Abstract
Recent studies have indicated that adoptive immunotherapy with autologous anti-tumor tumor-infiltrating lymphocytes (TILs) following non-myeloablative chemotherapy mediates tumor regression in approximately 50% of treated patients with metastatic melanoma, and that tumor regression is correlated with the degree of persistence of adoptively transferred T cells in peripheral blood. These findings, which suggested that the proliferative potential of transferred T cells may play a role in clinical responses, led to the current studies in which telomere length as well as phenotypic markers expressed on the administered TILs were examined. TILs that were associated with objective clinical responses following adoptive transfer possessed a mean telomere length of 6.3 kb, whereas TILs that were not associated with significant clinical responses were significantly shorter, averaging 4.9 kb (p<0.01). Furthermore, individual TIL-derived T cell clonotypes that persisted in vivo following adoptive cell transfer possessed telomeres that were longer than telomeres of T cell clonotypes that failed to persist (6.2 kb versus 4.5 kb respectively, p<0.001). Expression of the co-stimulatory molecule CD28 also appeared to be associated with long telomeres and T cell persistence. These results, indicating that the telomere length of transferred lymphocytes correlated with in vivo T cell persistence following adoptive transfer, and coupled with the previous observation that T cell persistence was associated with clinical responses in this adoptive immunotherapy trial, suggest that telomere length and the proliferative potential of the transferred T cells may play a significant role in mediating response to adoptive immunotherapy.
Keywords: Human, T cells, Tumor immunity
Introduction
Recent clinical trial results have demonstrated that cell transfer therapy with autologous anti-tumor tumor-infiltrating lymphocytes (TILs) following a conditioning nonmyeloablative chemotherapy regimen can cause the regression of large, vascularized tumors in patients with refractory metastatic melanoma (1, 2). Tumor regression in some patients was accompanied by a large in vivo expansion of the administered anti-tumor lymphocytes, which represented greater than 70% of the total lymphocytes in peripheral blood for more than five months after transfer. Sequence analysis of T cell receptor beta chain variable region gene (TRBV) products expressed in the administered TIL samples, tumor samples and PBMC samples obtained from the treated patients demonstrated that there was a significant correlation between tumor regression and the degree of persistence in peripheral blood of adoptively transferred T cell clonotypes (1, 3–5).
These observations lead to further consideration of the mechanisms involved with maintaining T cell persistence. Telomeres are specialized DNA-protein structures at the ends of eukaryotic chromosomes consisting in humans of 5–15 kb of tandemly repeated TTAGGG sequence with associated proteins (6). Chromosomal replication during normal cell division is incomplete and results in the loss of terminal telomeric sequences at a rate of 50–100 base pairs per cell division (7, 8). Telomere shortening has been observed in T cells as they differentiate from naïve to memory T cells and undergo extensive expansion (8, 9). Clonal expansion of T cells in vivo during immune responses to both foreign and autoantigens has been reported to be associated with telomere shortening (10, 11). The shortening of telomeres to a critical level leads to loss of telomere function resulting in increased cell death and altered cell functions, particularly loss of replicative capacity (senescence) (9, 12, 13). The loss of telomeres that occurs with cell division can be compensated by the action of telomerase, an enzyme that can catalyze the addition of telomeric end repeats (14). T cells up-regulate telomerase upon antigen stimulation (15); however, telomerase activity decreases following repeated antigen stimulation, resulting in corresponding decreases in telomere length upon further cell division, and ultimately leading to replicative senescence (16). Studies of naïve and memory T cells showed that naïve T cells, which have longer telomeres, can undergo increased numbers of population doublings in vitro (17). Conversely, stabilization of telomere length in T cells after over-expression of telomerase enhances replicative capacity (18, 19). Thus, it is possible that loss in telomere length during extensive in vitro expansion may act to decrease the residual replicative capacity of TILs in vivo, impair T cell persistence and limit responses to adoptive immunotherapy.
Phenotypic markers have also been associated with cells that have undergone extensive proliferation. Markers that are up-regulated on senescent T populations include CD57 (20, 21) and the killer cell inhibitory receptor KLRG1 (22). In addition, the loss of expression of the co-stimulatory markers CD27 (23, 24) and CD28 (8, 25) has been found on T cells that have undergone extensive proliferation.
Results presented in the current study indicated that the telomere length of the administered cells was significantly correlated with in vivo T cell persistence and tumor regression in melanoma patients. These findings suggest that the ability of adoptively transferred anti-tumor T cells to mediate tumor regression in vivo may be associated with their proliferative potential, and that the analysis of telomere lengths of populations of in vitro cultured tumor reactive T cells might facilitate the selection of TIL with enhanced in vivo efficacy.
Materials and Methods
Patient samples
All patients in this study signed an institutional review board–approved consent and were treated with autologous TILs following lymphodepletion chemotherapy as described in detail previously (1). The techniques of TIL growth have been described in detail (26). Briefly, explants of small (2mm3) tumor fragments or 1x106 viable cells of tumor tissue digests were used to initiate TIL culture in 2 ml of RPMI 1640 based medium (Invitrogen) containing 10% human serum and 6000 IU/ml IL-2 (Chiron Corp). After 2–4 weeks of culture, usually several million TIL cells were obtained and screened by IFN secretion assay for recognition of tumor cells. Anti-tumor TIL cultures were further expanded in AIM V medium (Invitrogen) supplemented with irradiated allogeneic feeder cells, anti-CD3 antibody (Ortho Biotech) and 6000 IU/ml IL-2. This expansion protocol typically resulted in 1000-fold expansions of cells by the time of administration 14–15 days after initiation of the expansions. All of the administered TIL secreted greater than 100 pg/ml of IFN and at least twice background in co-cultures with autologous melanoma cells, allogeneic HLA matched melanoma cell lines, or T2 cells pulsed with the dominant HLA-A2 restricted peptide epitopes MART-1:27–35 or gp100:209–217. Peripheral blood samples were obtained from the patients approximately one week and one month following adoptive transfer of autologous TILs and PBMC were separated from the peripheral blood samples using Lymphocyte Separation Medium (MP Biomedicals).
Clinical response evaluation
Patients underwent computed axial tomography of the brain, chest, abdomen, and pelvis prior to adoptive cell transfer and after the treatment. A partial response was defined as a decrease of at least 50% (but <100%) of the sum of the products of the longest perpendicular diameters of all tumors (World Health Organization Criteria) after therapy lasting at least 1 month with no new or enlarging tumors, and a complete response required the disappearance of all lesions with the appearance of no new lesions. All patients not achieving a complete or partial response were considered as non-responders.
Analysis of in vivo T cell persistence
Analysis of in vivo persistence in the patients after adoptive transfer of autologous TILs was carried out as previously described (3). Briefly, total RNA was isolated from TILs and PBMC samples and used in the analysis of TRBV gene expression using 5’RACE technique. The 5’RACE products were analyzed by an automated DNA sequencer (ABI PRISM 3100-Avant Genetic Analyze; Applied Biosystems). The sequence data were analyzed by comparison with known TRBV sequences using Vector NTI Suite 8 (Invitrogen).
Sorting of T cell clonotypes
Individual T cell clonotypes were sorted from the administered TILs using anti-PE MicroBeads (Miltenyi Biotec) for telomere flow-FISH assay. PE-conjugated TCR Vbeta antibodies (Beckman Coulter) including VB1, VB2, VB3, VB4, VB5.1, VB7, VB8, VB11, VB13.1, VB14, VB16 and VB17 as well as MART-1 tetramer (Beckman Coulter) were used to separate the corresponding T cell clonotypes.
Telomere assay of fluorescence in situ hybridization and flow cytometry (flow-FISH)
The average length of telomere repeats at chromosome ends in individual T cell clonotypes was measured by quantitative flow-FISH as previously described (8). FITC-conjugated telomere probe (FITC-OO-CCCTAACCCTAACCCTAA, O indicating a molecule linking FITC to DNA sequence) was from Applied Biosystems. FITC-labeled fluorescent calibration beads (Quantum TM-24 Premixed; Bangs Laboratories Inc.) were used to convert telomere fluorescence data to molecules of equivalent soluble fluorescence (MESF) units. The following equation was performed to estimate the telomere length in basepair from telomere fluorescence in MESF units (bp = MESF × 0.495) (8). Aliquots of the SUP-T1 human T lymphoblastic leukemia cell line (ATCC) were used as daily positive control to normalize telomere lengths. Unstained cells were included as negative controls.
FACS analysis of phenotypic marker expression
TIL samples were co-stained with FITC, PE, allophycocyanin or biotin-conjugated antibodies against phenotypic markers (Table III) and corresponding FITC or PE-conjugated TCR Vbeta antibodies including VB1, VB2, VB3, VB4, VB5.1, VB7, VB8, VB11, VB13.1, VB14, VB16 and VB17 as well as MART-1 tetramer (Beckman Coulter). Antibodies against CD8β, CD30, CD48 and CD127 were purchased from Beckman Coulter. Antibodies against CD27 and ICOS were purchased from eBioscience. Anti-CD102 antibody was purchased from SouthernBiotech. Anti-CD178 antibody was purchased from ALEXIS Biochemicals. Anti-DR3 antibody was purchased from NeoMarkers. Anti-HVEM antibody was purchased from R & D Systems. CD8α expression was detected by TL-tetramer, a gift from Dr. Hilde Cheroutre (La Jolla Institute of Allergy & Immunology). Antibodies against the other phenotypic markers were purchased from BD Biosciences. Phenotypic marker expression was analyzed using FACS Calibur (BD Biosciences).
Table III.
Phenotypic analysis of persistent and nonpersistent T cell clonotypesa
| Marker distinguishing Persistent from Nonpersistent T cellsb | |||
|---|---|---|---|
| CD28
| |||
| Percent of positive T cells (mean + SEM, %)
| |||
| Persistent clonotypes
|
Nonpersistent clonotypes
|
||
| 65+10 | 34+10 | ||
| Markers that fail to distinguish persistent from nonpersistent T cellsc | |||
| 4-1BB | CD30 | CD70 | CXCR4 |
| CCR5 | CD40L | CD71 | DR3 |
| CCR6 | CD45RA | CD80 | HVEM |
| CCR7 | CD45RO | CD86 | ICOS |
| CD2 | CD48 | CD94 | NKB1 |
| CD5 | CD50 | CD95 | NKG2D |
| CD8αα | CD54 | CD102 | Notch1 |
| CD8β | CD56 | CD122 | PD-1 |
| CD11a | CD57 | CD127 | TRAIL |
| CD16 | CD58 | CD161 | |
| CD25 | CD62L | CD178 | |
| CD27 | CD69 | CD231 | |
Phenotypic markers were analyzed by FACS. TCR Vβ Abs or MART-1 tetramer were used to distinguish different T cell clonotypes by direct staining of T cells.
Pairwise analysis of the percentages of persistent and nonpersistent T cells corresponding to dominant clonotypes from seven individual responding patients demonstrated no significant differences in the expression of 45 phenotypic markers using the Wilcoxon signed-ranks test (p>0.1).
Pairwise analysis of the percentages of persistent and nonpersistent T cells corresponding to dominant clonotypes from seven individual responding patients demonstrated a significant difference in the expression of CD28 using the Wilcoxon signed-ranks test (p<0.05) without correction for multiple comparisons.
Statistical analysis
Data were analyzed by Mann-Whitney U test or Wilcoxon signed-ranks test, and differences were considered significant at p<0.05. Linear regression analysis was utilized to determine the relationship between telomere length and characteristics of TIL and patient samples.
Results
Comparison of telomere lengths between responder-TILs and non-responder-TILs
The characteristics of autologous TILs used to treat patients with metastatic melanoma were studied in order to understand the mechanisms involved with maintaining T cell persistence and tumor regression. Prior studies have demonstrated that the proliferative potential of T cells was associated with telomere length (27), and an initial attempt was therefore made to determine whether or not telomere length of TIL was associated with their ability to persist when administered to patients and to mediate tumor regression. When the telomeres present in bulk samples of TIL were evaluated, there appeared to be a relatively narrow distribution of telomere lengths within these T cell populations (Fig. 1). The telomere lengths of the administered bulk TIL were not correlated with patient age (Fig. 2A) or with the length of in vitro culture prior to infusion (Fig. 2B). The telomere lengths determined for the in vitro cultured TIL were, however, correlated with the in vivo absolute lymphocyte counts (ALC) between 5 and 10 days following adoptive transfer (p<0.0005) (Fig. 2C) and between 19 and 37 days following transfer (p<0.0005) (Fig. 2D), whereas the ALC in the peripheral blood at these times following adoptive transfer was not correlated with the number of total transferred T cells (p>0.1). These observations suggest that the telomere lengths of bulk administered TIL may be related to their ability to proliferate and persist following adoptive transfer in vivo.
Figure 1.
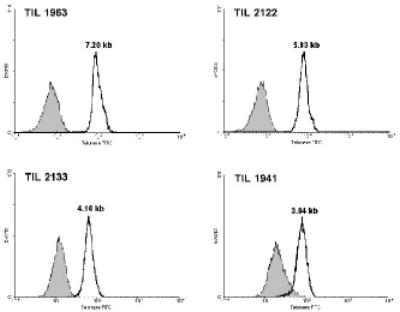
Representative histograms of telomere flow-FISH assays of bulk administered TILs. Administered TIL samples were thawed and used in telomere flow-FISH assays without further culture. Shaded area represents the unstained control.
Figure 2.
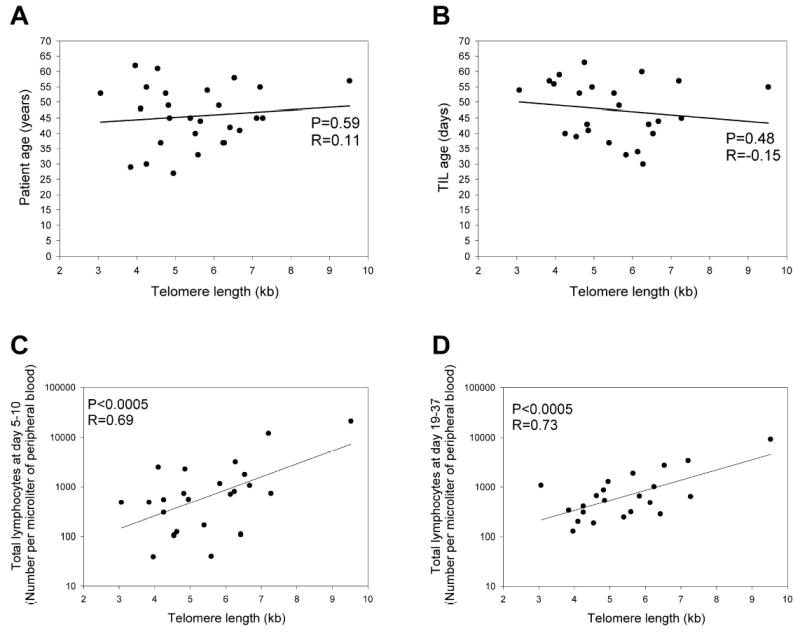
Telomere length of administered TIL is not correlated with age of TIL or patient age but is correlated with absolute lymphocyte count. The relationship was examined between telomere lengths of the administered TIL and patient age (A), or between the telomere lengths of the administered TILs and the TIL age (B). TIL age refers to the total number of days TIL were cultured in vitro. The association between the telomere lengths of the TIL prior to adoptive transfer and absolute lymphocyte counts in the peripheral blood obtained between 5 and 10 days following adoptive cell transfer (C) and between 19 and 37 days following adoptive cell transfer (D) was evaluated. All TIL samples were included if their corresponding data were available.
The telomere lengths of TILs that were administered to responding patients were then compared with TILs that were administered to patients who did not respond to adoptive immunotherapy. The results of this comparison indicated that the overall telomere lengths of TILs used to treat responding patients were significantly longer than those that were utilized to treat non-responding patients (6.31 ± 0.37 kb compared to 4.91 ± 0.29 kb respectively, p<0.01; Table I). Differences have been observed in the overall telomere length of T cells isolated from individual normal donors (28, 29); however, the overall telomere lengths of normal CD8+ PBMC isolated from responding patients (8.68 kb) was not significantly different from those of CD8+ PBMC cells isolated from non-responders (8.03 kb) (p>0.2). These observations suggest that response to therapy may be related to the proliferative potential of the adoptively transferred T cells.
Table I.
Comparison of telomere lengths between bulk responder-TIL samples and non-responder-TIL samples
| Responder-TIL sample | Telomere length (kb) | Non-responder-TIL sample | Telomere length (kb) | ||
|---|---|---|---|---|---|
| TIL 1913 | 6.27 | TIL 1866 | 5.52 | ||
| TIL 1931a | 9.52 | TIL 1890 | 4.75 | ||
| TIL 1954 | 6.53 | TIL 1907 | 5.65 | ||
| TIL 1963 | 7.20 | TIL 1909 | 3.06 | ||
| TIL 2026 | 5.59 | TIL 1941 | 3.84 | ||
| TIL 2035 | 4.62 | TIL 1945 | 6.24 | ||
| TIL 2109 | 4.95 | TIL 1979 | 6.67 | ||
| TIL 2122 | 5.83 | TIL 2009 | 4.82 | ||
| TIL 2149 | 5.39 | TIL 2037 | 4.85 | ||
| TIL 2154 | 6.13 | TIL 2075 | 3.96 | ||
| TIL 2162 | 6.42 | TIL 2080 | 7.11 | ||
| TIL 2208 | 7.27 | TIL 2119 | 4.25 | ||
| TIL 2127 | 4.54 | ||||
| TIL 2133 | 4.10 | ||||
| TIL 2195 | 4.25 | ||||
| Mean ± SE | 6.31 ± 0.37 | Mean ± SE | 4.91 ± 0.29 | ||
| Mann-Whitney U test | P < 0.01 | ||||
The telomere length of TIL 1931 was calculated by analyzing its predominant components, TIL 1931-F4 (10.78 kb telomere) representing 29% of total T cells and TIL 1931-F5 (8.83 kb) representing 53% of total T cells in the administered TIL.
Comparison of telomere lengths between persistent and non-persistent clonotypes
Previous observations indicated that TILs used to treat responding patients generally contained at least one dominant clonotype that persisted following adoptive transfer, whereas the dominant clonotypes present in TILs administered to non-responding patients generally failed to persist (5). Analysis of the mean telomere length of individual persistent T cell clonotypes indicated that these were significantly longer than those present in the clonotypes that did not persist (6.16 ± 0.43 kb versus 4.49 ± 0.25 kb respectively, p<0.001; Table II). Further, in six patients, all of whom responded to therapy, at least one persistent and one non-persistent clonotype were identified in the same administered TIL population. For every case in which a comparison could be carried out between clonotypes present in an individual patient, the telomere lengths of persistent clonotypes were longer than those of the non-persistent clonotypes, and pairwise analysis carried out for samples obtained from these patients indicated that the telomeres present in the persistent clonotypes were significantly longer than those present in the corresponding non-persistent clonotypes (p<0.05).
Table II.
Telomere lengths of persistent and non-persistent clonotypes from the administered TIL samples and their clinical responses
| Administered TIL sample | Patient clinical responsea | Persistent clonotypeb | Telomere length (Kb) | Non-persistent clonotypec | Telomere length (Kb) |
|---|---|---|---|---|---|
| TIL 2208 | CR | N/Ad | N/A | VB17a (48%→0%)e | 6.48f, g |
| VB17b (9%→0%) | |||||
| TIL 1913 | PR | N/A | N/A | VB1 (16%→0%) | 4.27 |
| TIL 1931 | PR | F4-MART-1 (20%→83%) | 8.91 | F5-MART-1 (24%→0%) | 4.61 |
| TIL 1954 | PR | VB8 (10%→5%) | 6.59 | VB3 (13%→0%) | 4.67 |
| VB14 (5%→0%) | 4.18 | ||||
| TIL 1963 | PR | VB5.1 (23%→7%) | 7.26 | N/A | N/A |
| VB7 (65%→77%) | 8.04 | ||||
| TIL 2026 | PR | VB2 (65%→8%) | 5.21 | N/A | N/A |
| TIL 2035 | PR | VB1 (1%→9%) | 5.32 | VB2 (9%→0%) | 4.73 |
| VB17 (31%→0%) | 4.56 | ||||
| TIL 2109 | PR | N/A | N/A | VB2 (32%→0%) | 3.49 |
| VB5.1 (17%→0%) | 2.94 | ||||
| TIL 2122 | PR | VB16 (45%→20%) | 4.50 | VB13.1 (6%→0%) | 4.34 |
| TIL 2149 | PR | VB8a (19%→7%) | 6.06 g | VB17 (5%→0%) | 4.12 |
| VB8b (6%→1%) | |||||
| TIL 2154 | PR | N/A | N/A | VB2 (15%→0%) | 6.76 |
| VB4 (26%→0%) | 4.11 | ||||
| TIL 2162 | PR | VB11 (50%→7%) | 5.00 | VB17 (13%→0%) | 4.31 |
| TIL 2037 | NR | VB5.1a (27%→9%) | 6.09 g | N/A | N/A |
| VB5.1b (26%→7%) | |||||
| VB7 (2%→17%) | 4.76 | ||||
| TIL 1941 | NR | N/A | N/A | VB3 (91%→0%) | 3.84 |
| Mean ± SE | 6.16 ± 0.43 | Mean ± SE | 4.49 ± 0.25 | ||
| Mann-Whitney U test | P<0.001 | ||||
Patient clinical response: CR, complete response; PR, partial response; NR, no response.
Persistent clonotypes are defined as TRBV Sequences, determined by 5’RACE analysis, that were derived from the administered TILs and present at levels ≥5% in PBMC samples obtained from melanoma patients at one month after TIL administration.
Non-persistent clonotypes are defined as TRBV sequences, determined by 5’RACE analysis, that were represented at levels ≥5% of the administered TILs and that were present at levels ≤ 1% in PBMC samples obtained at one month after TIL administration.
N/A: Either the corresponding antibodies were not available to detect and isolate the specific TRBV sequences, or none of TRBV sequences from the administered TILs met the criteria used to define either persistent clonotypes or non-persistent clonotypes.
The first number in the parenthesis represents the percentage of specific clonotype TRBV sequences in the administered TILs, and the second represents the percentage of specific clonotype TRBV sequences in PBMC samples obtained at one month after TIL administration.
Both persistent and non-persistent clonotypes were sorted from the administered TIL samples using magnetic beads as described in the materials and methods, and expressed the appropriate marker on >95% of the sorted cells.
Average of two clonotypes.
Since the mean telomere length of the heterogeneous bulk TILs correlated with post-treatment lymphocyte levels in vivo (Fig. 2), the association of telomere length with the degree of persistence of individual clonotypes that were observed following adoptive transfer, which may reflect their proliferative capacity, was examined. The results of this analysis indicated that the number of lymphocytes in peripheral blood corresponding to dominant persistent clonotypes was significantly correlated with the telomere length of the in vitro cultured T cells prior to transfer at both between 5 and 10 days following transfer (Fig. 3A; p<0.0005) and between 19 and 37 days following transfer (Fig. 3B; p<0.005). In an attempt to more directly measure of the extent of T cell proliferation that occurred following transfer, the number of cells corresponding to persistent clonotypes that were detected in peripheral blood relative to the number of cells corresponding to those clonotypes that were initially present in the infused TIL was calculated and expressed as relative cell number in PBMC (Fig. 3C, 3D). The relative cell numbers were significantly correlated with the telomere length between 5 and 10 days (p<0.005) as well as at the later time point (p<0.01), providing direct evidence that the proliferative capacity of adoptively transferred T cells may be correlated with telomere length.
Figure 3.
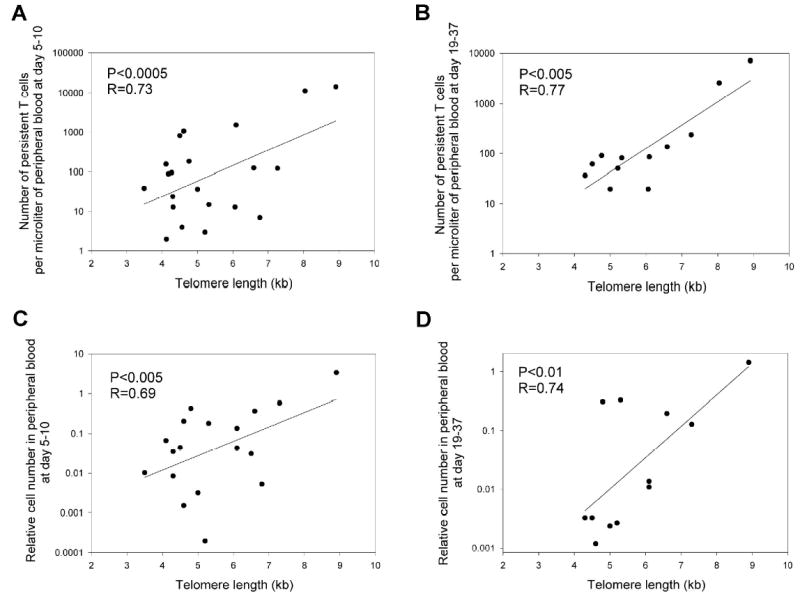
Correlation of telomere length of transferred lymphocytes with in vivo T cell persistence. The telomere lengths of transferred lymphocytes were compared with the total number of persistent T cells detected in patient peripheral blood samples obtained between 5 and 10 days following adoptive cell transfer (A) and between 19 and 37 days following adoptive transfer (B). In addition, the relative number of persistent T cells that were present in peripheral blood was calculated by determining the ratio of number of cells corresponding to individual persistent clonotypes that were originally present in the administered TIL to the number of those cells detected in peripheral blood at the two time points, assuming a total cell blood volume of 4 liters. A value of 1 indicates that the number of cells present in blood was equal to that present in the TIL. The relative cell numbers were calculated between 5 and 10 days (C) and between 19 and 37 days (D) following transfer. All clonotypes that were present in the administered TILs, as determined by sequencing the expressed TRBV gene products, and that were also detected in the peripheral blood at levels of 1% or greater in either sample, were included in this analysis.
Comparison of T cell phenotypic markers between the persistent and non-persistent clonotypes
Since phenotypic markers have also been associated with cells that have undergone extensive proliferation, additional studies were carried out to determine whether or not differences in the expression of cell surface phenotypic markers could be discerned on the persistent and non-persistent clonotypes that had been analyzed with regard to telomere length. Pair-wise analysis of T cell clonotypes from individual patients revealed that the expression of 45 markers that included co-stimulatory, cytokine and chemokine receptors, as well as markers associated with T cell senescence did not differ significantly between the persistent and non-persistent T cell clonotypes (Table III). Only one of the markers that were examined, CD28, appeared to be expressed at a significantly higher level on cells corresponding to persistent clonotypes than non-persistent clonotypes (Table III), although the expression of CD28 on individual clonotypes was heterogeneous and no distinct CD28 positive and negative subpopulations could be discerned among the cells that comprised individual clonotypes. Previous studies indicated that there was a significant correlation between CD28 expression on CD8 T cells and telomere length (8, 25). In agreement with these observations, there appeared to be a weak correlation between the expression of CD28 and the telomere length of individual clonotypes within the administered TIL (p<0.05, without correction for multiple comparisons), and the majority of the persistent clonotypes had long telomeres and expressed a relatively high level of CD28 (Fig. 4). These data suggest that the in vivo persistence of adoptively transferred T cells is associated both with telomere length and CD28 expression.
Figure 4.
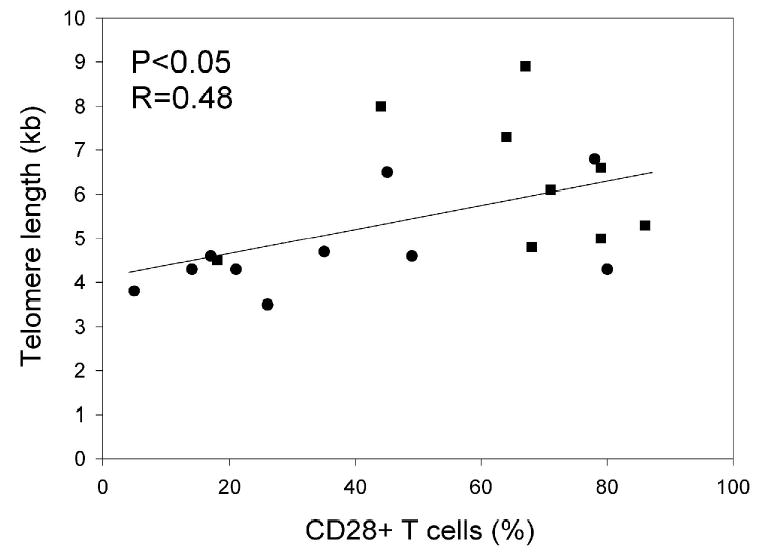
Relationship between telomere lengths and CD28 expression of transferred T cell clonotypes. The percentage of CD28 expression on cells corresponding to individual clonotypes was determined by co-staining with anti-TCR Vbeta antibodies. Closed squares represented persistent clonotypes and closed circles represented non-persistent clonotypes.
Discussion
The results presented in this study indicate that the telomere lengths of transferred lymphocytes significantly correlate with T cell persistence in vivo following adoptive cell transfer. Our previous study demonstrated that there was a significant correlation between tumor regression and the degree of persistence in peripheral blood of adoptively transferred T cell clonotypes (1, 3–5). These data suggest that telomere length of transferred lymphocytes also correlates with tumor regression in melanoma patients after adoptive cell transfer. Thus telomere length may represent a marker that could aid in the selection of cells utilized for adoptive immunotherapy. It is clear that long telomere length, however, is not sufficient to mediate tumor regression. The T cells that were administered to one of the non-responders, patient 2037, possessed relatively long telomeres and persisted to a high degree following adoptive transfer (Table II). In addition, TIL 2080, which failed to mediate significant tumor regression following adoptive transfer, possessed longer telomeres than many of the TIL from responding patients (Table I). Preliminary results, however, indicate that a dominant clonotype from TIL 2080 that recognized the MART-1:26–35 epitope was highly persistent following adoptive transfer (unpublished data). The lack of response to therapy that was observed in these patients in the face of what appeared to represent relatively high levels of persistence is unexplained, but could have resulted from tumor antigen or MHC antigen loss, as well as from loss of molecules involved with antigen processing in the tumor or with effector mechanisms required for anti-tumor effects.
The mean telomere length of bulk TIL that were administered to patients was associated with the post-transfer overall absolute lymphocyte count (Fig. 1), irrespective of the levels of T cell persistence that were observed in these patients (1, 3–5). The apparent association that was observed one month following transfer was unexpected, given that for many of the patient samples analyzed, only a minority of the total populations of lymphocytes present in the blood at this time appeared to comprise the transferred T cells (unpublished data). Persistence of individual clonotypes at the tumor site may be a better indication than clonotypes in peripheral blood; however, most patients had metastases in parenchymal organs and were thus not accessible to study. It is also possible that the persistence in peripheral blood was under-estimated due to the persistence of minor T cell clonotypes that were not accurately evaluated due to the limited number of sequences that were determined. Alternatively, some feature of the patient environment may act to promote T cell proliferation independently of the particular T cells that were administered.
Although persistence of adoptively transferred T cells following non-myeloablative chemotherapy has been associated with clinical response, widely varying levels of persistence have been observed in responding patients. In addition, individual clonotypes that possessed relatively long telomeres in some cases demonstrated little or no persistence following adoptive transfer. A variety of factors that include tumor histology, tumor burden, as well as antigen and MHC expression could play a significant a role in the outcome of these treatments, and could potentially have an impact on the degree of persistence of individual T cell clonotypes. The lack of clinical responses observed in patients who received cells that possessed telomeres shorter than 4 kb, however, may indicate that possession of relatively long telomeres represents an important factor that may be required for the proliferation as well as persistence of cells in vivo. Activated T cells up-regulate telomerase expression (30), and ectopic expression of the human telomerase reverse transcriptase gene in human CD8 positive T cells has been shown to result in telomere elongation and extension of lifespan (19, 31, 32). The results of previous studies have indicated that signaling through CD28 may lead to enhanced telomerase expression (27, 33), and a weak but potentially significant association was noted in the current study between CD28 expression and the persistence of individual clonotypes. The levels of telomerase expression in TIL are now being evaluated in an attempt to address this issue. Prior observations, taken together with the findings presented in this report, indicate that over-expression of telomerase could potentially enhance the in vivo persistence and the efficacy of cell transfer therapies in cancer patients, although again, this may not be sufficient in all cases to lead to the persistence of tumor reactive T cells and any associated clinical responses. Nevertheless, analysis of telomere length may provide a means to identify more effective TILs for adoptive immunotherapy and suggests that the proliferative potential of the transferred TIL is an important factor influencing T cell persistence and tumor regression.
Footnotes
Abbreviations used in this paper: ALC, absolute lymphocyte counts; flow-FISH, fluorescence in situ hybridization and flow cytometry; MESF, molecules of equivalent soluble fluorescence; TIL, tumor-infiltrating lymphocytes; TRBV, T cell receptor beta chain variable region genes.
References
- 1.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Selective growth, in vitro and in vivo, of individual T cell clones from tumor-infiltrating lymphocytes obtained from patients with melanoma. J Immunol. 2004;173:7622–7629. doi: 10.4049/jimmunol.173.12.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005;28:53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr, Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 7.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 8.Rufer N, Dragowska W, Thornbury G, Roosnek E, Lansdorp PM. Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat Biotechnol. 1998;16:743–747. doi: 10.1038/nbt0898-743. [DOI] [PubMed] [Google Scholar]
- 9.Hodes RJ, Hathcock KS, Weng NP. Telomeres in T and B cells. Nat Rev Immunol. 2002;2:699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- 10.Reed JR, Vukmanovic-Stejic M, Fletcher JM, Soares MV, Cook JE, Orteu CH, Jackson SE, Birch KE, Foster GR, Salmon M, Beverley PC, Rustin MH, Akbar AN. Telomere erosion in memory T cells induced by telomerase inhibition at the site of antigenic challenge in vivo. J Exp Med. 2004;199:1433–1443. doi: 10.1084/jem.20040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner UG, Koetz K, Weyand CM, Goronzy JJ. Perturbation of the T cell repertoire in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1998;95:14447–14452. doi: 10.1073/pnas.95.24.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 13.de Lange T. Protection of mammalian telomeres. Oncogene. 2002;21:532–540. doi: 10.1038/sj.onc.1205080. [DOI] [PubMed] [Google Scholar]
- 14.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 15.Weng NP, Levine BL, June CH, Hodes RJ. Regulated expression of telomerase activity in human T lymphocyte development and activation. J Exp Med. 1996;183:2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weng NP, Palmer LD, Levine BL, Lane HC, June CH, Hodes RJ. Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol Rev. 1997;160:43–54. doi: 10.1111/j.1600-065x.1997.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 17.Weng NP, Levine BL, June CH, Hodes RJ. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc Natl Acad Sci U S A. 1995;92:11091–11094. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth A, Yssel H, Pene J, Chavez EA, Schertzer M, Lansdorp PM, Spits H, Luiten RM. Telomerase levels control the lifespan of human T lymphocytes. Blood. 2003;102:849–857. doi: 10.1182/blood-2002-07-2015. [DOI] [PubMed] [Google Scholar]
- 19.Hooijberg E, Ruizendaal JJ, Snijders PJ, Kueter EW, Walboomers JM, Spits H. Immortalization of human CD8+ T cell clones by ectopic expression of telomerase reverse transcriptase. J Immunol. 2000;165:4239–4245. doi: 10.4049/jimmunol.165.8.4239. [DOI] [PubMed] [Google Scholar]
- 20.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28−CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 21.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 22.Beyersdorf NB, Ding X, Karp K, Hanke T. Expression of inhibitory "killer cell lectin-like receptor G1" identifies unique subpopulations of effector and memory CD8 T cells. Eur J Immunol. 2001;31:3443–3452. doi: 10.1002/1521-4141(200112)31:12<3443::aid-immu3443>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Hamann D, Kostense S, Wolthers KC, Otto SA, Baars PA, Miedema F, van Lier RA. Evidence that human CD8+CD45RA+CD27− cells are induced by antigen and evolve through extensive rounds of division. Int Immunol. 1999;11:1027–1033. doi: 10.1093/intimm/11.7.1027. [DOI] [PubMed] [Google Scholar]
- 24.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 25.Effros RB, Boucher N, Porter V, Zhu X, Spaulding C, Walford RL, Kronenberg M, Cohen D, Schachter F. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994;29:601–609. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 26.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Baarle D, Tsegaye A, Miedema F, Akbar A. Significance of senescence for virus-specific memory T cell responses: rapid ageing during chronic stimulation of the immune system. Immunol Lett. 2005;97:19–29. doi: 10.1016/j.imlet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 29.Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190:157–167. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cristofalo VJ, Lorenzini A, Allen RG, Torres C, Tresini M. Replicative senescence: a critical review. Mech Ageing Dev. 2004;125:827–848. doi: 10.1016/j.mad.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Rufer N, Migliaccio M, Antonchuk J, Humphries RK, Roosnek E, Lansdorp PM. Transfer of the human telomerase reverse transcriptase (TERT) gene into T lymphocytes results in extension of replicative potential. Blood. 2001;98:597–603. doi: 10.1182/blood.v98.3.597. [DOI] [PubMed] [Google Scholar]
- 32.Dagarag M, Evazyan T, Rao N, Effros RB. Genetic manipulation of telomerase in HIV-specific CD8+ T cells: enhanced antiviral functions accompany the increased proliferative potential and telomere length stabilization. J Immunol. 2004;173:6303–6311. doi: 10.4049/jimmunol.173.10.6303. [DOI] [PubMed] [Google Scholar]
- 33.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]


