Abstract
Background:
Nitrous oxide rapidly inflates gas-filled spaces such as the intestines; but whether the resulting bowel distension is clinically important remains unclear. We, therefore, tested the hypothesis that nitrous oxide produces clinically important bowel distension.
Methods:
Patients scheduled for colon resection were anesthetized with isoflurane and 35% oxygen and randomly assigned to 65% nitrous oxide (n=175) or 65% nitrogen in air (n=169). At the end of surgery, blinded surgeons rated the degree of bowel distension as none, mild, moderate, or severe. Patients reported pain, and nausea and vomiting (PONV) two hours after surgery. Data are reported as means (SDs). P < 0.05 was statistically significant.
Results:
Morphometric and demographic data were similar in the groups. The duration of surgery was 3.0 (1.2) hr in the nitrous oxide group and 3.4 (1.5) hr in the air group (P=0.017). Postoperative self-reported pain scores (visual analogue scale, 0–100 mm) were greater in the nitrous oxide group (43 [30] mm) than in the air group (35 [31] mm, P = 0.018). Although the incidence of PONV was similar in the groups, VAS scores for nausea were significantly greater in the nitrous oxide group (P = 0.040). Moderate-to-severe bowel distension was observed in 23% of nitrous oxide patients, but in only 9% of patients in the air group (P < 0.001). The number-needed-to-harm for moderate or severe bowel distension from nitrous oxide was thus seven.
Conclusions:
Our results suggest that avoiding nitrous oxide administration during prolonged bowel operations will minimize bowel distension and possibly reduce postoperative pain related to it.
Keywords: Anaesthesia, Bowel distension, Nitrous oxide, Surgery
Nitrous oxide has been used for more than 150 years and has been given to several billion patients. It justifiably remains a popular anaesthetic agent because it is inexpensive, analgesic, and extremely short acting. It reduces the need of maintenance anaesthetic, which has more significance when ventilators with larger fresh airflows are used. However, intestinal distension by nitrous oxide has been reported at least since the 1960’s (1), and it is well established that nitrous oxide inflates closed, gas-filled spaces (2).
Nitrous oxide will move into air-filled cavities in the body that normally contain nitrogen; so as nitrous oxide transfers from the blood into the space, nitrogen transfers out. However, nitrous oxide is 34 times more soluble than nitrogen in blood. Thus, substantial quantities of nitrous oxide leave the blood and enter the bowel, but not much nitrogen can leave the bowel to enter the blood. The result is that during exposure to nitrous oxide, like in other compliant spaces, the volume of gas in the bowel increases. The amount of the increase depends on the alveolar partial pressure of nitrous oxide, the intestinal blood flow, and the duration of nitrous oxide administration.
The evidence that nitrous oxide causes clinically important bowel distension is equivocal. Several studies have failed to identify any effect whatsoever of nitrous oxide on bowel distension(3–6) or postoperative intestinal function (6,7). In contrast, others have reported that nitrous oxide increases bowel distension in both animals (8) and humans (9). All of these studies were relatively small and some were seriously underpowered. We, therefore, tested the hypothesis that the use of nitrous oxide produces clinically important bowel distension in patients undergoing elective colon resection with isoflurane anaesthesia. We simultaneously quantified the magnitude of the effect by determining the number of patients in whom air must be substituted for nitrous oxide to avoid a single case of clinically important bowel distension (number-needed-to-harm).
Methods
With IRB approval and informed consent, we studied 344 ASA physical status I–III patients, 18 to 80 years, scheduled for elective colon resection scheduled to last more than two hours. This bowel distention protocol was a sub-study performed at two Viennese hospitals that participated in a larger trial of nitrous oxide and surgical wound infection. We had enrolled 344 patients in this sub-study at the time the primary study was terminated on the basis of a primary infectious endpoint. Patients with bowel obstruction or having minor colon surgery (e.g., polypectomy, isolated colostomy) were not admitted to the study; however, we did include patients undergoing abdominal-peritoneal pull-through procedures.
All patients received standard mechanical bowel preparation using an electrolyte solution the night before surgery. Anaesthetic management was standardized. Sodium thiopental (3–5 mg·kg−1) and vecuronium (0.1 mg·kg−1) were used for induction; anaesthesia subsequently was maintained with isoflurane (0.5–1.0% in nitrous oxide or air as described below), vecuronium, and remifentanil (0.2 μg·kg−1·min−1).
After induction of anaesthesia, patients were assigned to one of two groups using a reproducible set of computer-generated random numbers. The assignments were kept in sealed, sequentially numbered opaque envelopes that were opened after induction of anaesthesia. All patients were given 35% inspired oxygen during surgery, which was balanced by either 65% nitrous oxide (n=175) or nitrogen (n=169), until immediately before extubation, whereupon 100% oxygen was administered.
The anaesthesiologists were not blinded to group assignment; however, great care was exercised to prevent the surgeons from observing the administered gas mixture. Preventive measures included shielding the vaporizers and gas monitors from the surgeons. Surgeons were asked to rate bowel distension clinically at the end of surgery.
Isoflurane administration was adjusted by the attending anaesthesiologist with the goal of maintaining arterial blood pressure within 20% of pre-induction values. Additional vecuronium was administered as needed to maintain 1–2 mechanical twitches in response to supra-maximal stimulation of the ulnar nerve at the wrist. Ventilation was mechanically controlled to maintain end-tidal carbon dioxide tension near 35 mmHg. The endotracheal cuff was checked at 15-minute intervals, and gas volume was adjusted as necessary to keep the cuff pressure between 15 and 20 cm H2O. Postoperative pain was treated with patient-controlled opioid (piritramid): an initial 3 mg bolus followed by patient-controlled 3 mg boluses with 20 minutes lockout time. Postanaesthetic care nurses, who managed analgesic needs, were blinded to carrier gas randomisation. Patients who experienced PONV were given 4 mg ondansetron.
Measurements
Appropriate morphometric characteristics of each treatment group were tabulated. Detailed records of anaesthetic and fluid management were kept. Heart rate and arterial blood pressure were recorded at 15-min intervals during anaesthesia. End-tidal isoflurane, nitrous oxide, and carbon dioxide concentrations were measured with an infrared detector, also at 15-min intervals.
At the end of each operation, the surgeon who was blinded to anaesthesia management was asked to rate the degree of bowel distension: none, mild, moderate, or severe. None: no detectable distension. Mild: minimal distension which did not interfere with surgery. Moderate: clinically important distension requiring additional manoeuvres to provide an adequate surgical field. Severe: markedly distended bowel that made surgery far more difficult than usual. Moderate and severe grades thus indicated distension that interfered with surgery.
After two hours of postanaesthetic recovery, patients were asked to rate their pain on a 100-mm-long visual analogue scale (10). They were similarly asked to rate the worst nausea they had experienced during the first two postoperative hours. The number of emetic episodes during this period was recorded. Patients experiencing any nausea and/or vomiting were considered to have had postoperative nausea or vomiting (PONV).
Data Analysis
Morphometric and perioperative data were compared with unpaired t or chi square tests as appropriate. Distension in the nitrous oxide and air groups were compared with chi square analyses; P < 0.05 was considered statistically significant. Data are presented as counts (%), or means (SDs or 95% confidence intervals).
Results
Morphometric and demographic data were similar in both groups except that patients assigned to the nitrous oxide group were slightly, but significantly, younger than those given air were. Mean arterial pressure was significantly less in the nitrous oxide than in the air group. Duration of surgery was slightly shorter in the nitrous oxide group (3.0 [1.2] hr) than in the air group (3.4 [1.5] hr, Table 1).
Table 1.
Morphometric and Perioperative Data
| Nitrous Oxide | Air | P | |
|---|---|---|---|
| Patients, n | 175 | 169 | |
| Age, yr | 57 (15) | 60(14) | 0.047 |
| ASA status, I/II/III* | 56/66/32 | 46/65/39 | 0.444 |
| Sex, M/F | 90/85 | 94/75 | 0.433 |
| Weight, kg | 73 (4) | 74 (15) | 0.528 |
| Duration of surgery, h | 3.0 (1.2) | 3.4 (1.5) | 0.017 |
| IV fluids administered, L | 3.9 (1.5) | 3.9 (1.8) | 0.804 |
| End-tidal CO2, kPa | 4.25 (0.33) | 4.32 (0.35) | 0.489 |
| End-tidal Isoflurane, % | 0.60 (0.15) | 0.61 (0.15) | 0.664 |
| Mean arterial pressure, mmHg | 80 (9) | 85 (11) | <0.001 |
| Heart rate, beats per min | 71 (11) | 74 (13) | 0.061 |
Values below line are averaged over the duration of surgery. Data presented as number of patients or means (SDs). * ASA status not recorded for all patients.
Piritramid use in the first two postoperative hours was virtually identical in the treatment groups (Table 2); however, pain scores were significantly greater in patients given nitrous oxide (43 [30] mm) than in those given air (35 [31] mm, P = 0.02, Fig. 1).
Table 2.
Pain, PONV, and Bowel Distension as Rated by Surgeons Blinded to Anaesthesia Group.
| Nitrous Oxide (n=175) | Air (n=169) | P | |
|---|---|---|---|
| Piritramid, mg | 8.6 (8.1) | 8.6 (6.4) | 0.942 |
| PONV, # of patients | 75 (43%) | 71 (42%) | 0.874 |
| Emesis, # of patients | 7 (4%) | 5 (3%) | 0.600 |
| Nausea Score, VAS | 15 (25) | 10 (20) | 0.040 |
| Bowel Distension, # of patients | < 0.001 | ||
| None | 92 (53%) | 120 (71%) | |
| Mild | 42 (24%) | 34 (20%) | |
| Moderate | 30 (17%) | 12 (7%) | |
| Severe | 11 (6%) | 3 (2%) |
Results are presented as number of patients (%) or means (SDs).
Fig. 1.
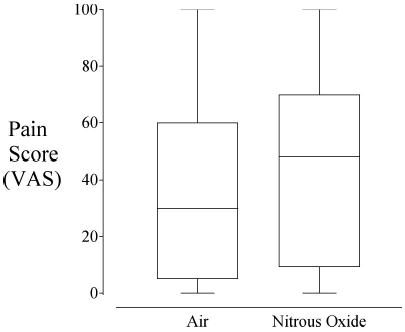
A box and whiskers plot showing the pain scores for patients receiving nitrous oxide or air. The whiskers show the minimum and maximum values. The top and bottom sides of the box show the 25th and 75th percentiles, and the line in the box shows the median. The groups were significantly different (P = 0.018)
Nausea or vomiting during the first two postoperative hours was observed in 42% of the patients given nitrous oxide and 43% of the patients given air(P = 0.874). However, qualitatively, VAS scores for nausea were significantly greater in the nitrous oxide group (Table 2).
Bowel distension, as rated by surgeons blinded to group assignment, was significantly greater in the nitrous oxide group than in the air group (Table 2). Moderate-to-severe bowel distension was observed in 23% of the nitrous oxide patients, but in only 9% of the patients in the air group (P<0.001). The 95% confidence intervals (CI) for this 14% absolute risk reduction were 8% to 21%. The number-needed-to-harm for a case of moderate or severe bowel distension from air was 7 (95% CI 5 to 13 cases).
Discussion
Patients given nitrous oxide were more than twice as likely (23% vs. 9%) to be rated by blinded surgeons as having moderate or severe distension as those given air were. Since the number-needed-to-harm is the reciprocal of the absolute risk reduction, nitrous oxide needed to be avoided in only seven patients to avoid clinically important bowel distension in a single patient. These results suggest that avoiding nitrous oxide administration during prolonged bowel operations will minimize bowel distension, and thus, facilitate surgery.
Although our results are consistent with several previous reports (8,9), they differ markedly from others. For example, Taylor et al. were unable to detect additional bowel distension when nitrous oxide was substituted for air during laparoscopic cholecystectomy with isoflurane anaesthesia in 50 patients (3). Furthermore, in 20 patients nitrous oxide at concentrations of 60% had little effect on bowel size following intestinal surgery lasting less than 90 minutes (4). Krogh et al. also failed to identify bowel distension related to nitrous oxide administration in 139 patients who, like ours, had colonic surgery (5). And finally, Pedersen, et al. (6) failed to identify adverse effects of nitrous oxide in 36 women having hysterectomies.
The critical distinction between our study and previous ones is that we randomized 344 patients, more than twice as many patients as the previous largest study of bowel distension with nitrous oxide (5). Furthermore, our study was more than seven times larger than several other previous studies (3,9). One study of nitrous oxide and bowel distension had only 20 patients (4) and another only 36 (6). Our study, unlike some previous ones, thus had sufficient statistical power to identify significant bowel distension resulting from nitrous oxide administration.
However, other important differences surely contributed to previous negative results. The most important is that nitrous oxide diffusion into the intestinal space is obviously time-dependent. We evaluated bowel distension only at the end of surgery; we were thus unable to determine from our data the extent to which nitrous oxide may have distended the bowel during shorter procedures. Even though the patients given nitrous oxide in our study had a shorter duration of surgery than those given air, surgery duration averaged 3 hours or longer in both groups. Some of the previous negative results were observed in shorter procedures, and it remains likely that nitrous oxide does not produce clinically important bowel distension in sufficiently short operations.
We did not quantify bowel distension by measuring abdominal or intestinal circumference; instead, the surgeons rated it in terms of the clinically relevant parameter (i.e., the extent to which distension interfered with surgery). We are thus confident that we have identified an effect of nitrous oxide that is both statistically significant and clinically important. Bowel distension, of course, is less of an issue in non-abdominal surgery — although it may contribute to postoperative nausea and vomiting that is associated with nitrous oxide administration (11). In an earlier meta-analysis of 37 studies, Divatia et al. showed that avoidance of nitrous oxide could reduce the risk for PONV by 28% (12).
Postoperative pain was slightly, but significantly, increased in patients given nitrous oxide, although analgesic use was virtually identical in the groups. Presumably, the increased pain resulted from bowel distension. Although increased surgical pain is the logical sequela to bowel distension, it is not a widely recognized consequence of nitrous oxide administration.
Nitrous oxide administration did not increase the overall incidence of postoperative nausea and vomiting in our patients, and it only minimally increased the severity of nausea as self-reported by the patients. However, PONV was evaluated only for two postoperative hours rather than over 24 hours as is standard. The distinction is important because a considerable amount of nausea and vomiting occurs after patients leave the postanaesthesia care unit. An additional factor to consider is that our study was not large enough to preclude a type II statistical error for PONV. In light of these limitations, it is unsurprising that previously mentioned large meta-analysis have identified a statistically significant (although modest) contribution of nitrous oxide on PONV (12).
In conclusion, including nitrous oxide in the anaesthesia regimen more than doubled the number of patients with moderate or severe bowel distension in patients undergoing colon surgery lasting an average of ≈3 hours. Nitrous oxide needs to be avoided in only seven patients to prevent clinically important bowel distension in a single patient. Avoiding nitrous oxide administration during prolonged bowel operations thus minimizes bowel distension and facilitates surgery.
Acknowledgments
Supported by NIH Grants GM 061655 and DE 014879-01A1 (Bethesda, MD), the Joseph Drown Foundation (Los Angeles, CA), and the Commonwealth of Kentucky Research Challenge Trust Fund (Louisville, KY). Dr. Akça is the recipient of a Research Training Grant from the Foundation for Anesthesia Education and Research We appreciate the statistical assistance of Gilbert Haugh, M.S., and the editorial assistance of Nancy L. Alsip, Ph.D., both of the University of Louisville.
References
- 1.Eger EI, 2nd, Saidman LJ. Hazards of nitrous oxide anesthesia in bowel obstruction and pneumothorax. Anesthesiology. 1965;26:61–66. doi: 10.1097/00000542-196501000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Reinelt H, Schirmer U, Marx T, Topalidis P. Diffusion of xenon and nitrous oxide into the bowel. Anesthesiology. 2001;94:475–77. doi: 10.1097/00000542-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Taylor E, Feinstein R, White PF, Soper N. Anesthesia for laparoscopic cholecystectomy. Is nitrous oxide contraindicated? Anesthesiology. 1992;76:541–3. doi: 10.1097/00000542-199204000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Boulanger A, Hardy JF. [Intestinal distention during elective abdominal surgery: should nitrous oxide be banished?] Can J Anaesth. 1987;34:346–50. doi: 10.1007/BF03010131. [DOI] [PubMed] [Google Scholar]
- 5.Krogh B, Jorn Jensen P, Henneberg SW, Hole P, Kronborg O. Nitrous oxide does not influence operating conditions or postoperative course in colonic surgery. Br J Anaesth. 1994;72:55–7. doi: 10.1093/bja/72.1.55. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen FM, Wilken-Jensen C, Knudsen F, Lindekaer AL, Svare EI. The influence of nitrous oxide on recovery of bowel function after abdominal hysterectomy. Acta Anaesthesiol Scand. 1993;37:692–6. doi: 10.1111/j.1399-6576.1993.tb03791.x. [DOI] [PubMed] [Google Scholar]
- 7.Jensen AG, Kalman SH, Nystrom PO, Eintrei C. Anaesthetic technique does not influence postoperative bowel function: a comparison of propofol, nitrous oxide and isoflurane. Can J Anaesth. 1992;39:938–43. doi: 10.1007/BF03008343. [DOI] [PubMed] [Google Scholar]
- 8.Cundy RL, Aldrete JA, Thomas J. Intestinal distention produced by nitrous oxide or ethylene inhalation. Surg Gynecol Obstet. 1969;129:108–12. [PubMed] [Google Scholar]
- 9.Scheinin B, Lindgren L, Scheinin TM. Peroperative nitrous oxide delays bowel function after colonic surgery. Br J Anaesth. 1990;64:154–8. doi: 10.1093/bja/64.2.154. [DOI] [PubMed] [Google Scholar]
- 10.Myles PS, Troedel S, Boquest M, Reeves M. The pain visual analog scale: is it linear or nonlinear? Anesth Analg. 1999;89:1517–20. doi: 10.1097/00000539-199912000-00038. [DOI] [PubMed] [Google Scholar]
- 11.Apfel CC, Roewer N. [Risk factors for nausea and vomiting after general anesthesia: fictions and facts] Anaesthesist. 2000;49:629–42. doi: 10.1007/s001010070080. [DOI] [PubMed] [Google Scholar]
- 12.Divatia JV, Vaidya JS, Badwe RA, Hawaldar RW. Omission of nitrous oxide during anesthesia reduces the incidence of postoperative nausea and vomiting. A meta-analysis Anesthesiology. 1996;85:1055–62. doi: 10.1097/00000542-199611000-00014. [DOI] [PubMed] [Google Scholar]


