Abstract
Streptococcus pneumoniae is an important human pathogen that contains single copies of genes encoding the ClpP and FtsH ATP-dependent proteases but lacks the Lon and HslV proteases. We constructed and characterized the phenotypes of clpP, clpC, and clpX deletion replacement mutants, which lack the ClpP protease subunit or the putative ClpC or ClpX ATPase specificity factor. A ΔclpP mutant, but not a ΔclpC or ΔclpX mutant, of the virulent D39 type 2 strain of S. pneumoniae grew poorly at 30°C and failed to grow at 40°C. Despite this temperature sensitivity, transcription of the heat shock regulon determined by microarray analysis was induced in a ΔclpP mutant, which was also more sensitive to oxidative stress by H2O2 and to puromycin than its clpP+ parent strain. A ΔclpP mutant, but not a ΔclpC mutant, was strongly attenuated for virulence in the murine lung and sepsis infection models. All of these phenotypes were complemented in a ΔclpP/clpP+ merodiploid strain. Consistent with these complementation patterns, clpP was found to be in a monocistronic operon, whose transcription was induced about fivefold by heat shock in S. pneumoniae as determined by Northern and real-time reverse transcription-PCR analyses. Besides clpP, transcription of clpC, clpE, and clpL, but not clpX or ftsH, was induced by heat shock or entry into late exponential growth phase. Microarray analysis of ΔclpP mutants showed a limited change in transcription pattern (≈80 genes) consistent with these phenotypes, including repression of genes involved in oxidative stress, metal ion transport, and virulence. In addition, transcription of the early and late competence regulon was induced in the ΔclpP mutant, and competence gene expression and DNA uptake seemed to be constitutively induced throughout growth. Together, these results indicate that ClpP-mediated proteolysis plays a complex and central role in numerous pneumococcal stress responses, development of competence, and virulence.
Four distinct classes of ATP-dependent proteases have been found in bacteria, including the multimeric Clp (ClpAP, ClpXP, or ClpCP) and HslUV (ClpYQ) proteases and the single-chain AAA (FtsH) and Lon family proteases (Lon) (19). The Clp proteases (caseinolytic proteases) were first identified in Escherichia coli and consist of an ATPase specificity factor (ClpA or ClpX in E. coli and ClpX or ClpC in Bacillus subtilis) and a proteolytic domain (ClpP) that contains a consensus serine protease active site (17, 19, 36). This multimeric enzyme complex assembles into a structure that is remarkably similar to that of the eukaryotic proteosome (7). In E. coli, ClpP-mediated proteolysis, which is regulated by heat shock, removes abnormal proteins that accumulate during stress conditions, recycles amino acids from nonessential proteins during starvation, and contributes to the clearance of truncated peptides produced from stalled ribosomes by the SsrA system (20, 47). In addition, ClpP-mediated proteolysis in E. coli controls the half-life of regulatory proteins, including the stationary phase sigma factor σs (59), the heterodimeric UmuD SOS protein (11), and several phage proteins (reviewed in reference 17). Despite the significant roles played by the ClpP system in stress responses in E. coli, the phenotype of E. coli clpP mutants is essentially identical to that of wild-type cells (5), especially in the degradation of abnormal proteins (18). Presumably, this results from the presence of other redundant ATP-dependent proteases that likely play a more critical role in this process (i.e., Lon and HslUV).
ClpP-mediated proteolysis appears to be more central for stress tolerance and global regulation in bacteria other than E. coli. For example, clpP mutants of B. subtilis and Lactococcus lactis are impaired for survival during heat shock and other stress conditions and exhibit clear defects in the degradation of abnormal proteins (12, 14, 39). Microbial developmental processes, such as natural genetic competence and sporulation in B. subtilis (39), biofilm development in Pseudomonas fluorescens (41), and viability and cell cycle progression in Caulobacter crescentus (25), are dependent on ClpP-mediated proteolysis. Moreover, a growing number of studies demonstrate a requirement for ClpP-mediated proteolysis for virulence and disease progression of important bacterial pathogens. For example, ClpP function is required for virulence factor expression, intracellular survival, and pathogenesis of Listeria monocytogenes in mice (13). ClpP function has also been linked to the acid tolerance response and virulence of the intracellular pathogen Salmonella enterica serovar Typhimurium (21, 56) and to virulence factor expression in Yersinia enterocolitica (42). In some of these cases, the association between ClpP function and virulence may reflect the general role played by ClpP-mediated proteolysis in stress survival.
The S. pneumoniae genome has recently been shown to contain only single copies of clpP and ftsH genes and to lack lon and hslUV genes (23). In this regard, S. pneumoniae is unique among eubacteria, which contain both clpP and lon or multiple copies of clpP when lon is absent (7). S. pneumoniae contains putative orthologs of the ClpC, ClpE, ClpL, and ClpX ATPase specificity factors that presumably associate with the ClpP protease subunit (reviewed in reference 48). S. pneumoniae is a serious human pathogen that is a causal agent of bacterial pneumonia, bronchitis, meningitis, and otitis media and is particularly devastating to the elderly and the very young (55). Antibiotic-resistant clinical isolates of S. pneumoniae have recently become common, and multiple-antibiotic-resistant strains are now found worldwide (27, 52). Normal disease progression for S. pneumoniae begins with entry and colonization of the nasopharnyx followed by progression to other sites, such as the lung (55). The infection process exposes S. pneumoniae to numerous stress conditions, including temperature shift between the upper respiratory tract (30°C) and deeper tissues (37°C), pH changes, exposure to reactive oxygen species generated by host phagocytes, and nutritional deprivation (60).
The role of ClpP- or FtsH-mediated ATP-dependent proteolysis in the stress responses and virulence of S. pneumoniae is largely unknown. Recently, signature-tagged mutagenesis was used to identify ClpC as a putative virulence factor of S. pneumoniae in mice (45); however, ClpP did not turn up in this study. Pneumococcal ClpC was reported to regulate growth inhibition at high temperature, autolysis in the presence of antibiotics, transformation, and adherence (3); however, another report did not confirm these phenotypes (4). Chastanet and coworkers recently reported that ClpP and ClpE play a role in the thermotolerance of S. pneumoniae (4). Their biochemical and genetic evidence in a heterologous B. subtilis system suggest that CtsR regulates the heat-shock response of clpC, clpL, clpE, and clpP and unexpectedly groESL in S. pneumoniae (4). They further demonstrated that the expression of two competence genes is induced in a clpP mutant, suggesting a negative regulatory role for ClpP in competence development under inappropriate conditions (4). Here, we report further characterization of the phenotypes of S. pneumoniae clpP, clpC, and clpX mutants, including global microarray analysis of transcription patterns of clpP mutants and the heat shock regulon of S. pneumoniae. Our results show that ClpP-mediated proteolysis plays complex roles in the response of S. pneumoniae to thermal and oxidation stress, the regulation of the natural competence pathway, and virulence.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. pneumoniae strains were cultivated statically in brain heart infusion (BHI) broth or on trypticase soy agar II blood agar plates (TSA-BA; Becton Dickson BBL) at 37°C in an atmosphere of 5% CO2 unless otherwise stated. Chemically defined medium (CDM) for the growth of S. pneumoniae cultures (50) lacking methionine and glucose was formulated by JRH Biosciences (Denver, Pa.). CDM was supplemented with 1.0% (wt/vol) glucose, 0.1% (wt/vol) choline chloride, 0.075% (wt/vol) l-cysteine HCl, and 0.25% (wt/vol) NaHCO3. For antibiotic selection, media was supplemented with 0.3 μg of erythromycin per ml, 100 μg of spectinomycin per ml, 2.5 μg of chloramphenicol per ml, or 2.5 μg of novobiocin per ml as appropriate.
The virulent type 2 encapsulated S. pneumoniae strain D39 and its unencapsulated derivative R6 (49) used in these studies were assigned unique strain designations to track isogenic derivatives (Table 1). Strains CPM7 [CP1250 ssbB::(pEVP3)::ssbB] and CP1500 were used as the ssbB::lacZ reporter strain and the source of genomic donor DNA in transformation experiments, respectively (Table 1) (34). S. pneumoniae mutants were constructed by transformation of linear deletion replacement amplicons following treatment with competence stimulatory peptide (CSP) (34). Briefly, 1.0- to 1.5-kb DNA fragments corresponding to the 5′ and 3′ regions of target genes to be deleted (≈60 to 80% of internal coding sequences) and either the 1.0-kb ermAM (erythromycin resistance [Err]) (35) or aad9 (spectinomycin resistance [Spr]) (33) cassettes were amplified by PCR using oligonucleotide primers containing short stretches of DNA sequences that were complementary to a DNA fragment designed to be adjoining (Table 2). The resulting three DNA fragments were mixed, allowed to anneal at the overlapping complementary sequences, and extended and amplified using a second round of PCR (22). Recombinant rTth polymerase and XL reaction buffer (Applied Biosystems) were used in these amplifications. Resultant linear gene amplicons containing deletion replacements were transformed into early exponential phase cultures of S. pneumoniae (OD620 of ∼0.05 to 0.1) diluted 1 to 10 in BHI medium containing 10% heat-inactivated horse serum (Sigma), 10 mM glucose, and 100 ng of synthetic CSP-1 per ml and incubated for 15 min at 30 or 37°C to induce competence. Following transformation, bacteria were allowed to recover for 2 h to allow phenotypic expression of the antibiotic resistance marker, and recombinant clones were recovered on TSA-BA in the presence of selective antibiotics in nutrient broth soft-agar overlay (0.8% [wt/vol] Bacto Nutrient Broth, 0.4% [wt/vol] Bacto Agar) at 30 or 37°C.
TABLE 1.
Bacterial strains and DNA amplicons used in strain construction
| Strain | Genotype and/or phenotypea | Description | Source or reference |
|---|---|---|---|
| EL59 | R6, avirulent unencapsulated parent | Derived from D39 isolate | 49 |
| EL161 | D39, virulent encapsulated type 2 parent | Subclone of original clinical isolate | 49 |
| CP1500 | hex nov-r1 bry-r str-1 ery-r1 ery-r2 Nvr | Donor of novobiocin resistance point marker | 34 |
| CPM7 | CP1250 ssbB′::(pEVP3)::ssbB+ssbB::lacZ Cmr SsbB+ | Rx derivative with ssbB::lacZ reporter construct | 34 |
| EL539b | EL59 ΔclpP::aad9 Spr | EL59 transformed with linear ΔclpP::aad9 amplicon | This study |
| EL555 | EL59 ΔbgaA::ermAM Err | EL59 bearing a ΔbgaA::ermAM mutation | D. LeBlanc strain collection |
| EL556 | EL59 ΔlytA::aad9 Spr | EL59 transformed with linear ΔlytA::aad9 amplicon | This study |
| EL677 | EL161 ΔclpP::aad9 Spr | EL161 transformed with linear ΔclpP::aad9 amplicon | This study |
| EL824 | CPM7 ΔclpP::aad9 Spr | CPM7 transformed with linear ΔclpP::aad9 amplicon | This study |
| EL854 | EL59 ΔclpC::ermAM Err | EL59 transformed with linear ΔclpC::ermAM amplicon | This study |
| EL856 | EL161 ΔclpC::ermAM Err | EL161 transformed with linear ΔclpC::ermAM amplicon | This study |
| EL873 | EL59 ΔclpX::ermAM Err | EL59 transformed with linear ΔclpX::ermAM amplicon | This study |
| EL923 | EL677 ΔbgaA::ermAM::T1T2::clpP+ Spr Err ClpP+ | EL677 transformed with linear ΔbgaA::ermAM::T1T2::clpP+ | This study |
| EL925 | EL539 ΔbgaA::ermAM::T1T2::clpP+ Spr Err ClpP+ | EL539 transformed with linear ΔbgaA::ermAM::T1T2::clpP+ | This study |
| EL961 | EL161 ΔbgaA::ermAM Err | EL161 transformed with linear ΔbgaA::ermAM from EL555 | This study |
| Amplicons | |||
| clpP-a | EL59 genomic DNA,c (clpPa1, clpPa2),d partial construct | This study | |
| P-aad9-b | pDL278, (clpPb1, clpPb2), partial construct | This study | |
| clpP-c | EL59 genomic DNA, (clpPc1, clpPc2), partial construct | This study | |
| ΔclpP::aad9 | clpP-a, P-aad9-b, clpP-c, (clpPa1, clpPc2), completed construct | This study | |
| clpC-a | EL59 genomic DNA, (clpCa1, clpCa2), partial construct | This study | |
| C-erm-b | pVA838, (clpCb1, clpCb2), partial construct | This study | |
| clpC-c | EL59 genomic DNA, (clpCc1, clpCc2), partial construct | This study | |
| ΔclpC::ermAM | clpC-a, C-erm-b, clpC-c, (clpCa1, clpCc2), completed construct | This study | |
| clpX-a | EL59 genomic DNA, (clpXa1, clpXa2), partial construct | This study | |
| X-erm-b | pVA838, (clpXb1, clpXb2), partial construct | This study | |
| clpX-c | EL59 genomic DNA, (clpXc1, clpXc2) | This study | |
| ΔclpX::ermAM | clpX-a, X-erm-b, clpX-c, (clpXa1, clpXc2), completed construct | This study | |
| bgaA-a | EL59 genomic DNA, (bgaAa1, bgaAa2), partial construct | This study | |
| B-erm-b | pVA838, (ermAMb1, ermAMb2), partial construct | This study | |
| T1T2-c | pDL278, (terMc1, terMc2), partial construct | This study | |
| clpP-d | EL59 genomic DNA, (clpPd1, clpPd2), partial construct | This study | |
| bgaA-e | EL59 genomic DNA, (bgaAe1, bgaAe2), partial construct | This study | |
| ΔbgaA::ermAM::T1T2::clpP+ | bgaA-a, B-erm-b, T1T2-c, clpP-d, bgaA-e (bgaAa1, bgaAe2), completed construct | This study | |
| lytA-a | EL59 genomic DNA, (lytAa1, lytAa2), partial construct | This study | |
| L-aad9-b | pDL278, (lytAb1, lytAb2), partial construct | This study | |
| lytA-c | EL59 genomic DNA, (lytAc1, lytAc2), partial construct | This study | |
| ΔlytA::aad9 | lytA-a, L-aad9-b, lytA-c, (lytAa1, lytAc2), completed construct | This study | |
| ΔbgaA::ermAM | EL555 genomic DNA, (bgaAa1, bgaAe2), completed construct | This study |
Nvr, resistant to novobiocin; Cmr, resistant to chloramphenicol; Spr, resistant to spectinomycin; Err, resistant to erythromycin.
Strain construction was carried out by transformation of indicated recipient with linear double stranded synthetic PCR amplicon.
Template DNA for amplicon generation.
Primer pair set used to generate a given amplicon (see Table 2).
TABLE 2.
Synthetic oligonucleotides used in these studies
| Name of primer | Sequence (5′ → 3′)a |
|---|---|
| Primer set for clpP probe generation for Northern blotting | |
| clpP-5′ | GCCGTGGAGAACGTTCTTA |
| clpP-3′ | GGGCGCTCATCCAGTTA |
| Primer/probe sets used for quantitative real-time RT-PCR | |
| S.pn. 16S-forward | GACGATACATAGCCGACCTG |
| S.pn. 16S-reverse | GAGTCTGGGCCGTGTCTCA |
| S.pn. 16S-probe | 6FAM-TCCCAGTGTGGCCGATCACCCT-TAMRA |
| S.pn. clpP-forward | GGCTGCATCTATGGGAACAGTC |
| S.pn. clpP-reverse | GCTTTTGTATCATTTAGCATCGCA |
| S.pn. clpP-probe | 6FAM-TCGCATCAAGTGGAGCAAAAGGCA-TAMRA |
| S.pn. fabk-forward | CTGCAGGAGGAATTGCGG |
| S.pn. fabk-reverse | CAGCCTCTGCACCTAGCATAAA |
| S.pn. fabk-probe | 6FAM-TGGTGAAGGTGCTGCGGCTGG-TAMRA |
| Primer sets used for deletion replacement cassette construction by overlap-extension PCR | |
| clpPa1 | GCGAATCGTTCCACTTGTA |
| clpPa2 | CGAACGAAAATCGATCTCCACGGCTTGTTTGTT |
| clpPb1 | AACAAACAAGCCGTGGAATCGATTTTCGTTCGTGAAT |
| clpPb2 | TTCTGCATCTGCATGGACTTAGAATGAATATTTCC |
| clpPc1 | GGAAATATTCATTCTAAGTCCATGCAGATGCAGAA |
| clpPc2 | CAGCAGTTGCTTGTTGTA |
| clpCa1 | CCCGAGCGGATAAAATT |
| clpCa2 | TTATTTCCATAACTTCTTTTATACGAGTCGCCAAGGCA |
| clpCb1 | TGCCTTGGCGACTCGTATAAAAGAAGTTATGGAAATAA |
| clpCb2 | TCAAAGAGGAGAACGGAATAGTTAGCTCCTTGGAAGCTGT |
| clpCc1 | ACAGCTTCCAAGGAGCTAACTATTCCGTTCTCCTCTTTGAT |
| clpCc2 | GGTTTTGTCACATGATCAT |
| clpXa1 | CCGTTACTTGTCAGAACTTT |
| clpXa2 | TTATTTCCATAACTTCTTTTGTGGTTCAAGATATGGA |
| clpXb1 | TCCATATCTTGAACCACAAAAGAAGTTATGGAAAT |
| clpXb2 | TAAGAAAGCAAGGTTTGGTACTTTAGCTCCTTGGAAGCTGT |
| clpXc1 | ACAGCTTCCAAGGAGCTAAAGTACCAAACCTTGCTTTCTTA |
| clpXc2 | CTCTGCGATGACTTCAATT |
| bgaAa1 | TCAATTGATTACTCACTCTACTCTTG |
| bgaAa2 | CAGCTTCCAAGGAGCTAATAAACCTAGTTCTGCTGCGATTTGCAA |
| ermAMb1 | TCGCAGCAGAACTAGGTTTATTAGCTCCTTGGAAGCTGTCAGTA |
| ermAMb2 | CGTTTTATTTGATGCAAGAAGTTATGGAAATAAGACTTAG |
| terMc1 | TTTCCATAACTTCTTGCATCAAATAAAACGAAAGGCTCAGT |
| terMc2 | CGTGACTGGAAAYACTTTTCCAAGTCGTAGAAACGCAAAAAGGCCAT |
| clpPd1 | GCCTTTTTGCGTTTCTACGACTTGGAAAAGTATTTCCAGTCACGAAA |
| clpPd2 | ATGAGAAAGTAAGTTCTTAAGAAATCTCTCCCTATTATA |
| bgaAe1 | GGAGAGATTTCTTAAGAACTTACTTTCTCATAAACCAGTT |
| bgaAe2 | GCTAAAGAATGTCAGGCGCTTTTATCT |
| lytAa1 | GGCATGGGGATTGTCATT |
| lytAa2 | CGAACGAAAATCGATGTTGAGTGTGCGTGTA |
| lytAb1 | TACACGCACACTCAAATCGATTTTCGTTCGTGAAT |
| lytAb2 | TTTGAGGTAGTACCAGCCTGTTAGAATGAATATTTCC |
| lytAc1 | GGAAATATTCATTCTAACAGGCTGGTACTACCTCAAA |
| lytAc2 | CCCTTAGCTACCAACTGA |
Abbreviations: 6FAM, 6-carboxyfluorescein; TAMRA, N, N, N′, N′-tetramethyl-6-carboxyrhodamine. The underlined portion of each oligonucleotide represents short heterologous extensions that are complementary to the fragments of DNA to be linked by overlap-extension PCR.
Phenotypic characterization of S. pneumoniae clpP mutants.
The ability of S. pneumoniae to grow at reduced and elevated temperatures was determined in triplicate on TSA-BA plates incubated at 30, 37, or 40°C for 24 h. The sensitivity of S. pneumoniae EL161 (D39 parent) and its derivatives to puromycin, H2O2, or HCl was evaluated using a disk diffusion assay, as EL59 (R6 parent) was incapable of forming an adequate lawn on TSA plates without blood supplementation. Bacteria were harvested from TSA-BA plates following overnight incubation at 37°C in an atmosphere of 5% CO2 and suspended in sterile 0.9% (wt/vol) NaCl to yield an optical density at 620 nm (OD620) of ∼0.13 (∼1 × 107 CFU per ml). One milliliter of suspension was mixed with 3 ml of nutrient broth soft agar and poured onto the surfaces of BHI agar plates. Sterile 8-mm-diameter disks were placed in the center of the lawns of test organisms and saturated with 10 μl of 4.8 mg of puromycin (Sigma) per ml, 4.9 M H2O2 (Mallinckrodt), or 1 N HCl (Red Bird Inc.). Average zones of inhibition were determined after 24 h of growth at 37°C under 5% CO2, and statistical comparisons were made using one-way analysis of variance (ANOVA), where P values of <0.05 were considered significant. The effects of clpP, clpC, and clpX mutations on autolysis of pneumococcus was determined by measuring the loss of OD of 6-ml BHI cultures at mid-exponential phase (OD660 of ∼0.13) over time following addition of a final concentration of 0.01% (wt/vol) Triton X-100.
Infection of ICR outbred mice.
In conducting experiments with animals, the investigators adhered to the Guide for the Care and Use of Laboratory Animals, prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (40a).
Bacteria grown overnight on TSA-BA plates were harvested into 0.9% (wt/vol) NaCl and adjusted to a MacFarland standard of 0.5 (∼1.0 × 108 CFU per ml) which was further diluted fivefold in 0.9% NaCl. After brief isofluorane anesthetization, male ICR outbred mice (Harlan Sprague Dawley) were infected by intratracheal inoculation with 50 μl of bacterial suspension. At 0, 8, 24, and 48 h following inoculation, four mice per group were sacrificed by CO2 asphyxiation and the lungs aseptically harvested and homogenized in 0.9% NaCl. Total numbers of viable bacteria per lung were determined by serial dilution and spreading onto TSA-BA plates. Statistical comparisons were made using ANOVA where P values of <0.05 were considered significant. For 50% lethal dose (LD50) studies, bacteria were grown as described above with the exception that 0.5 MacFarland standard suspensions were serially diluted 10-fold in BHI medium to yield infection doses ranging from ∼102 to 106 CFU per ml. Male ICR outbred mice were injected in the peritoneal cavity with 50 μl of bacterial suspensions and observed daily for clinical signs of disease. Moribund animals were sacrificed by CO2 asphyxiation, and the LD50 was calculated based on the mean infecting dose required to promote evidence of morbidity in 50% of test subjects (10 mice per dose per strain) in a given period of time.
Extraction of total RNA from S. pneumoniae.
Total RNA was extracted from S. pneumoniae by using a variation of the hot-phenol method described by Tsui et al. (53). Briefly, 10 ml of S. pneumoniae culture was mixed directly with 5 ml of 100°C lysis buffer containing 2% (wt/vol) sodium dodecyl sulfate (SDS) and 16 mM EDTA (pH 8.0) and held for 2 min at 100°C to induce cell lysis. The lysed culture was extracted with 10 ml of acidified phenol (Gibco BRL) at 65°C for 5 min with vigorous agitation. The aqueous phase was further extracted once with phenol-chloroform-isoamyl alcohol (25:24:1; Gibco BRL) and once with chloform-isoamyl alcohol (Gibco BRL). Total RNA was concentrated from the aqueous phase by using Centriplus YM-30 cartridges (Millipore) and purified using an RNeasy (Qiagen) column with on-column DNase I treatment performed according to the manufacturer's instructions.
Heat shock of S. pneumoniae.
S. pneumoniae cells were cultivated statically overnight in CDM at 37°C in an atmosphere of 5% CO2. Cultures were diluted to an OD620 of ∼0.03 in 40 ml of prewarmed CDM and allowed to incubate without shaking at 37°C until the culture reached early to middle exponential growth phase (OD620 of ∼0.3). Cultures were divided into three 10-ml portions which were heated at 37°C or for 5 and 15 min at 45°C, after which RNA was extracted as described above.
Northern analysis and quantitative real time reverse transcription (RT)-PCR.
Aliquots of 5 μg of total S. pneumoniae RNA, purified as described above, were separated by formaldehyde agarose gel electrophoresis and transferred using a Northern Max kit (Ambion, Inc.) according to the manufacturer's instructions. A 0.5-kb internal fragment of clpP generated by PCR with the primer set clpP-5′ and clpP-3′ (Table 2) was labeled with psoralen-biotin by using a Brightstar psoralen-biotin nonisotopic labeling kit (Ambion) and used as a probe for Northern analysis according to the conditions given for the Northern Max kit. Bands on the exposed audioradiographic film were quantified using Sigma Scan version 2.0 (Jandel). Relative increases (n-fold) in clpP band intensities of RNA isolated from cultures following a shift from 37 to 45°C for 5 and 15 min were compared to that of the 37°C control.
S. pneumoniae clpP mRNA was quantified by a real-time, 5′ exonuclease PCR (TaqMan) assay (31) using a primer-probe combination that recognized a portion of the S. pneumoniae clpP gene. For comparison purposes, primer-probe sets for fabK and 16S rRNA were also employed. The primers and probes are listed in Table 2. Primer-probe sets were selected using Primer Express software (PE Biosystems, Foster City, Calif.) and were obtained from PE Biosystems. The primers were used in concentrations of 300 nM and the probes were used in concentrations of 200 nM in 25-μl reaction mixtures. TaqMan reactions were set up using an EZ RT-PCR kit (PE Biosystems). Each reaction contained twofold serial dilutions of either 200 ng or 20 ng of total RNA, depending on the primer-probe set used. The quantitative RT-PCRs were performed on a ABI PRISM 7700 (PE Biosystems) and included the following steps: 2 min treatment with uracil N-glycosylase at 50°C, 30 min of reverse transcription at 60°C, 5 min at 95°C, and then 40 cycles of amplification using 95°C for 15 seconds followed by 60°C for 60 s. Three replicates were performed for each sample. The results were calculated using the comparative critical threshold (ΔΔCT) method (user bulletin no. 2 [http://docs.appliedbiosystems.com/pebiodocs/04303859.pdf]; Applied Biosystems), in which the amount of target is normalized to a reference (non-heat shock control) relative to an internal calibrator target RNA (16S rRNA). Transcript amounts from a control target gene (fabK) were analyzed using the same parameters. Data are presented as the relative changes in experimental target amounts compared to those of the 37°C control following 5- and 15-min shifts from 37 to 45°C or in the clpP mutant (EL539).
Microarray analysis.
Fragmentation and labeling of RNA was performed as instructed by Affymetrix (Santa Clara, Calif.). Briefly, 60 μg of total RNA was fragmented in 1.1× T4 polynucleotide kinase buffer (New England Biolabs) for 10 min at 95°C. The fragmented RNA was then end-labeled with 0.1 mM [γ-S]ATP by using T4 polynucleotide kinase for 1 h at 37°C. Labeled RNA was precipitated and allowed to react with 2 mM PEO-iodoacetyl-biotin (Pierce) in 30 mM MOPS (morpholinepropanesulfonic acid) (pH 7.5) for 1 h at 37°C. The biotinylated RNA was purified using a DNA/RNA midi kit (Qiagen) according to the manufacturer's instructions. RNA was concentrated by precipitation with isopropanol. An aliquot of 10 μg of fractionated biotin-labeled RNA was used for hybridization with each S. pneumoniae R6 microarray, which were custom designed and manufactured by Affymetrix. The custom arrays represent >95% of S. pneumoniae R6 predicted open reading frames (ORFs) (23) tiled onto two low-density 50-μm feature gene arrays. The genes that were not represented on the chip included one copy of those from identical or redundant ORFs (i.e., comX1 and comX2), ORFs that were <200 bp in length, and ORFs encoding tRNAs. Each gene on the microarray was represented by a probe set which consisted of 20 pairs of perfect-match and mismatch oligonucleotides. Hybridization, staining with R-phycoerythrin (Molecular Probes), and washing conditions were performed as suggested by Affymetrix. Stained microarrays were scanned in a Hewlett-Packard GeneArray Scanner at 570 nm. Data were analyzed using Affymetrix Microarray Suite 4.0. The absence, presence, and relative expression level of a given gene is subject to multiple standardized algorithms, which can be viewed in detail at the Affymetrix website. The hybridization intensity of each probe pair was calculated based on the difference in signal between the perfect-match and the mismatch oligonucleotides. The relative change (n-fold) of the normalized hybridization intensity of the experimental (e.g., R6 ΔclpP mutant) probe set is relative to that of the control (e.g., R6 parent) probe set. Only probe pairs having hybridization intensities that were within the standard deviation of the mean of the hybridization intensity of the entire probe set were included in the relative change (n-fold) calculation. For microarray analysis of heat shock gene induction, the results represent a single microarray analysis and only changes in predicted heat-inducible genes are reported.
Competence and β-galactosidase assays.
For an assay of natural competence development, bacterial cultures of similar ODs after overnight incubation in BHI broth at 37°C were diluted 1 to 10 in 8 ml of BHI medium or CDM to yield an OD620 of ∼0.01. Cultures were incubated at 37°C in an atmosphere of 5% CO2, and growth was monitored by direct measurement of changes at OD620. At 30-min intervals, 100 μl of cell suspension was removed and mixed with 0.2 μg of CP1500 genomic DNA carrying a marker for novobiocin resistance (Table 1). Cultures were incubated for 15 min at 37°C and then treated with 20 U of DNase I (Sample Buffer II; Genomic Solutions) to remove remaining extracellular DNA. Cultures were incubated at 37°C for an additional 45 min to allow phenotypic expression of the antibiotic marker. Nvr clones were recovered from dilutions of the cell suspensions in novobiocin-containing nutrient broth soft-agar overlays poured onto TSA-BA plates. The number of Nvr colonies per milliliter of culture was recorded following overnight incubation at 37°C in an atmosphere of 5% CO2.
For ssbB::lacZ expression studies and experimental induction of competence with CSP-1, bacteria were grown overnight in BHI broth at 37°C and diluted into 3 ml of fresh BHI medium to an OD620 of ∼0.002. Cultures were incubated until the OD620 reached 0.07, at which point genomic DNA from donor strain CP1500 (Table 1) was added to 0.2 μg per ml with and without 50 ng of synthetic CSP-1 per ml. Cultures were incubated for 20 min at 37°C and then treated with 20 U of DNase I to remove remaining extracellular DNA. Thirty minutes after addition of donor DNA, 1 ml of cell suspension was removed, lysed with 0.1% (vol/vol) Triton X-100 at 37°C for 2 min, and assayed for β-galactosidase activity by using ONPG (o-nitrophenyl-β-d-galactopyranoside) as the substrate. Results of β-galactosidase assays are expressed as Miller units normalized to the OD620 of the culture when the assay was initiated. The remaining culture was allowed to incubate in BHI medium at 37°C for an additional 90 min to allow phenotypic expression of the antibiotic resistance marker, and Nvr clones were recovered as described above.
Database searches for HrcA and CtsR DNA binding motifs.
The 5′ leader regions of genes whose transcriptions were induced in the absence of clpP were searched using Vector NTI version 5.0 to find the consensus nucleotide binding sequences for CtsR (RGTCAAWnAnRGTCARn) (9) and HrcA (TTAGCACTC-N9-GAGTGCTAA) (40), where R equals G or A, W equals A or T, and n equals any base. Additional searches were performed on the genome sequence of S. pneumoniae R6 by using the nucleic acid pattern searching program fuzznuc, developed and distributed through the European Molecular Biology Open Software Suite (EMBOSS [http://www.uk.embnet.org/Software/EMBOSS/index.html]).
RESULTS
Temperature requirements for construction of S. pneumoniae clp mutants.
The lack of multiple classes of ATP-dependent proteases in S. pneumoniae R6 led to investigation of the contribution of ClpP to stress response and pathogenesis of this bacterium. Genes predicted to encode homologs of ClpP, ClpX, and ClpC were readily identifiable in the S. pneumoniae R6 genome. The predicted S. pneumoniae clpP and clpX gene products share >76% amino acid similarity with their counterparts in B. subtilis and E. coli (14, 18, 37). The gene encoding the ClpC protein was previously cloned and characterized in S. pneumoniae (3) and shares 63% amino acid similarity with its ortholog from B. subtilis (28). Individual deletion replacement mutations of clpP, clpX, and clpC were constructed by transforming S. pneumoniae with linear deletion cassettes for each gene bearing a selective antibiotic marker; thus, mutations were confined to the limited regions in the amplicons used for transformation. ΔclpC mutants were obtained using standard transformation conditions at 37°C for both the R6 (EL59) and D39 (EL161) parents. In contrast, isolation of ΔclpP strains was significantly enhanced following transformation and recovery at 30°C in the R6 background and was absolutely essential for isolation of these mutants in the D39 background (data not shown). ΔclpX mutants of R6 were obtained at a lower-than-expected frequency at 37°C, and the isolation of a ΔclpX mutation in D39 was achieved only by high-efficiency transformation using genomic DNA isolated from the R6 ΔclpX strain (EL539). At present, the basis for the apparent decrease in the recovery of ΔclpX and ΔclpP transformants in the D39 background is not known. All mutants were confirmed by diagnostic PCR analysis of genomic DNA from isolated clones (data not shown).
ClpP, but not ClpX or ClpC, is required for growth at reduced and elevated temperatures.
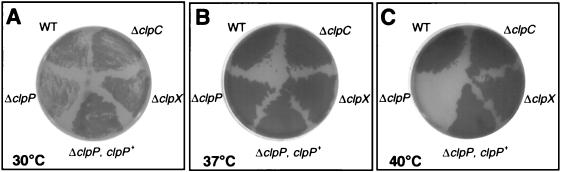
The requirement for a reduced temperature for the efficient recovery of clpP mutants of S. pneumoniae suggested that ClpP proteolysis may be needed for growth under certain stress conditions. To determine whether ClpP-mediated proteolysis was required for growth under heat stress conditions, pneumococcal clp mutants or parental strains were tested for their ability to grow on solid media at 30, 37, and 40°C. Unlike the R6 (EL59) or D39 (EL161) parent strains (Fig. 1) (data not shown), the ΔclpP mutants did not grow at 40°C. In contrast, clpC and clpX single mutants were not restricted for growth at 40°C. These findings are consistent with those reported recently by Chastanet and coworkers for S. pneumoniae clpP mutants in liquid media (4).
FIG. 1.
Impaired growth of S. pneumoniae R6 ΔclpP mutant at reduced and elevated temperatures. WT (R6 parent [EL59]), ΔclpP (R6 ΔclpP::aad9 [EL539]), ΔclpC (R6 ΔclpC::ermAM [EL854]), ΔclpX (ΔclpX::ermAM [EL873]), or ΔclpP, clpP+ (R6 ΔclpP::aad9/ΔbgaA::ermAM::T1T2::clpP+ [EL925]) were streaked in quadrants of TSA-BA plates (see Materials and Methods), which were incubated for 24 h at 30°C (A), 37°C (B), or 40°C (C).
We observed a slight reduction in colony size of ΔclpP strains on TSA-BA plates and a somewhat slower doubling time (78 min versus 63 min) in CDM for the R6 ΔclpP (EL539) mutant compared to its parent (EL59) at 37°C. Surprisingly, the D39 ΔclpP::aad9 mutant (EL677) showed a marked reduction in colony size at 30°C on TSA-BA plates, despite the fact that 30°C incubation was required for efficient initial recovery of the D39 ΔclpP mutant (see above). Taken together, these findings suggest that loss of the pneumococcal clpP gene product effects growth at temperature extremes and reducing growth rate may enhance the initial recovery of these mutants. Last, complementation of clpP mutant phenotypes had not been demonstrated previously (4). Therefore, we introduced an ectopic copy of the clpP+ gene and its promoter into the dispensable (58) bgaA locus of ΔclpP mutants (EL925 and EL923; Table 1). Growth of the ΔclpP/clpP+ merodiploids was indistinguishable from that of the R6 and D39 parent strains on different media at 30, 37, and 40°C (Fig. 1; data not shown). Thus, loss of clpP function alone is responsible for all of the observed growth defects at reduced and elevated temperatures.
Sensitivity of S. pneumoniae clpP mutant to H2O2 and puromycin stress.
The ClpP proteases participate in the degradation of abnormal or damaged proteins that arise during times of stress or in response to chemical or antibiotic treatment (12, 18, 29). The failure of clpP mutants of S. pneumoniae to grow at elevated temperatures is consistent with an absolute requirement for ClpP proteolysis to deal with conditions that are known to cause proteins to misfold. To test this idea further, we determined the sensitivity of S. pneumoniae D39 ΔclpP::aad9 (EL677) to the translational inhibitor puromycin, which binds to the peptidyl transferase site in ribosomes and prematurely truncates translation to generate randomly folded puromycyl peptides (16). Compared to its clpP+ D39 parent (EL161), the ΔclpP mutant was more sensitive to puromycin in disk diffusion assays (Table 3). Consistent with this result, treatment of S. pneumoniae R6 (EL59) with subinhibitory concentrations of puromycin caused a strong induction of the pneumococcal heat shock regulon as determined by DNA microarray analysis (unpublished result). The ΔclpP strain was also more sensitive to H2O2 challenge than its clpP+ parent (Table 3). Both strains were equally sensitive to mild acid stress (Table 3). Again, the sensitivity of the ΔclpP mutant to puromycin and H2O2 was complemented in the ΔclpP/clpP+ merodiploid strain (EL923; Table 3).
TABLE 3.
Increased sensitivity of S. pneumoniae D39 ΔclpP mutant to puromycin and hydrogen peroxide
| Strainb | Diameter of zone of inhibition (mm) (mean ± SDa) on:
|
||
|---|---|---|---|
| Puromycin | Hydrogen peroxide | HCl | |
| EL161 | 22.7 ± 0.9 | 46.6 ± 1.0 | 9.9 ± 0.6 |
| EL677 | 36.6 ± 0.6**c | 57.5 ± 1.5** | 9.5 ± 0.6 |
| EL923 | 22.9 ± 0.8 | 45.1 ± 0.9 | NT |
Results represent the average of four determinations and were repeated in at least two separate experiments. NT, not tested.
Genotypes: EL161 (D39 parent); EL677 (D39 ΔclpP::aad9); EL923 (D39 ΔclpP::aad9 ΔbgaA::ermAM::T1T2::clpP+).
**, P < 0.001 (compared to D39).
Autolysis is not perturbed in clpP mutants.
ClpC was implicated in the autolysis of S. pneumoniae following drug treatment or entrance into stationary phase (3). This defect was thought to have arisen because of the loss of expression of lytA, which encodes the major pneumococcal autolysin. In contrast to this earlier conclusion, it was recently reported that mutations in ctsR, clpC, clpE, or clpP did not alter penicillin- or desoxycholate-induced autolysis of S. pneumoniae (4). To resolve this apparent discrepancy, we tested whether S. pneumoniae strains with individual mutations in clpP, clpX, or clpC were defective in nonionic detergent-induced autolysis. We found that autolysis of the mutants was indistinguishable from that of the parent strain and occurred almost immediately when 0.01% (vol/vol) Triton X-100 was added to cultures (see Materials and Methods; data not shown). In contrast, cultures of a ΔlytA mutant (EL556) stopped growing but did not significantly decrease in optical density after treatment with Triton X-100 (data not shown). Our results confirm and extend those of Chastanet and coworkers (4) and argue that the normal LytA autolytic pathway is not perturbed in the absence of the clpP, clpC, or clpX genes.
The ClpP protease contributes to survival of pneumococcus in ICR outbred mice.
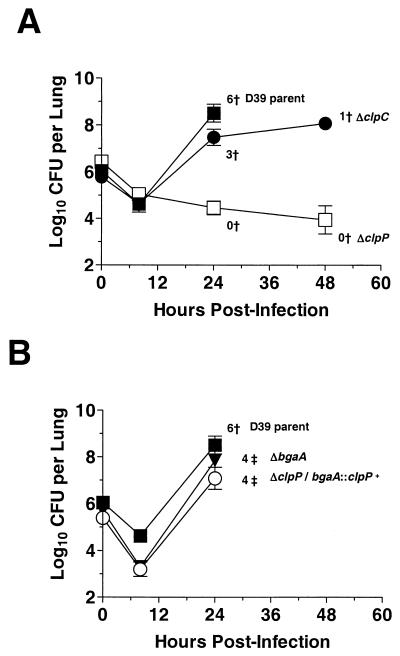
The sensitivity of the D39 clpP mutant to H2O2, puromycin, and temperature suggested that ClpP may either directly or indirectly affect the capacity of this catalase-negative bacterium to act as a respiratory pathogen. To test this hypothesis, male ICR outbred mice were infected by intratracheal challenge with the D39 clpP+ parent (EL161), D39 ΔclpP::aad9 (EL677), and the merodiploid D39 ΔclpP::aad9 ΔbgaA::ermAM::T1T2::clpP+ (EL923). Mice were also infected with D39 ΔbgaA::ermAM (EL961) and D39 ΔclpC::ermAM (EL856) as controls for insertion in the bgaA locus and to assess the effects of the lack of ClpC, which was reported to contribute to pneumococcal virulence (45), respectively. In repeated experiments, the ΔclpP mutant failed to colonize the lungs of infected animals to significant levels and produced no mortality in these animals through 48 h of infection (Fig. 2A). In contrast, the D39 parent (EL161), clpP+ ΔbgaA::ermAM control strain (EL961), and the ΔclpP/bgaA::clpP+ merodiploid (EL923) were highly virulent, replicated to high levels, and yielded no surviving infected animals by 48 h (Fig. 2B). Both the D39 ΔbgaA::ermAM (EL961) and the ΔclpP/bgaA::clpP+ merodiploid (EL923) strains showed slight, but significant, decreases in lung colonization at 8 h compared to that of the D39 parent (EL161) (Fig. 2B). This decrease likely reflected the absence of functional bgaA, which has been suggested to participate in pneumococcus pathogenesis (58). In contrast to the strong attenuation exhibited by the ΔclpP mutant (EL677), the ΔclpC::ermAM mutant (EL856) was only marginally attenuated, replicated to high levels, and produced significant morbidity in infected ICR mice (Fig. 2A). The attenuation of the ΔclpP mutant (EL677) was further evaluated using a murine model of septicemia. In these studies, we determined the average infecting dose of the D39 parent (EL161) and the ΔclpP mutant (EL677) required to produce signs of morbidity in 50% of the test subjects (LD50) within 24 h following direct intraperitoneal injection. The LD50s of the D39 parent (EL161) and the ΔclpP mutant were 10 to 100 and >106, respectively (data not shown), again indicating the strong requirement for clpP function in the pathogenicity of type 2 encapsulated pneumococcus in a murine host.
FIG. 2.
Impaired virulence of S. pneumoniae D39 ΔclpP mutant in lung model of infection. Male ICR outbred mice were infected by intratrachael inoculation with the indicated strains (see Materials and Methods). At each time point, four mice per group were sacrificed, and the CFU per lung were determined by serial dilution and plating on TSA-BA (see Materials and Methods). Symbols: ▪, D39 parent (EL161); □, D39 ΔclpP::aad9 (EL677); •, D39 ΔclpC::ermAM (EL856); ○, D39 ΔclpP::aad9/ΔbgaA::ermAM::T1T2::clpP+ merodiploid (EL923); ▾, D39 ΔbgaA::ermAM (EL961); vertical bars, standard deviations; †, number of moribund animals at each time point; ‡, number of animals that became moribund between the 24- and 48-h time points.
clpP transcript amounts increased in S. pneumoniae in response to heat shock.
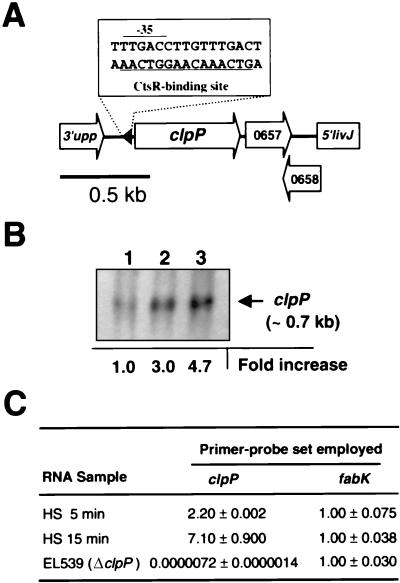
To date, all reported clpP gene products from nonphotosynthetic bacteria are regulated by heat shock (46). Chastanet et al. (4) located a consensus binding site for CtsR (class three stress gene repressor) (9) 67 base pairs upstream of the pneumococcal clpP ATG start codon (Fig. 3A; Table 5), and they showed that purified CtsR binds to this region (4). They also reported a 30-fold induction of a S. pneumoniae clpP-bgaB fusion in a heterologous B. subtilis system in response to heat shock at 48°C (4). The magnitude of this heat shock response was considerably greater than that reported for other stress-response proteases (15, 18). Therefore, we determined the extent of clpP heat shock induction by Northern analysis and real-time quantitative RT-PCR (TaqMan) in the R6 parent strain (EL59) after a shift to 45°C for 5 and 15 min (Fig. 3B and 3C). Transcription of pneumococcal clpP increased in response to heat shock by 2.2- to 7.1-fold by real-time RT-PCR (Fig. 3C) and 3.0- to 4.7-fold by Northern analysis (Fig. 3B). In addition, the Northern analysis indicated that clpP is a monocistronic operon in S. pneumoniae. The transcript length of 0.7 kb corresponds to that expected for the 5′-leader and clpP reading frame alone and not a bicistronic transcript of >0.9 kb containing both the clpP and orf-0657 reading frames (Fig. 3A).
FIG. 3.
Transcription of clpP in S. pneumoniae R6 at 37°C and after heat shock at 45°C. (A) Structure of the clpP locus of S. pneumoniae R6 (drawn to scale). Arrows indicate predicted directions of transcription. The predicted −35 and CtsR-binding sites (underlined) are indicated. See text for details. (B) Northern blot of total RNA isolated from S. pneumoniae R6 (EL59) probed for clpP (see Materials and Methods). Relative expression levels are indicated below the autoradiograph (see Materials and Methods). Lanes: 1, 37°C; 2, 45°C for 5 min; 3, 45°C for 15 min. (C) Quantitative real-time RT-PCR of clpP and fabK transcription following heat shock (HS) at 45°C. Data represent the relative change (n-fold) in mRNA amounts compared to that of the 37°C control, calibrated to 16S rRNA amounts (see Materials and Methods). fabK transcript amounts were included as a control of a gene not known to be regulated by heat shock. No clpP transcript was detected from the ΔclpP mutant (R6 clpP::aad9) (EL539).
TABLE 5.
Organization and transcriptional induction of R6 heat shock genes by temperature shift to 45°C, deletion of clpP, and entry of cultures into late exponential phasea
| Regulatory element and operon organizationd | Fold induction ofb:
|
|||
|---|---|---|---|---|
| Heat shock (shift to 45°C)
|
ΔclpPc | Entry into late exponential phase | ||
| 5 min | 15 min | |||
| HrcA-binding site (CIRCE) | ||||
| TTAGCACTCcagttcaaaGAGTGCTAA—30 nt—ATG—groES—groEL | 5.6 | 5.8 | 2.7/5.4 | 3.2 |
| TTAGCACTCtttataaaaGAGTGCTAA—109 nt—ATG—hrcA—grpE—dnaK—dnaJ | 9.6 | 12.1 | ≈4.2/8.6 | 1.6 |
| TTAGCACTCnnnnnnnnnGAGTGCTAA (consensus)e | ||||
| CtsR-binding site | ||||
| GGTCAAAAAAGGTCAAA (rev)f—67 nt—ATG—clpP | 1.3 | 1.6 | (≈−22.0)/(−21.8) | 1.7 |
| AGTCAAACAAGGTCAAg—25 nt—ATG—ctsR—clpC | 1.7 | 2.5 | 3.7/3.4 | 1.7 |
| GGTCAAAAATAGTCAAA (rev)—40 nt—ATG—clpE | 2.0 | 3.2 | 2.4/1.8 | 2.8 |
| GGTCAAAGATAGTCAAt—36 nt—ATG—clpL | 10.9 | 38.1 | 24.9/13.2 | 9.2 |
| GGTCAgAAATAGTCAAg (rev)—79 nt—ATG—groES—groEL | 5.6 | 5.8 | 2.7/5.4 | 3.2 |
| RGTCAAAnAnRGTCAAA (consensus)g | ||||
| NA—clpX | (−1.6) | (−2.0) | 1.2/(−1.1) | (−1.1) |
| NA—ftsH | (−1.1) | (−1.6) | 1.2/(−1.3) | 1.1 |
Growth of cultures, RNA extraction, and Affymetrix microarray analysis were performed as described in Materials and Methods.
Relative induction (n-fold) was calculated for the first gene of each operon. ≈, relative change (n-fold) calculated relative to that of the background instead of 37°C control.
Data are from each of two independent experiments.
Number of nucleotides (nt) between the 3′ end of the putative regulatory element and the start codon for the first gene of each operon is given. NA, neither regulatory element was detected for clpX and ftsH.
The consensus sequence was previously determined (9). Capitalized bases are strictly conserved. Lower case bases are not conserved.
(rev), listed sequence is located on the noncoding strand.
The consensus sequence was previously determined (39). (R), conservation of either A or G.
Global transcriptional profile of ΔclpP mutant of strain R6.
The phenotypes of clpP mutants suggest that the ClpP protease plays fundamental roles in the normal physiology, stress survival, and pathogenesis of S. pneumoniae. To begin to address the genetic basis for these phenotypes, we performed an analysis of global transcription of the R6 ΔclpP mutant compared to the R6 parent during exponential growth in CDM by using a custom Affymetrix oligonucleotide microarray (Materials and Methods). As an internal control of this method, clpP transcripts were not detected (called absent, with a decrease of at least 22-fold) from the R6 ΔclpP mutant (EL539) compared to the R6 parent strain (EL59) (Table 4. Furthermore, the level of transcription from the upp and orf-0657 genes, which flank clpP (Fig. 3A), was the same in the R6 ΔclpP and its clpP+ parent, indicating a lack of polarity on the surrounding genes.
TABLE 4.
Transcription profile of S. pneumoniae R6 ΔclpP mutant growing exponentially in CDM at 37°Ca
| Role and gene name | Description/putative function | Fold change relative to R6 clpP+ parentb |
|---|---|---|
| Heat shock and proteolysis | ||
| clpC | ATP-dependent Clp protease, ATP-binding subunit | 2.6/3.8 |
| ctsR | Transcriptional regulator, class III heat shock genes | 3.7/3.4 |
| clpL | ATP-dependent Clp protease, ATP-binding subunit | 24.9/13.2 |
| clpE | ATP-dependent Clp protease, ATP-binding subunit | 2.4/1.8 |
| hrcA | Transcriptional regulator, class I heat shock genes | 4.2/8.6 |
| grpE | Heat shock cochaperone | 2.7/4.9 |
| dnaK | Heat shock chaperone HSP70 | 2.9/6.4 |
| dnaJ | Heat shock cochaperone | 3.7/≈27.5 |
| groEL | Heat shock chaperone | 2.8/7.8 |
| groES | Heat shock cochaperone | 2.7/5.4 |
| nisP | Subtilisin-like serine protease | ≈4.2/4.4c |
| clpP* | ATP-dependent Clp protease, proteolytic subunit | (≈−22.0)/(−21.8)d |
| Competence and DNA metabolism | ||
| comD | Competence factor dependent histidine kinase | ≈17.0/≈15.1 |
| comE | Competence factor dependent response regulator | 24.7/31.6 |
| comX1 | Competence-specific sigma factor | 3.5/3.4 |
| spr1859 | ComYA-like protein | 20.5/12.1 |
| cglD | ComG-like protein | 6.8/6.8 |
| cglC | Pilin-like wall structure | 1.6/5.3 |
| cglB | Prepilin transport ATPase | 52.8/28.9 |
| cglA | Prepilin transport pore | 34.1/≈31.8 |
| comF | Competence protein | 2.4/≈3.0 |
| celA | DNA binding | ≈5.7/4.9 |
| celB | DNA uptake pore | 2.4/2.5 |
| ssbB, cilA | ssDNA binding | 10.6/10.7 |
| dinF | Efflux pump | 4.8/3.1 |
| recA | DNA recombination | 5.4/2.4 |
| cinA | Unknown | 4.7/4.1 |
| comA | Transport, ATP binding protein | 4.1/1.5 |
| comB | Transport | ≈23.3/11.9 |
| Peptide/amino acid transport | ||
| aliA | ABC transporter, oligopeptide transport | (−2.2)/(−2.7) |
| pncP | ABC transporter, unknown substrate | ≈11.4/≈3.3 |
| spr0100 | ABC transporter, amino acid transport | 2.8/≈2.9 |
| spr1773 | ABC transporter, ATP-binding protein | ≈8.9/≈12.6 |
| Sugar transport and metabolism | ||
| malR | Transcriptional repressor, maltose operon | ≈3.8/2.1 |
| manN | PTS-EIIAB, mannose specific | 2.1/2.2 |
| bglA | 6-Phospho-β-glucosidase | (−12.8)/(−2.9) |
| spr0277 | Conserved hypothetical | (−7.6)/(−3.2) |
| spr0278 | PTS-EIIB, sugar specific | (−7.6)/(−2.4) |
| bglG | Transcriptional antiterminator, BglG family | (−6.0)/(−2.3) |
| spr0280 | PTS-EIIA, sugar specific | (−6.7)/(−2.6) |
| spr0281 | Hypothetical | (−7.9)/(−3.1) |
| spr0282 | PTS-EIIC, sugar specific | (−7.4)/(−2.9) |
| Gene regulation | ||
| rr06 | Unknown, response regulator | ≈24.3/2.8 |
| hk06 | Unknown, sensor histidine kinase | 9.7/3.1 |
| plcR | Transcriptional regulator | ≈4.2/3.5 |
| Cell surface | ||
| lytA | Major autolysin | 2.7/1.7 |
| dlytC | d-alanyl carrier protein | ≈8.3/3.0 |
| cbpD | Choline-binding protein D | ≈9.4/4.0 |
| htrA | Putative cell surface serine protease | (−2.0)/(−4.5) |
| Oxidative stress resistance | ||
| sodA | Superoxide dismutase, manganese dependent | (−2.1)/(−2.0) |
| tpx | Thiolredoxin-linked thiol peroxidase | (−2.3)/(−3.1) |
| Metal transport/metabolism | ||
| copY | Transcriptional regulator, copper transport | ≈11.1/3.7 |
| ctpA | P-type ATPase, copper transport | ≈13.4/2.7 |
| psaB | ATP-binding protein, Mn2+ transport | (−2.4)/(−2.0) |
| psaC | Membrane spanning permease, Mn2+ transport | (≈−2.5)/(−2.4) |
| Transposon or insertion sequence | ||
| spr1984 | Transposase H, truncation | ≈5.5/3.8 |
| Hypothetical/unassigned | ||
| spr1856 | Unknown | 23.6/13.8 |
| spr1858 | Unknown | 9.5/14.8 |
| spr1996 | Probable integral membrane protein | 2.3/2.3 |
| spr0020 | Unknown | ≈8.2/2.9 |
| spr0030 | Unknown | ≈9.5/≈8.3 |
| spr0031 | Unknown | 4.0/≈8.7 |
| spr0141 | Unknown | (≈−86.9)/(−27.7) |
| spr0142 | Unknown | (−13.0)/(−13.9) |
| spr0143 | Unknown | (−9.2)/(−8.0) |
| spr0144 | Conserved hypothetical | (−8.6)/(−9.9) |
| spr0182 | Unknown | 2.1/2.5 |
| spr0328 | Conserved hypothetical | 2.3/2.3 |
| spr0785 | Unknown | (≈−4.9)/(−2.9) |
| spr0912 | Unknown | (≈−5.6)/(−3.1) |
| spr0946 | Conserved hypothetical | 4.1/3.5 |
| spr1549 | Unknown | (−7.3)/(−2.8) |
| spr1629 | Probable DNA binding protein | ≈5.9/≈16.1 |
| spr1630 | Unknown | ≈8.0/4.2 |
| spr1762 | Unknown | ≈4.3/≈4.0 |
| spr1765 | Unknown | ≈24.3/≈34.1 |
| spr1767 | Probable component, lantibiotic production | ≈8.9/≈12.1 |
| spr1768 | Probable component, lantibiotic production | 9.6/6.2 |
| spr1772 | Unknown | 7.5/6.2 |
Affymetrix microarray analysis of purified RNA was performed as described in Materials and Methods.
Data from each of the two independent experiments.
≈, relative difference (n-fold) calculated relative to background instead of the RNA signal for clpP+ parent.
Decreased transcription relative to the control is bracketed and indicated with a minus sign.
Changes in relative transcript amounts of ≥2.0-fold were observed for 150 and 200 genes, respectively, in two separate and independent experiments. Of these transcriptional changes, 79 were common to both experiments and thus were considered reproducible (see Materials and Methods). Some of the identified genes were linked in the chromosome and showed similar changes in expression; however, additional experiments would be necessary to demonstrate polycistronic operons for these genes. The majority of altered genes could be clustered into several major classes (Table 4), including the majority of the known competence and heat shock regulons (all induced), genes required for virulence and oxidative stress resistance (all repressed), a large group of hypothetical genes of unknown functions, and several genes associated with metabolism and cell surface components. Repressed genes that mediate resistance to oxidative stress included tpx (thioredoxin-linked thiol peroxidase) and sodA (manganese-dependent superoxide dismutase). Repressed genes that influence pneumococcal virulence included sodA and psaABC (manganese transport) (2, 57). It is noteworthy that the transcription of several cell surface proteins that may mediate pathogenesis were induced or repressed in the R6 clpP mutant, including lytA (major autolysin), dlytC (d-alanyl carrier protein), and cbpD (choline-binding protein D) (Table 4).
Altered competence gene expression in clpP mutants.
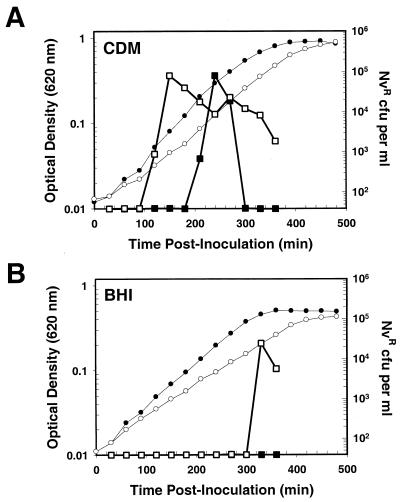
Chastanet and coworkers recently reported that expression of fusion constructs of the early comDCE and late ssbB competence genes was increased in a clpP insertion mutant (4). This observation led to the conclusion that ClpP may act as a negative regulator that prevents competence gene expression under inappropriate conditions. Our global transcription analysis showed that transcription of the majority of genes in the S. pneumoniae competence regulon is highly induced in a ΔclpP mutant during mid-exponential growth phase (Table 4). To test whether this induction was transient, we assayed the assimilation of donor genomic DNA in a ΔclpP mutant derived from the competence indicator strain CPM7 (Table 1) (34). In agreement with the microarray studies, the ΔclpP mutant (EL824) became competent for transformation in CDM at a much lower cell density than did its parental control (CPM7) and remained competent throughout logarithmic growth (Fig. 4A). In contrast, competence in the parental CPM7 strain was transient, resulting in typical pneumococcal transformation kinetics (44) (Fig. 4A). We also found that natural genetic competence did not develop for CPM7 grown in BHI but that this block in competence development could eventually be overcome in a ΔclpP strain (EL824) (Fig. 4B). Independent experiments with BHI medium revealed strong ssbB::lacZ expression (fivefold increase above the parental control) in the ΔclpP mutant or after addition of synthetic CSP-1 to the clpP+ strain, and this was accompanied by a comparable increase in the transformation frequency (data not shown). Maximum expression of ssbB::lacZ (ninefold increase compared to the untreated parental control) and transformation frequency occurred in the ΔclpP mutant treated with synthetic CSP-1 (data not shown). Thus, our microarray and direct determinations of competence development support and extend the conclusion of Chastanet et al. (4) that ClpP proteolysis plays a negative regulatory role in natural competence development of S. pneumoniae. Our results further suggest that ClpP proteolysis plays a role in the termination of competence as well.
FIG. 4.
ClpP negatively regulates competence development in S. pneumoniae. Cells grown in CDM (A) or BHI medium (B) were monitored for changes in OD620 and for the capacity to assimilate genomic donor DNA encoding resistance to novobiocin (see Materials and Methods). •, OD620 of the ssbB::lacZ parent strain (CPM7); ○, OD620 of the ΔclpP::aad9 ssbB::lacZ strain (EL824); ▪, Nvr transformants recovered from the ssbB::lacZ parent strain (CPM7); □, Nvr transformants recovered from the ΔclpP::aad9 ssbB::lacZ strain (EL824). The data are representative of two independent experiments.
Transcriptional induction of major heat shock genes in S. pneumoniae R6.
Microarray analysis of the S. pneumoniae clpP strain during normal exponential growth revealed a strong induction of genes that classically comprise the bacterial heat shock regulon (Table 4). Computer searches had previously demonstrated the presence of consensus HrcA binding sites (putative class I heat shock genes) (40) immediately upstream of the putative groES-groEL and hrcA-grpE-dnaK-dnaJ operons and CtsR consensus binding sites (putative class III heat shock genes) (9) preceding the clpP, ctsR-clpC, clpE, clpL, and groES-groEL loci (Table 5) (4, 26). Putative HcrA and CtsR binding sites were not identified upstream of other pneumococcal genes in the R6 genome (data not shown). Microarray analysis revealed significant transcriptional induction of these pneumococcal classes I and III heat shock genes in bacteria shifted to 45°C (Table 5). In contrast, transcription of clpX (putative specificity factor for ClpP) and ftsH (ATP-dependent protease) were not induced by thermal shock (Table 5), despite the fact that they are class IV heat shock genes in B. subtilis (48). Last, de Saizieu and coworkers (8) found that expression of the clpL and groEL heat shock genes was induced as pneumococcus entered the late exponential phase of growth. We found that transcription of the remaining class I and class III heat shock genes were induced in late exponential compared to early exponential cultures of S. pneumoniae R6 grown in CDM (Table 5). Unlike the heat shock genes, expression of clpX and ftsH was not affected by growth phase (Table 5).
DISCUSSION
Unlike most other bacterial species, S. pneumoniae contains only two ATP-dependent proteases, ClpP and FtsH, which are encoded by single-copy genes (23). We report here that clpP plays complex, pleiotropic roles in pneumococcal stress resistance, gene expression, regulation of genetic competence, and virulence in a murine host. clpP mutants of virulent S. pneumoniae strain D39 exhibited a very limited range of growth centered around 37°C. As noted before (4), clpP mutants of S. pneumoniae do not grow at 40°C, and we show further that this temperature sensitivity is complemented by a wild-type copy of clpP+ (Fig. 1). We found that D39 clpP mutants were cold sensitive and grew poorly at 30°C (see Results), whereas R6 clpP mutants were only moderately impaired for growth at 30°C (Fig. 1). clpX and clpC mutants, which lack ATPase specificity factors that bind to ClpP and that have been implicated in stress tolerance in other bacteria (14, 28), were not impaired for growth compared to their parent strains (Fig. 1; data not shown).
Chastanet and coworkers (4) recently reported that clpE mutants of S. pneumoniae are temperature sensitive, implying a role for ClpE or possibly ClpEP in resistance to thermal shock. Microarray analysis showed that transcription of clpP, clpC, clpL, and clpE, but not clpX or ftsH, are subject to heat shock regulation (Table 5). Consistent with this result, clpP, clpC, clpL and clpE were members of class III heat shock operons regulated by CtsR protein (Table 5) (4). This finding distinguishes S. pneumoniae from B. subtilis, in which clpX and ftsH are class IV heat shock genes whose transcription is induced by elevated temperature (reviewed in reference 48). Combinations of clpC, clpL, and clpX mutations have not been tested for temperature sensitivity.
S. pneumoniae clpP mutants were temperature sensitive despite the fact that transcription of the class I and class III heat shock regulons was induced compared to that of the clpP+ parent in bacteria growing exponentially at 37°C (Table 5). The class I and class III heat shock regulons were also induced as S. pneumoniae R6 entered late exponential growth phase, which precedes the onset of autolysis (Table 5). Misfolded proteins, which likely accumulate in clpP mutants, and possibly during late phase growth of the R6 parent, are thought to be a signal for heat shock induction (40). Consistent with this interpretation, clpP mutants were more sensitive to puromycin than their clpP+ parents (Table 3). Similar temperature and puromycin sensitivity were reported for a clpP mutant of L. lactis, which is a close relative of S. pneumoniae (12). Alternatively, ClpP could regulate the heat shock response directly. In B. subtilis, the McsA and McsB proteins, which are cotranscribed with clpC, modulate the targeting of CtsR by the ClpCP protease during stress (30). However, this exact mechanism is unlikely to occur in S. pneumoniae, because the mcsA and mcsB genes are absent by BLAST analysis (1).
The clpP operon is monocistronic on the basis of Northern analysis (Fig. 3) and the observation that ΔclpP insertion mutations did not diminish the amount of transcript synthesized from the downstream orf-0657 (see Results). Moreover, Δorf-0657 insertion mutants did not exhibit the phenotypes of the ΔclpP insertion mutants (data not shown), and the ΔclpP phenotypes were complemented by an ectopic copy of wild-type clpP+ (Table 3; Fig. 1), indicating that these phenotypes were due solely to lack of clpP function. clpP is regulated by heat shock in all of the nonphotosynthetic bacteria reported to date (46). Previous work established that S. pneumoniae clpP is regulated by heat shock and is a member of the class III CtsR regulon (Table 5) (4). We found that the extent of clpP heat shock regulation in S. pneumoniae was considerably less than the 30-fold reported for heterologous expression in B. subtilis (4). Northern analysis and quantitative real-time RT-PCR indicated that clpP transcription was induced two- to threefold or five- to sevenfold in S. pneumoniae shifted to 45°C for 5 or 15 min, respectively (Fig. 3). In contrast, transcription of the fabK gene, which is involved in fatty acid biosynthesis (23) and is not part of a heat shock regulon (Table 5), was not induced by this temperature shift (Fig. 3).
ClpP proteases do not generally seem to mediate the degradation of oxidatively damaged proteins (6). Therefore, it was somewhat unexpected to find that a S. pneumoniae ΔclpP mutant is more sensitive to H2O2 compared to its clpP+ parent (Table 3). Global transcriptional analysis of this catalase-lacking bacterium showed that expression of the tpx (thioredoxin-linked thiol peroxidase) and sodA (manganese-dependent superoxide dismutase) genes was decreased in the ΔclpP mutant compared to its clpP+ parent (Table 4). This repression could possibly account for the H2O2 sensitivity of the ΔclpP mutant. Further experiments are required to understand the link between ClpP function and sensitivity to H2O2.
The microarray and direct determination of competence development reported here support and extend the general conclusion of Chastanet and coworkers that ClpP acts as a negative regulator of competence expression (4). Moreover, our results are consistent with a role of ClpP in the termination of competence, which is normally transient in S. pneumoniae (44). We found that transcription of the majority of the known competence regulon is strongly induced in the ΔclpP mutant at a time during exponential growth when the pathway is normally repressed (Table 4). Expression of an ssbB-lacZ reporter gene (data not shown) and DNA uptake (Fig. 4) were derepressed without the addition of CSP-1, and addition of synthetic CSP-1 further increased derepression and DNA uptake (data not shown). These results could be explained by increased expression of the ComDE two-component system, which senses CSP-1 levels and indirectly activates transcription of late genes in the competence pathway, or increased expression of the CSP-1 pheromone, which is cotranscribed in the comCDE operon (43). A direct role for ClpP-mediated proteolysis in regulating the cellular amounts of ComDE is a testable hypothesis, although the effect of ClpP could also be indirect. There is a precedent for ClpP-mediated proteolysis of a competence transcription factor in B. subtilis in which a complex forms containing the MecA adaptor protein, the ComK competence transcription factor, and ClpCP (54). S. pneumoniae encodes a MecA homolog (73% amino acid similarity to B. subtilis), but lacks other factors, such as ComK and ComS, involved in the B. subtilis MecA switch. Furthermore, our data combined with that of Chastanet and coworkers (4) suggest that ClpC and ClpE do not singly mediate the effect of ClpP on competence induction. The potential role of MecA and ClpX or ClpL in the regulation of S. pneumoniae competence by ClpP will be tested in future experiments.
Finally, we found that a D39 ΔclpP mutant was strongly attenuated for virulence in the mouse lung (Fig. 2) and sepsis models (>104 to 105 increase in LD50; see Results). This attenuation phenotype was complemented by an ecotopic copy of the clpP+ gene (Fig. 2). In contrast, attenuation by a ΔclpC mutation was mild compared to that of the ΔclpP mutation (Fig. 2). It is unlikely that this attenuation is due to the temperature sensitivity of the D39 ΔclpP mutant, because the body temperature of mice ranges between 37.1 to 37.4°C (24), which supports the growth of the ΔclpP mutants in vitro (Fig. 1). Visual inspection of colonies of the D39 ΔclpP mutant on plates did not suggest any diminution of capsule production, which is strongly linked to S. pneumoniae virulence (38). Global transcription analysis showed that the transcription levels of several likely pathogenicity factors was reduced in the ΔclpP mutant compared to that of the clpP+ strain, including sodA (manganese-dependent superoxide dismutase), tpx (thioredoxin-linked thiol peroxidase), and psaABC (manganese transporter) (Table 4). sodA and psaABC have previously been linked with the virulence of S. pneumoniae (2, 57), and antioxidizing enzymes, such as Tpx and Sod, have been linked to phagocyte resistance and virulence in several pathogens (10, 32). In addition, the transcription of several genes encoding cell surface proteins, which have been suggested to play possible roles in virulence (38), and proteins of unknown functions (23) are altered in ΔclpP mutants. Knockout mutants of the hk06/rr06 two-component system, whose transcription is induced in the ΔclpP mutant (Table 4), are attenuated for virulence in our lung and sepsis animal models (data not shown) and those reported elsewhere (51). Together, these data imply that the large attenuation in virulence of ΔclpP mutants is a complex phenotype with many contributing factors. It remains to be determined how the loss of this major ATP-dependent protease affects gene expression in S. pneumoniae at the level of protein stability and amount.
Acknowledgments
We thank Alexander Tomasz, Janet Yother, Donald LeBlanc, and Donald Morrison for bacterial strains, Donald Morrison (University of Illinois at Chicago) for helpful discussions on competence, Yong Yang for bioinformatic assistance, John Glass and Jennifer Glass for assistance with quantitative real-time RT-PCR analyses, Genshi Zhao and Dan Mytelka for reviewing the manuscript, and John Richardson for technical assistance.
This work was supported by resources provided by Lilly Research Laboratories, and Gregory T. Robertson was supported by a Lilly Postdoctoral Fellowship.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Berry, A. M., and J. C. Paton. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 64:5255-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charpentier, E., R. Novak, and E. Tuomanen. 2000. Regulation of growth inhibition at high temperature, autolysis, transformation and adherence in Streptococcus pneumoniae by clpC. Mol. Microbiol. 37:717-726. [DOI] [PubMed] [Google Scholar]
- 4.Chastanet, A., M. Prudhomme, J. P. Claverys, and T. Msadek. 2001. Regulation of Streptococcus pneumoniae clp genes and their role in competence development and stress survival. J. Bacteriol. 183:7295-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damerau, K., and A. C. St. John. 1993. Role of Clp protease subunits in degradation of carbon starvation proteins in Escherichia coli. J. Bacteriol. 175:53-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies, K. J., and S. W. Lin. 1988. Oxidatively denatured proteins are degraded by an ATP-independent proteolytic pathway in Escherichia coli. Free Radic. Biol. Med. 5:225-236. [DOI] [PubMed] [Google Scholar]
- 7.De Mot, R., I. Nagy, J. Walz, and W. Baumeister. 1999. Proteasomes and other self-compartmentalizing proteases in prokaryotes. Trends Microbiol. 7:88-92. [DOI] [PubMed] [Google Scholar]
- 8.de Saizieu, A., U. Certa, J. Warrington, C. Gray, W. Keck, and J. Mous. 1998. Bacterial transcript imaging by hybridization of total RNA to oligonucleotide arrays. Nat. Biotechnol. 16:45-48. [DOI] [PubMed] [Google Scholar]
- 9.Derré, I., G. Rapoport, and T. Msadek. 1999. CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in gram-positive bacteria. Mol. Microbiol. 31:117-131. [DOI] [PubMed] [Google Scholar]
- 10.Fang, F. C., M. A. DeGroote, J. W. Foster, A. J. Baumler, U. Ochsner, T. Testerman, S. Bearson, J. C. Giard, Y. Xu, G. Campbell, and T. Laessig. 1999. Virulent Salmonella typhimurium has two periplasmic Cu, Zn-superoxide dismutases. Proc. Natl. Acad. Sci. USA 96:7502-7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank, E. G., D. G. Ennis, M. Gonzalez, A. S. Levine, and R. Woodgate. 1996. Regulation of SOS mutagenesis by proteolysis. Proc. Natl. Acad. Sci. USA 93:10291-10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frees, D., and H. Ingmer. 1999. ClpP participates in the degradation of misfolded protein in Lactococcus lactis. Mol. Microbiol. 31:79-87. [DOI] [PubMed] [Google Scholar]
- 13.Gaillot, O., E. Pellegrini, S. Bregenholt, S. Nair, and P. Berche. 2000. The ClpP serine protease is essential for the intracellular parasitism and virulence of Listeria monocytogenes. Mol. Microbiol. 35:1286-1294. [DOI] [PubMed] [Google Scholar]
- 14.Gerth, U., E. Kruger, I. Derré, T. Msadek, and M. Hecker. 1998. Stress induction of the Bacillus subtilis clpP gene encoding a homologue of the proteolytic component of the Clp protease and the involvement of ClpP and ClpX in stress tolerance. Mol. Microbiol. 28:787-802. [DOI] [PubMed] [Google Scholar]
- 15.Goff, S. A., and A. L. Goldberg. 1985. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell 41:587-595. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg, A. L. 1972. Correlation between rates of degradation of bacterial proteins in vivo and their sensitivity to proteases. Proc. Natl. Acad. Sci. USA 69:2640-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottesman, S. 1996. Proteases and their targets in Escherichia coli. Annu. Rev. Genet. 30:465-506. [DOI] [PubMed] [Google Scholar]
- 18.Gottesman, S., W. P. Clark, V. de Crecy-Lagard, and M. R. Maurizi. 1993. ClpX, an alternative subunit for the ATP-dependent Clp protease of Escherichia coli. Sequence and in vivo activities. J. Biol. Chem. 268:22618-22626. [PubMed] [Google Scholar]
- 19.Gottesman, S., and M. R. Maurizi. 1992. Regulation by proteolysis: energy-dependent proteases and their targets. Microbiol. Rev. 56:592-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottesman, S., E. Roche, Y. Zhou, and R. T. Sauer. 1998. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 12:1338-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hensel, M., J. E. Shea, C. Gleeson, M. D. Jones, E. Dalton, and D. W. Holden. 1995. Simultaneous identification of bacterial virulence genes by negative selection. Science 269:400-403. [DOI] [PubMed] [Google Scholar]
- 22.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 23.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacoby, R. O., and J. G. Fox. 1984. Biology and diseases of mice, p. 31-89. In J. G. Fox, B. J. Cohen, and F. M. Loew (ed.), Laboratory animal medicine. Academic Press, Orlando, Fla.
- 25.Jenal, U., and T. Fuchs. 1998. An essential protease involved in bacterial cell-cycle control. EMBO J. 17:5658-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, S. N., S. W. Kim, S. N. Pyo, and D. K. Rhee. 2001. Molecular cloning and characterization of groESL operon in Streptococcus pneumoniae. Mol. Cell 11:360-368. [PubMed] [Google Scholar]
- 27.Klugman, K. P., and C. Feldman. 2001. Streptococcus pneumoniae respiratory tract infections. Curr. Opin. Infect. Dis. 14:173-179. [DOI] [PubMed] [Google Scholar]
- 28.Kruger, E., U. Volker, and M. Hecker. 1994. Stress induction of clpC in Bacillus subtilis and its involvement in stress tolerance. J. Bacteriol. 176:3360-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kruger, E., E. Witt, S. Ohlmeier, R. Hanschke, and M. Hecker. 2000. The clp proteases of Bacillus subtilis are directly involved in degradation of misfolded proteins. J. Bacteriol. 182:3259-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruger, E., D. Zuhlke, E. Witt, H. Ludwig, and M. Hecker. 2001. Clp-mediated proteolysis in Gram-positive bacteria is autoregulated by the stability of a repressor. EMBO J. 20:852-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruse, N., M. Pette, K. Toyka, and P. Rieckmann. 1997. Quantification of cytokine mRNA expression by RT PCR in samples of previously frozen blood. J. Immunol. Methods 210:195-203. [DOI] [PubMed] [Google Scholar]
- 32.Kwatia, M. A., D. J. Botkin, and D. L. Williams. 2000. Molecular and enzymatic characterization of Schistosoma mansoni thioredoxin peroxidase. J. Parasitol. 86:908-915. [DOI] [PubMed] [Google Scholar]
- 33.LeBlanc, D. J., L. N. Lee, and A. Abu-Al-Jaibat. 1992. Molecular, genetic, and functional analysis of the basic replicon of pVA380-1, a plasmid of oral streptococcal origin. Plasmid 28:130-145. [DOI] [PubMed] [Google Scholar]
- 34.Lee, M. S., and D. A. Morrison. 1999. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J. Bacteriol. 181:5004-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macrina, F. L., J. A. Tobian, K. R. Jones, R. P. Evans, and D. B. Clewell. 1982. A cloning vector able to replicate in Escherichia coli and Streptococcus sanguis. Gene 19:345-353. [DOI] [PubMed] [Google Scholar]
- 36.Maurizi, M. R. 1992. Proteases and protein degradation in Escherichia coli. Experientia 48:178-201. [DOI] [PubMed] [Google Scholar]
- 37.Maurizi, M. R., W. P. Clark, Y. Katayama, S. Rudikoff, J. Pumphrey, B. Bowers, and S. Gottesman. 1990. Sequence and structure of ClpP, the proteolytic component of the ATP-dependent Clp protease of Escherichia coli. J. Biol. Chem. 265:12536-12545. [PubMed] [Google Scholar]
- 38.Mitchell, T. J. 2000. Virulence factors and the pathogenesis of disease caused by Streptococcus pneumoniae. Res. Microbiol. 151:413-419. [DOI] [PubMed] [Google Scholar]
- 39.Msadek, T., V. Dartois, F. Kunst, M. L. Herbaud, F. Denizot, and G. Rapoport. 1998. ClpP of Bacillus subtilis is required for competence development, motility, degradative enzyme synthesis, growth at high temperature and sporulation. Mol. Microbiol. 27:899-914. [DOI] [PubMed] [Google Scholar]
- 40.Narberhaus, F. 1999. Negative regulation of bacterial heat shock genes. Mol. Microbiol. 31:1-8. [DOI] [PubMed] [Google Scholar]
- 40a.National Research Council. 1985. Guide for the care and use of laboratory animals. NIH publication no. 86-23. National Institutes of Health, Bethesda, Md.
- 41.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 42.Pederson, K. J., S. Carlson, and D. E. Pierson. 1997. The ClpP protein, a subunit of the Clp protease, modulates ail gene expression in Yersinia enterocolitica. Mol. Microbiol. 26:99-107. [DOI] [PubMed] [Google Scholar]
- 43.Pestova, E. V., L. S. Havarstein, and D. A. Morrison. 1996. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol. Microbiol. 21:853-862. [DOI] [PubMed] [Google Scholar]
- 44.Peterson, S., R. T. Cline, H. Tettelin, V. Sharov, and D. A. Morrison. 2000. Gene expression analysis of the Streptococcus pneumoniae competence regulons by use of DNA microarrays. J. Bacteriol. 182:6192-6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polissi, A., A. Pontiggia, G. Feger, M. Altieri, H. Mottl, L. Ferrari, and D. Simon. 1998. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect. Immun. 66:5620-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porankiewicz, J., J. Wang, and A. K. Clarke. 1999. New insights into the ATP-dependent Clp protease: Escherichia coli and beyond. Mol. Microbiol. 32:449-458. [DOI] [PubMed] [Google Scholar]
- 47.Reeve, C. A., A. T. Bockman, and A. Matin. 1984. Role of protein degradation in the survival of carbon-starved Escherichia coli and Salmonella typhimurium. J. Bacteriol. 157:758-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schumann, W., Hecker, M., and T. Msadek. 2002. Regulation and function of heat inducible genes in Bacillus subtilis, p. 359-368. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 49.Smith, M. D., and W. R. Guild. 1979. A plasmid in Streptococcus pneumoniae. J. Bacteriol. 137:735-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Talkington, D. F., D. C. Voellinger, L. S. McDaniel, and D. E. Briles. 1992. Analysis of pneumococcal PspA microheterogeneity in SDS polyacrylamide gels and the association of PspA with the cell membrane. Microb. Pathog. 13:343-355. [DOI] [PubMed] [Google Scholar]
- 51.Throup, J. P., K. K. Koretke, A. P. Bryant, K. A. Ingraham, A. F. Chalker, Y. Ge, A. Marra, N. G. Wallis, J. R. Brown, D. J. Holmes, M. Rosenberg, and M. K. Burnham. 2000. A genomic analysis of two-component signal transduction in Streptococcus pneumoniae. Mol. Microbiol. 35:566-576. [DOI] [PubMed] [Google Scholar]
- 52.Tomasz, A. 1999. New faces of an old pathogen: emergence and spread of multidrug-resistant Streptococcus pneumoniae. Am. J. Med. 107:55S-62S. [DOI] [PubMed] [Google Scholar]
- 53.Tsui, H.-C. T., A. J. Pease, T. M. Koehler, and M. E. Winkler. 1994. Detection and quantitation of RNA transcribed from bacterial chromosomes. Methods Mol. Genet. 3:179-204. [Google Scholar]
- 54.Turgay, K., J. Hahn, J. Burghoorn, and D. Dubnau. 1998. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 17:6730-6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson, D. A., D. M. Musher, and J. Verhoef. 1995. Pneumococcal virulence factors and host immune responses to them. Eur. J. Clin. Microbiol. Infect. Dis. 14:479-490. [DOI] [PubMed] [Google Scholar]
- 56.Webb, C., M. Moreno, M. Wilmes-Riesenberg, R. Curtiss III, and J. W. Foster. 1999. Effects of DksA and ClpP protease on sigma S production and virulence in Salmonella typhimurium. Mol. Microbiol. 34:112-123. [DOI] [PubMed] [Google Scholar]
- 57.Yesilkaya, H., A. Kadioglu, N. Gingles, J. E. Alexander, T. J. Mitchell, and P. W. Andrew. 2000. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect. Immun. 68:2819-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zahner, D., and R. Hakenbeck. 2000. The Streptococcus pneumoniae beta-galactosidase is a surface protein. J. Bacteriol. 182:5919-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou, Y., and S. Gottesman. 1998. Regulation of proteolysis of the stationary-phase sigma factor RpoS. J. Bacteriol. 180:1154-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zugel, U., and S. H. Kaufmann. 1999. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin. Microbiol. Rev. 12:19-39. [DOI] [PMC free article] [PubMed] [Google Scholar]