragon is expressed in gonadal germ cells and in reproductive tract epithelial cells. Moreover, we have previously shown that Dragon enhances the BMP2 response in the HepG2 liver cell and LLC-PK1 kidney cell lines (17). To examine whether Dragon has a similar role in reproductive cells, Ishikawa and KGN cells were transfected with Dragon cDNA together with BRE-Luc, a BMP-responsive luciferase reporter construct. Dragon dose-dependently increased BRE luciferase activity in both Ishikawa and KGN cells in the absence of added BMPs (Fig 6A). While Dragon induced similar reporter activity in both cell lines at lower doses (0.1–10 ng), it was more effective in Ishikawa cells at higher doses. Although Dragon protein was undetectable in Ishikawa cells before transfection, Dragon was detectable after transfection with 100 ng cDNA (Fig 6A, inset), indicating that the effects of transfected Dragon are mediated by an increase in Dragon protein.
Treatment of Ishikawa cells with noggin-containing conditioned medium (100 ng/ml) resulted in partial inhibition of Dragon-dependent BRE-Luc activity, and completely inhibit signaling by BMP-2 (10 ng/ml) (Fig 6B). Conditioned medium from mock transfected 293 cells had no inhibitory activity (Fig 6C), indicating that the BMP-inhibiting activity in the noggin conditioned medium was due to noggin itself. These results also suggest that the effect of Dragon on BMP reporter activity is dependent on endogenous ligand. We next examined the effect of Dragon on BMP signaling in the presence of added BMP ligands. Ishikawa cells were transfected with Dragon cDNA and treated with increasing doses of BMP ligands. Dragon significantly increased BMP2 signaling at 11 to 700 pM BMP2 doses (P < 0.05), but had no effect at 2800 pM (Fig 6D). Thus, at lower BMP2 doses (i.e. 11 or 44 pM), Dragon transfection results in detectable reporter activity that is not seen in the absence of Dragon (Fig 6D). Similar results were observed with BMP4 and in KGN cells (data not shown). These results indicate that Dragon acts to increase sensitivity of BMP responsive cells to low concentrations of endogenous or exogenous BMP ligands.
Discussion
Although originally discovered as a GPI-anchored cell-surface protein involved in neuronal differentiation and cell-cell contact in the developing nervous system (13), it is now evident that Dragon can also act as a co-receptor for BMP 2 and 4 (17). In addition to previously reported sites of expression in neural tissues (13;17), our studies demonstrate that Dragon is also expressed in many specific cell types throughout the reproductive system. In addition, we found that Dragon was expressed in numerous cell lines derived from reproductive tissues, and that Dragon expression enhanced responsiveness of Ishikawa and KGN cells to BMP2 and 4. Thus, our results suggest that Dragon may have important roles in mediating BMP signaling in reproduction.
To define specific sites where Dragon-mediated BMP signaling might be important in reproduction, we explored cell-specific expression in the male and female reproductive tracts. In males, BMP 8a and b are expressed in maturing spermatocytes, and BMP8b knockout males are infertile due to developmental arrest and degeneration of spermatocytes (11;12), suggesting that these BMP’s are critical for normal spermatocyte development. BMP receptors ALK3 and BMPRII are localized in postnatal spermatogonia and BMP4 is produced by Sertoli cells very early in postnatal development, consistent with an ongoing requirement for BMP signaling in the testis (30). In addition, BMP2 primarily stimulates spermatogonial proliferation while BMP7 acts mainly on Sertoli cells in the testis from 7-day-old mice (31). Our results extend these findings to a novel BMP co-receptor which enhances BMP signaling, since in 3 day old male mice, Dragon was highly expressed in gonocytes before and after they migrated from the tubule lumen to their basal position, with this immunoreactivity being maintained as spermatogonia in Day 9 animals. By day 21, staining in spermatogonia was substantially diminished but gonocytes remaining within the tubule lumen were still positive. In adults, Dragon staining appeared in maturing spermatocytes but not in other testicular cell types. The shift in expression from gonocytes and spermatogonia in juvenile animals to spermatocytes in mature males suggests that the role of BMPs may change as the testes mature to produce active sperm. Taken together, these results suggest a critical role for BMPs in regulating testis development and spermatogenesis and suggest that Dragon may be an important mediator of these processes.
BMP 4, 7 and 8A are expressed in the epididymis, and knockout of each gene by itself resulted in degeneration of the epididymal epithelium (10–12). These results indicate a role for BMPs in the control of epididymal function. Interestingly, Dragon was strongly expressed on the apical surface of polarized epididymal epithelium in immature and mature males consistent with a role for Dragon in enhancing this essential BMP signaling.
In females, both BMPs and their receptors have been identified in numerous ovarian cell types, including oocytes and granulosa cells (8). In vitro studies have demonstrated that BMP 2, 4, 6 7 and 15 regulate granulosa cell functions and BMP4 and 7 promote the primordial-to-primary follicle transition during follicle maturation (reviewed in (8)). The significance of BMP signaling in ovarian function is also underscored by the altered ovulation rates in Inverdale sheep with a natural point mutation in the BMP 15 gene (32) and in Booroola sheep with a point mutation in the BMPR-IB gene (33). Our results demonstrate that in the ovary, Dragon is expressed exclusively in oocytes, and most prominently in oocytes within secondary follicles. This is a time of oocyte growth and cytoplasmic maturation (34), suggesting that BMP signaling in general, and Dragon enhancement of this signaling in particular, may be important for growth and maturation of oocytes.
BMP signaling components are expressed in a variety of cells within the rat uterus (9). BMP2 mRNA is restricted to periluminal stroma and BMP7 is expressed in periluminal stroma and glandular epithelial cells while BMP 4 and BMP 6 are expressed in blood vessels in the uterus. BMPIA, BMPRIB and BMPRII are expressed in a number of cell types in the uterus including luminal and glandular epithelial cells. We observed Dragon expression in luminal and glandular epithelial cells of the mouse endometrium, suggesting that Dragon may enhance BMP signals involved in regulating uterine maturation in preparation for implantation.
BMP 2, 4, 6, 7 and 15 are expressed in mouse pituitary and BMP 6, 7 and 15 have been shown to stimulate FSH synthesis and secretion (25;27;35). BMP 6 and 7 can also stimulate FSH mRNA biosynthesis in LβT2 mouse pituitary cells in culture (27). We observed Dragon expression in LβT2 cells, as well as in numerous cells within the mouse pituitary, some of which also stained for FSH. These results suggest that BMPs may act in an autocrine manner to modulate FSH biosynthesis and that Dragon may enhance this process.
In cell culture studies using cell lines from the reproductive tract, we found that Dragon expression enhanced the response to endogenous BMP ligand, as well as low doses of exogenous BMP2 and 4, results that agree with our earlier observations in non-reproductive cell lines (36). While the precise mechanism for this signaling enhancement has not yet been fully elucidated, our immunocytochemical analysis indicates that Dragon is located on the plasma membrane in discrete patches, consistent with its belonging to the class of GPI-anchored proteins which are known to localize in lipid rafts (37). Moreover, our earlier studies indicate that Dragon can interact directly with BMPRII, ActRII, and the Alk3 and Alk6 type I receptors (36). Taken together, these results suggest a model for enhanced BMP signaling in which Dragon acts as a BMP co-receptor in collecting type II and type I receptors into lipid rafts where they are optimized to respond to low doses of BMP ligands. Since Dragon can bind BMP 2 and 4 directly, it is also possible that Dragon acts to stabilize the ligand-receptor complex in lipid rafts, thereby facilitating endocytosis and signaling. Of course, these two possibilities are not mutually exclusive. Based on the localized expression of Dragon in developing and maturing germ cells, as well as specific epithelial cells within the reproductive tract that are known to be BMP-responsive, our results support the concept that BMP’s play an important role in regulating reproduction in mammals and that this role may be regulated by Dragon.
Acknowledgments
Dr. Ernestina Schipani and Janet Saxton provided expertise and access to critical facilities for the immunocytochemical and in situ hybridization analyses. Dr. Carla Boitani provided helpful suggestions for germ cell classifications in the testis from newborn mice. Cell lines were generously provided by Dr. Martin Dym (S4 spermatogonial cells), Dr. Pamela Mellon (LβT2 cells) and Dr. Hajime Nawata (KGN cells). Dr. Peter ten Dijke provided the BRE-Luc reporter.
Footnotes
Supported in part by NIH grants: HD039777 and DK055838 (ALS); HD038533 and NS038253 (CJW); and GM075267, DK071837, and DK069533 (HYL).
References
- 1.Welt C, Sidis Y, Keutmann H, Schneyer A. Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med (Maywood ) 2002;227:724–752. doi: 10.1177/153537020222700905. [DOI] [PubMed] [Google Scholar]
- 2.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 3.Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 4.Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- 5.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 6.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 7.Shimasaki S, Zachow RJ, Li D, Kim H, Iemura S, Ueno N, Sampath K, Chang RJ, Erickson GF. A functional bone morphogenetic protein system in the ovary. Proc Nat Acad Sci USA. 1999;96:7282–7287. doi: 10.1073/pnas.96.13.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- 9.Erickson GF, Fuqua L, Shimasaki S. Analysis of spatial and temporal expression patterns of bone morphogenetic protein family members in the rat uterus over the estrous cycle. J Endocrinol. 2004;182:203–217. doi: 10.1677/joe.0.1820203. [DOI] [PubMed] [Google Scholar]
- 10.Hu J, Chen YX, Wang D, Qi X, Li TG, Hao J, Mishina Y, Garbers DL, Zhao GQ. Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity. Dev Biol. 2004;276:158–171. doi: 10.1016/j.ydbio.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Zhao GQ, Deng K, Labosky PA, Liaw L, Hogan BL. The gene encoding bone morphogenetic protein 8B is required for the initiation and maintenance of spermatogenesis in the mouse. Genes Dev. 1996;10:1657–1669. doi: 10.1101/gad.10.13.1657. [DOI] [PubMed] [Google Scholar]
- 12.Zhao GQ, Liaw L, Hogan BL. Bone morphogenetic protein 8A plays a role in the maintenance of spermatogenesis and the integrity of the epididymis. Development. 1998;125:1103–1112. doi: 10.1242/dev.125.6.1103. [DOI] [PubMed] [Google Scholar]
- 13.Samad TA, Srinivasan A, Karchewski LA, Jeong SJ, Campagna JA, Ji RR, Fabrizio DA, Zhang Y, Lin HY, Bell E, Woolf CJ. DRAGON: a member of the repulsive guidance molecule-related family of neuronal- and muscle-expressed membrane proteins is regulated by DRG11 and has neuronal adhesive properties. J Neurosci. 2004;24:2027–2036. doi: 10.1523/JNEUROSCI.4115-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidtmer J, Engelkamp D. Isolation and expression pattern of three mouse homologues of chick Rgm. Gene Expr Patterns. 2004;4:105–110. doi: 10.1016/s1567-133x(03)00144-3. [DOI] [PubMed] [Google Scholar]
- 15.Niederkofler V, Salie R, Sigrist M, Arber S. Repulsive guidance molecule (RGM) gene function is required for neural tube closure but not retinal topography in the mouse visual system. J Neurosci. 2004;24:808–818. doi: 10.1523/JNEUROSCI.4610-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldekamp J, Kramer N, varez-Bolado G, Skutella T. Expression pattern of the repulsive guidance molecules RGM A, B and C during mouse development. Gene Expr Patterns. 2004;4:283–288. doi: 10.1016/j.modgep.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Samad TA, Rebbapragada A, Bell E, Zhang Y, Sidis Y, Jeong SJ, Campagna JA, Perusini S, Fabrizio DA, Schneyer AL, Lin HY, Brivanlou AH, Attisano L, Woolf CJ. DRAGON, a Bone Morphogenetic Protein Co-receptor. J Biol Chem. 2005;280:14122–14129. doi: 10.1074/jbc.M410034200. [DOI] [PubMed] [Google Scholar]
- 18.Sidis Y, Fujiwara T, Leykin L, Isaacson K, Toth T, Schneyer AL. Characterization of inhibin/activin subunit, activin receptor, and follistatin messenger ribonucleic acid in human and mouse oocytes: evidence for activin’s paracrine signaling from granulosa cells to oocytes. Biol Reprod. 1998;59:807–812. doi: 10.1095/biolreprod59.4.807. [DOI] [PubMed] [Google Scholar]
- 19.Xia Y, Sidis Y, Schneyer A. Overexpression of follistatin-like 3 in gonads causes defects in gonadal development and function in transgenic mice. Mol Endocrinol. 2004;18:979–994. doi: 10.1210/me.2003-0364. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee A, Arnaud L, Cooper JA. Lipid-dependent recruitment of neuronal Src to lipid rafts in the brain. J Biol Chem. 2003;278:40806–40814. doi: 10.1074/jbc.M306440200. [DOI] [PubMed] [Google Scholar]
- 21.Vacca F, Amadio S, Sancesario G, Bernardi G, Volonte C. P2X3 receptor localizes into lipid rafts in neuronal cells. J Neurosci Res. 2004;76:653–661. doi: 10.1002/jnr.20069. [DOI] [PubMed] [Google Scholar]
- 22.Korchynskyi O, Ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- 23.Keutmann HT, Schneyer AL, Sidis Y. The role of follistatin domains in follistatin biological action. Mol Endocrinol. 2004;18:228–240. doi: 10.1210/me.2003-0112. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 25.Paez-Pereda M, Giacomini D, Refojo D, Nagashima AC, Hopfner U, Grubler Y, Chervin A, Goldberg V, Goya R, Hentges ST, Low MJ, Holsboer F, Stalla GK, Arzt E. Involvement of bone morphogenetic protein 4 (BMP-4) in pituitary prolactinoma pathogenesis through a Smad/estrogen receptor crosstalk. Proc Natl Acad Sci U S A. 2003;100:1034–1039. doi: 10.1073/pnas.0237312100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otsuka F, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J Biol Chem. 2001;276:11387–11392. doi: 10.1074/jbc.M010043200. [DOI] [PubMed] [Google Scholar]
- 27.Huang HJ, Wu JC, Su P, Zhirnov O, Miller WL. A novel role for bone morphogenetic proteins in the synthesis of follicle-stimulating hormone. Endocrinology. 2001;142:2275–2283. doi: 10.1210/endo.142.6.8159. [DOI] [PubMed] [Google Scholar]
- 28.Feng LX, Chen Y, Dettin L, Pera RA, Herr JC, Goldberg E, Dym M. Generation and in vitro differentiation of a spermatogonial cell line. Science. 2002;297:392–395. doi: 10.1126/science.1073162. [DOI] [PubMed] [Google Scholar]
- 29.Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K, Takayanagi R, Kashimura Y, Haji M, Nawata H. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142:437–445. doi: 10.1210/endo.142.1.7862. [DOI] [PubMed] [Google Scholar]
- 30.Pellegrini M, Grimaldi P, Rossi P, Geremia R, Dolci S. Developmental expression of BMP4/ALK3/SMAD5 signaling pathway in the mouse testis: a potential role of BMP4 in spermatogonia differentiation. J Cell Sci. 2003;116:3363–3372. doi: 10.1242/jcs.00650. [DOI] [PubMed] [Google Scholar]
- 31.Puglisi R, Montanari M, Chiarella P, Stefanini M, Boitani C. Regulatory role of BMP2 and BMP7 in spermatogonia and Sertoli cell proliferation in the immature mouse. Eur J Endocrinol. 2004;151:511–520. doi: 10.1530/eje.0.1510511. [DOI] [PubMed] [Google Scholar]
- 32.Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25:279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- 33.Fabre S, Pierre A, Pisselet C, Mulsant P, Lecerf F, Pohl J, Monget P, Monniaux D. The Booroola mutation in sheep is associated with an alteration of the bone morphogenetic protein receptor-IB functionality. J Endocrinol. 2003;177:435–444. doi: 10.1677/joe.0.1770435. [DOI] [PubMed] [Google Scholar]
- 34.Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996;8:485–489. doi: 10.1071/rd9960485. [DOI] [PubMed] [Google Scholar]
- 35.Otsuka F, Shimasaki S. A Novel Function of Bone Morphogenetic Protein-15 in the Pituitary: Selective Synthesis and Secretion of FSH by Gonadotropes. Endocrinology. 2002;143:4938–4941. doi: 10.1210/en.2002-220929. [DOI] [PubMed] [Google Scholar]
- 36.Costigan M, Samad TA, Allchorne A, Lanoue C, Tate S, Woolf CJ. High basal expression and injury-induced down regulation of two regulator of G-protein signaling transcripts, RGS3 and RGS4 in primary sensory neurons. Mol Cell Neurosci. 2003;24:106–116. doi: 10.1016/s1044-7431(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 37.Fullekrug J, Simons K. Lipid rafts and apical membrane traffic. Ann N Y Acad Sci. 2004;1014:164–169. doi: 10.1196/annals.1294.017. [DOI] [PubMed] [Google Scholar]


