Abstract
Artificial joints are subject to chronic infections associated with bacterial biofilms, which only can be eradicated by the traumatic removal of the implant followed by sustained intravenous antibiotic therapy. We have adopted an engineering approach to develop electrical–current-based approaches to bacterial eradication and microelectromechanical systems that could be embedded within the implanted joint to detect the presence of bacteria and to provide in situ treatment of the infection before a biofilm can form. In the former case we will examine the combined bactericidal effects of direct and indirect electrical fields in combination with antibiotic therapy. In the latter case, bacterial detection will occur by developing a microelectromechanical–systems-based biosensor that can “eavesdrop” on bacterial quorum–sensing-based communication systems. Treatment will be effected by the release of a cocktail of pharmaceutical reagents contained within integral reservoirs associated with the implant, including a molecular jamming signal that competitively binds to the bacteria’s quorum sensing receptors (which will “blind” the bacteria, preventing the production of toxins) and multiple high dose antibiotics to eradicate the planktonic bacteria. This approach is designed to take advantage of the relatively high susceptibility to antibiotics that planktonic bacteria display compared with biofilm envirovars. Here we report the development of a generic microelectromechanical systems biosensor that measures changes in internal viscosity in a base fluid triggered by a change in the external environment.
Introduction
Orthopaedic implant infection is a devastating disease because of the physical and emotional trauma the patient encounters that is associated with revisional surgery, compounded by the long-term postoperative treatment needed.9,30 Current rates of infection for artificial joints (for the lifetime of an implant) vary by hospital, surgeon, and study, but the best estimates suggest an infection rate of 1 to 2%, which produces a figure of 4000 to 8000 infected arthroplasties requiring surgical revision annually. Implant infection is not limited to orthopaedic implants; it is conservatively estimated that there are 1.32 million prosthetic devices that become infected each year in the United States (Table 1). The cost is enormous, as is the morbidity and patient suffering caused by these persistent infections. Chronic infections are associated with bacterial biofilms which are characterized by microcolonies of bacteria encased in a protective extracellular polymeric matrix. Bacterial biofilms can form on any artificial surface that has been introduced into the human body, as well as on tissues adjacent to the implanted surface. It is important to emphasize that artificial joints of any type (hips, knees, elbows, etc); orthopaedic screws, bolts and rods are all vulnerable to hosting a biofilm.30 Implant infections can result acutely when infectious bacteria enter the implant site during surgery or recovery. However, many implant infections are subacute or chronic (20, 31) and result from systemic seeding of the implant following a septic event. Once a biofilm is established within the body, it is almost impossible to eradicate it, even with high doses of antibiotics.7,10 The biofilm also can produce periodic planktonic showers of bacteria (ie, bacteria shedding from the biofilm) into the bloodstream, which can result in episodic acute systemic infection in addition to the chronic infectious nidus for the patient.10 The biofilm model introduces a novel paradigm into microbiology and infectious disease, ie, the concept that bacteria have a life cycle just as many eukaryotes do.11 This knowledge provides us with a framework for understanding persistence and chronicity in bacterial infections, but more importantly it provides us with a starting place for the development of new approaches to the treatment of what have been intractable infections. Like all abiotic systems introduced into the human body, arthroplasties are prone to bacterial biofilm infections and even mixed-kingdom biofilm infections composed of bacterial and fungal species (Fig 1).
Table 1.
Device-Related Infections of Prosthetic Devices in the United States
| Device | Usage/Year | Infection Risk (%) |
|---|---|---|
| Central venous catheters | 5 million | 3–8 |
| Bladder catheters | Tens of millions | 10–30 |
| Prosthetic heart valves | 85,000 | 1–3 |
| Vascular grafts | 450,000 | 2–10 |
| Cardiac pacemakers | 400,000 | 1–5 |
| Cardiac assist devices | 700 | 50–100 |
| Penile implants | 15,000 | 2–10 |
| Joint prostheses | 600,000 | 1–3 |
| Fracture fixators | 2 million | 5–10 |
| Dental Implants | 2 million | 5–10 |
Data in the table were obtained from a variety of sources by the Center for Biofilm Engineering at Montana State University.
Fig 1.
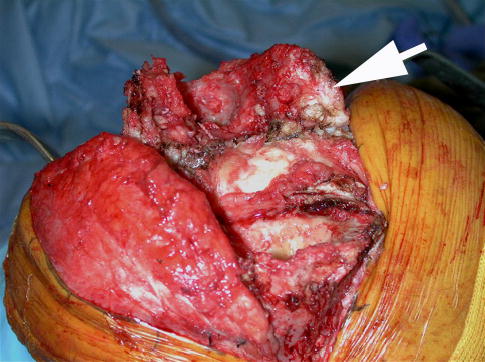
An intraoperative photograph taken during excisional surgery to remove an infected prosthetic joint shows a bacterial biofilm on an infected arthroplasty. The white material that the arrow is pointing to is pus that contains huge numbers of micro-organisms and host-derived leukocytes.
Biofilm bacteria, once firmly established on a nonliving surface within a host, essentially become a permanent feature of that surface7,9. There is no means, short of removing the infected device or killing the host, to eradicate the biofilm. Biofilms are not simply collections of individual bacteria, but rather are complex co-operative communities composed of one or more species of bacteria (and/or fungi) embedded within an extracellular matrix, displaying discrete temporal and spatial organizational properties and possessing a wide range of environmental sensing mechanisms linked to adaptive responses that operate at the population level rather than at the individual cell level (7, 9,13). This introduces an important concept with respect to biofilm pathogenesis in that the biofilm as a whole is acting as an organism instead of the bacterial cells acting individually (8,11). This realization provides for a fundamental change in our consideration of the evolutionary pressures that are operative on the bacteria. This duality of existence provides for an unprecedented level of adaptability and fitness for bacterial species that can transition between planktonic and biofilm environmental variants (envirovars). 9
From a medical perspective, the most important aspect of biofilm bacteria is their near imperviousness to elimination by either host defense mechanisms or even intensive long-term antimicrobial therapy. We have shown that these resistances to host and pharmacologic attack result in part from the fact that bacterial biofilms contain many different environmental niches with respect to nutrient availability and pH and O2 tension. The bacteria composing the biofilm express multiple phenotypes in response to these substrate gradients.4,24 Therefore, the bacteria possess a phenotypic plurality that ensures that some subpopulation of them will survive any type of antibiotic treatment based on metabolic considerations.7,9,10,26 Moreover, the bacteria within a biofilm function cooperatively, analogous to a simple metazoan. At this stage of their life cycle, natural selection pressures will occur at the population level, as opposed to the individual cell during planktonic growth. We posit that it is this duality of existence modes that provide bacteria with their extraordinary fitness.11 In the presence of abundant nutrient sources and the absence of severe environmental threats, bacteria will grow as rapid planktonic blooms, but in the face of adversity they will adapt and persist as biofilms.
In this paper we describe two engineering-based approaches for the detection and control of biofilm infections associated with orthopaedic implants that can be deployed in situ. The economics of the treatment of individual infected arthroplasties multiplied by the large numbers of people affected by implant infections justify the increased costs associated with the development and manufacture of such “intelligent implants.” Such devices would be engineered to have multifunctional capabilities including bacterial diagnostics, treatment regimens with automonitoring of dispensed pharmaceuticals, and telemetry to provide feedback regarding the microbiologic and pharmacologic state of the joint.
An engineering approach to prevent infected arthroplasties
We have organized a multidisciplinary biomedical-engineering approach to develop a self-diagnosing, self-treating, self-monitoring artificial joint (Fig 2; an “intelligent implant”) to combat the devastating problem of postimplantation bacterial biofilm infections that form on artificial joint prostheses. A meeting about such implant (Designing Intelligent Orthopaedic Implants for Biofilm Control) was held on April 10 to 12, 2003 in Big Sky, MT, which was attended by surgeons, microbiologists, biochemists, mechanical engineers, electrical engineers, and microelectromechanical systems (MEMS) engineers.
Fig 2.
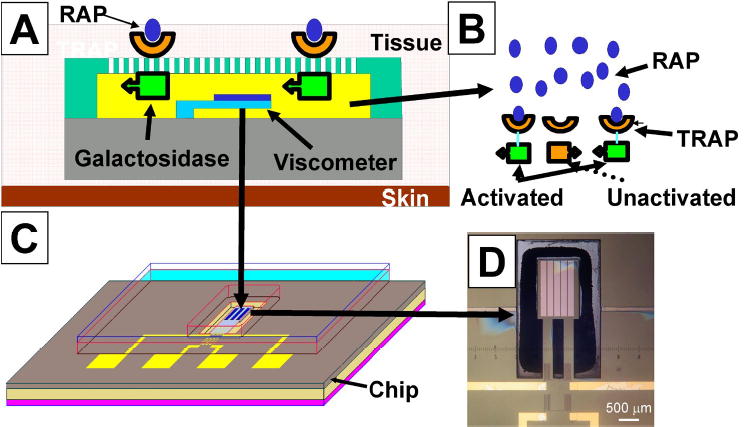
A–D. The basic concept of the MEMS biosensing device is illustrated showing how the binding of staphylococci-derived quorum sensing peptides on the outside will trigger an internal change in viscosity. (A) A cantilever viscometer is positioned within a microchamber filled with a high-viscosity glucose polysaccharide, and a dextran gel cross-linked with Con-A. (B) The bacterial quorum sensing molecule (RAP, blue ovals) binds to an engineered chimeric receptor protein, (TRAP, orange parabolas) embedded in an artificial membrane. The binding of TRAP induces a conformational change in the transmembrane portion of the chimeric protein(orange rectangles changing to green rectangles) which activates a galactosidase function which cleaves glucose monomers from a polysaccharide substrate. The glucose displaces dextran on the Con-A resulting in a drop in viscosity which is registered by increased deflection of the cantilever. (C) shows the entire unit which would be flush mounted into the artificial joint. (D) photograph of a functional cantilever-based viscometer with scale bar.
The most salient point made by the surgeons (with regard to implant design) was that a majority (> 60%) of arthroplasty infections result from two related staphylococcal species, Staphylococcus aureus and S. epidermidis. The microbiologists were united in their view that the infection should ideally be controlled before the formation of a biofilm because treatment regimens currently do not exist that can eradicate a biofilm on an abiotic surface implanted into human tissue. From the biosensor/MEMS engineers it was learned that the most difficult technical challenge is to overcome biofouling of an implanted device that would contain a membrane through which ligands would pass to induce a signal cascade. The electrical engineers indicated that the most problematic area for them was the need to provide power to the device for years without external leads which could become a source of infection. These consensus viewpoints, together with a preferred embodiment of what would be required in the design of an intelligent implant, were subsequently published 9
The overall concept in the design of an intelligent implant is to produce an arthroplasty that contains a MEMS-type biosensing device that can “eavesdrop” on bacterial communication systems associated with autoinduction, quorum sensing, and biofilm formation. Most pathogenic bacterial species (and most nonpathogenic species) respond to and produce intercellular signaling molecules that are designed to detect either the concentration of bacteria in a given locale (quorum sensing)23 or to determine the rate of diffusion within the ecosystem in which the bacteria find themselves.25 Depending on the bacterial species and the environment, quorum sensing serves to provide coordination of metabolic switching among a population of like bacteria so that they act in concert for the benefit of the population instead of as individual organisms. In the case of many pathogens, the detection of a quorum of bacteria induces the production of virulence factors and toxins. This co-ordinate-inducible phenomenon occurring on a population level has been interpreted as a survival strategy for pathogens wherein they try to remain “below the radar” of the host’s pathogen detection systems until such time because their numbers are great enough to overwhelm the host’s initial response. A classic example is S. aureus-induced toxic shock syndrome.19
On intercepting the bacterial signals, the MEMS biosensor will send a signal to a pair of integral gated reservoirs that will: 1) release inhibitory compounds that will prevent biofilm formation; and 2) release antibiotics at very high concentrations locally that will eradicate all planktonic bacteria that are in proximity to the joint before they establish a biofilm. The ability to provide site-specific dosing with antibiotics would have the benefit of being able to deliver much higher concentrations of antibiotics at the locus of infection than could be tolerated by the host through systemic treatment regimens. Moreover, local dosing would potentially permit the use of highly efficacious antimicrobials that have specific organ system toxicity profiles that makes them unusable with current systemic dosing regimens. Therefore, not only could this strategy provide for higher dosing levels of systemically tolerated antibiotics, but it could also increase the size of the available pharmacopoeia—a considerable advantage considering the high percentage of pathogenic strains that have acquired one or more planktonically active antibiotic resistance genes.
The MEMS biosensors and the drug reservoirs would be connected to a telemetry system embedded within the prostheses that would be accessible to the patient and physician using a handheld Bluetooth monitoring device, which in turn would be able to communicate with the wireless web. Therefore, patients would be able to take a reading anywhere in the world and upload the data to the internet, from which their physician could monitor the condition of the joint, regardless of location.
Steps towards the development of MEMS biosensor
The critical development components upon which the fate of the Intelligent Implant system hangs are the MEMS-based biosensors. This and the integration of the various modules are expected to consume the bulk of the developmental process. As noted above, the predominant bacterial species associated with infections in artificial joints are S. aureus and S. epidermidis, which collectively account for a the majority of all implant infections. Fortunately these staphylococci use a well-characterized peptide-based quorum sensing system that is amenable to manipulation (2). Therefore, a decision was made to focus exclusively on these organisms for the development of a first-generation MEMS biosensor. Both of these species produce a quorum-sensing peptidyl autoinducer (ligand) termed RAP (Ribonucleic acid [RNA] III-activating protein) and a cognate cell-surface based receptor termed TRAP (target of RNA III-activating protein), which becomes activated through phosphorylation on binding RAP. The TRAP activation triggers up-regulation of a secondary two-component cell-signaling system encoded by the accessory gene regulator (agr) locus. Activation of agr results in production of AIP (autoinducing peptide), which is produced by cleavage of the prepeptide AgrD by the AgrB protein and AgrC, its cognate receptor encoded within the agr locus. Binding of AIP to AgrC initiates a phosphorylation cascade that results in up-regulation of RNA III synthesis from the agr locus. The RNA III is a central pleiotropic regulator that controls the expression of numerous virulence factors. The agr locus contains divergent transcriptional systems controlled by the promoters P2 and P3 that encode RNA II and RNA III, respectively. Promoter P2 is activated by the RAP-TRAP system and P3 is activated by the AIP-AgrC system. The first MEMS biosensor is being developed to detect the earliest stage of staphylococcal intercellular communication (RAP-TRAP) to provide the greatest lead time before biofilm formation for treatment.
Interference with the RAP-TRAP signaling system by the peptide RIP (RNA III-inhibiting peptide) already has been shown to produce a beneficial effect in terms of reducing staphylococcal-based pathogenesis.3 Ribonucleic acid III-inhibiting peptide is an octapeptide that is synthesized by S. warnerii and S. xylosus, coagulase-negative staphylococci. Native and synthetic forms of RIP have been shown to competitively inhibit RAP’s binding to TRAP, making RIP highly effective in inhibiting RNA III synthesis in vitro and suppressing pathogenesis in vivo.2,16 Moreover, mice vaccinated with RAP show protection from challenge with S. aureus in direct correlation with their titers of anti-RAP antibodies.16 Therefore, we have chosen the RAP-TRAP system as a validated target for the initial focus of our MEMS-based biosensor development, with the objective of creating a biosensor based on TRAP that would detect bacterially-produced RAP (Fig 2).
Ribonucleic acid III-activating protein binding to the biosensor, just as in the bacteria, would result in a conformational switch in a chimeric TRAP molecule that would activate an enzymatic moiety triggering a signal transduction cascade within the MEMS device. This cascade would result in the release of RIP and anti-RAP antibodies from the implant’s reservoirs. The three-dimensional space proximal to the prosthesis then would be flooded with a bivalent bolus of bacterial blinding agents that would prevent toxin production, biofilm development and quorum sensing by the planktonic staphylococci present in the area. Simultaneously, a second set of reservoirs would release a cocktail of potent antibiotics including, for example, nafcillin and perhaps vancomycin to kill the planktonic staphylococci. The release of the various specific signaling inhibitors and antimicrobials would, in turn, be monitored by a second set of MEMS-based sensors to ensure that an adequate release had occurred in situ. Finally, all the activity of the various biosensors and reservoirs would be stored within a memory module embedded in the implant that would be available for uploading upon signaling from a remote hand-held unit that would be provided to the patient.
Development of a Generic Biosensing Core
For design simplicity and cost control it would be advantageous to have a single generic MEMS-based biosensing core that could be easily modified to produce a family of biosensors each with a unique specificity. With that in mind, we have chosen to work on the development of a generic MEMS device that will detect an internal change in viscosity. In this model system, the sensor actually is a cantilever-based viscometer. We successfully have constructed a microviscometer sensing unit (Fig 2) by adapting a system developed by Jeckelmann and Seibold(14) for monitoring the blood glucose levels of people with diabetes. In their system (non-MEMS) changes in viscosity are measured based on competition between glucose and dextran (a glucose polymer) for binding with the quadrivalent concanavalin A (conA) molecule. In the absence of free glucose, four large dextran molecules will bind to each molecule of ConA producing a high viscosity gel-like matrix. If glucose enters the system, then it will compete for the ConA binding sites resulting in a drop in viscosity. The MEMS-type viscosity biosensor that we developed works by measuring the deflection of a cantilever in inverse proportion to the viscosity (Fig 3). Therefore, the greater the deflection of the cantilever, the lower the viscosity and the greater the signal recorded by the system. For the device we are designing, enzymatic glucose production (generated from a polysaccharide) will be the signal that the TRAP-based biosensor has been triggered by RAP binding.
Fig 3.
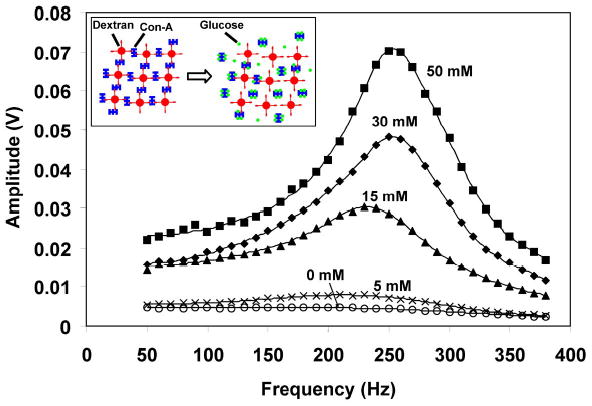
The viscosity (measured as amplitude) of the dextran-Con-A hydrogel as a function of glucose concentration is measured by the microviscometer.
As mentioned above, in an effort to circumvent the universal problem of biofouling that befalls all implantable biosensors, we have developed a strategy in which we will not attempt to pass the ligand to be detected (RAP) into the biosensor. Instead we will attempt to transduce a signal into the biosensor using a transmembrane-based conformational protein switch, in essence mimicking the natural process by which most cells receive information about their environment. To accomplish this we are taking a protein-engineering approach to the problem in which the extracellular domain of TRAP will be fused to a transmembrane conformational switching domain that in turn is fused to a glucosidase. When the hybrid TRAP molecule is unbound, the glucosidase on the interior of the biosensor will be in an inactive conformation and the biosensor will remain in high viscosity status, indicating that there are no bacteria nearby. However, if staphylococcal produced RAP is present near the joint, it will bind to the engineered TRAP proteins on the surface of the biosensor and the molecular switch will trigger activation of the glucosidase moiety. The activated glucosidase will release free glucose from a substrate glucose polymer which will, in turn, competitively bind to the concanavalin A, displacing the dextran and resulting in a decrease in viscosity which will initiate release of the multicomponent treatment regimen described above.
A similar protein engineering approach will be used to monitor the concentration of the released pharmacological reagents. For each ligand to be monitored, a hybrid receptor-switch-glucosidase protein will be engineered to function as a “front end” for the MEMS glucose-based viscometer. Therefore the most difficult developmental process associated with this project will serve multiple masters.
Technical challenges to implementing a MEMS-based biosensor
The number and placement of the biosensors, treatment reservoirs and telemetry unit(s) is far from established. Additionally, the communication between the multiple biosensors and among the several types of units must be developed and fitted with an intelligent decision-making algorithm that can determine if a signal likely is a false alarm. Moreover, the operating system must be capable of deciding between a local release from a single reservoir and a global release from all reservoirs. Because of the size of the implants, it is thought that multiple biosensors should be used, providing as broad an area of coverage for the prosthesis as possible. It may not be possible, however, to place the sensors on the articulating surfaces and we don’t know if it will be efficacious or even possible to position biosensors on the parts of the implants which are seated directly in existing bone. One model has part of the prosthesis hollowed out for reservoirs, but mechanical engineering constraints may mandate the use of auxiliary reservoirs that would have to be placed with the implant. Two other major challenges that must be overcome are (1) designing a system that will be stable and functional for an extended period of time in situ at 37 °C; and (2) resetting the biosensor to baseline after exogenous signal is no longer present. It is understood that these and other major developmental hurdles will be have to be overcome at all levels and stages of this project.
The Bioelectric Effect
In addition to delivery of antibiotics to the site of infection we also are designing ways of increasing antibiotic efficacy against biofilms using the “bioelectric effect.” The bioelectric effect is the synergistic killing effect observed on biofilm cells that are exposed to antibiotics in the presence of an electric direct current (DC) or alternating current (AC) field. The use of electric currents and electromagnetic fields to modulate biologic processes has become an increasingly popular subject of scientific enquiry in recent years. Although many such studies are still in their infancy in orthopaedics, at least one avenue of investigation has shown substantial progress: the use of electromagnetic stimulation to promote healing of problematic bony fractures. Aaron et al1 discuss the results from several trials that indicate electromagnetic fields can be used to accelerate bone formation and healing. Nelson et al21 discuss pulsed electromagnetic fields, capacitive coupled fields (electric fields rather than magnetic fields are used to induce currents), and low-intensity ultrasound as methods that are used to stimulate bone healing and bone formation. Cell studies are reported by Guerkov et al.12. They suggest a cascade of regulatory events is stimulated by the pulsed electromagnetic fields in the human hypertrophic and atrophic nonunion tissues. Ryaby27 presents a review article on the clinical use of electric and electromagnetic fields that are being used in the clinic to assist fracture healing, and a review paper by Otter et al (22) covers a number of the applications of electromagnetic fields to the field of bone healing.
With this background of practical success, efforts are now also underway to determine whether deployment of electric currents and fields against biofilms can become an effective therapy against infection. Early reports in in vitro systems have been encouraging, most particularly in observing the success of the bioelectric effect in potentiating the efficacy of concomitant antibiotic action against biofilm bacteria (Table 2). Costerton et al7 reported that application of a low-intensity direct current (max. 2.1 mA/cm2) to a flow cell in which Pseudomonas aeruginosa biofilm had been established was relatively ineffective in reducing bacterial numbers alone, but increased the killing efficacy of tobramycin by over four orders of magnitude when the two modalities were applied concurrently. The concentration of antibiotic necessary to treat biofilm bacteria was effectively reduced to within the same order of magnitude as that required to achieve MBC values in planktonic bacteria, rather than the thousandfold or more increased concentrations typically required to kill biofilms. Importantly, this increased efficacy brings antibiotic doses down to physiologically and clinically acceptable levels.
Table 2.
The Bioelectric Effect Increases Antibiotic Killing of Biofilm Bacteria.
| Strain | P. aeruginosa | P. aeruginosa | P. aeruginosa | K. pneumoniae | E. coli | S. gordonii |
|---|---|---|---|---|---|---|
| Antibiotic | Tobramycin | Tobramycin | Tobramycin | Tobramycin | Gentamicin/Oxytetracycline | Gentamicin |
| Reference | Costerton et al 7 | McLeod et al17 | Wellman et al31 | Wellman et al31 | Caubet et al6 | Wattanakaroon et al31. |
| Abx alone | 1.53 | 2.88 | 0 | 0 | 2.11/1.9 | 0.84 |
| DC alone | 0.81 | 0.65 | NT | 2.45 | 0.91 | 1.9 |
| AC alone | NT | NT | NT | NT | 0.5 | NT |
| DC and Abx | 6.02 | 5.77 | 6.7 | 3.9 | 4.27/> 5.15 | 4.3 |
| AC and Abx | NT | NT | NT | NT | 3.43/2.80 | NT |
Abx = Antibiotic; DC = Direct current; AC = Alternating current; NT = Not tested
These results were confirmed by McLeod et al,18 who again found that direct electric current potentiated the action of tobramycin against pseudomonal biofilms by an almost thousand-fold reduction in bacterial viability in an exposure chamber system (Table 2, Fig. 4). McLeod and co-workers, however, kept current constant for the full duration of treatment and were thus able to compile a dose-response curve for current efficacy, with the lowest optimal current value appearing at 1 mA. Similar encouraging results have been noted for the bioelectric effect against Klebsiella pneumoniae treated with tobramycin, S. epidermidis treated with tobramycin, Streptococcus gordonii treated with gentamicin, and Candida albicans treated with cycloheximide (15,33).
Fig 4.
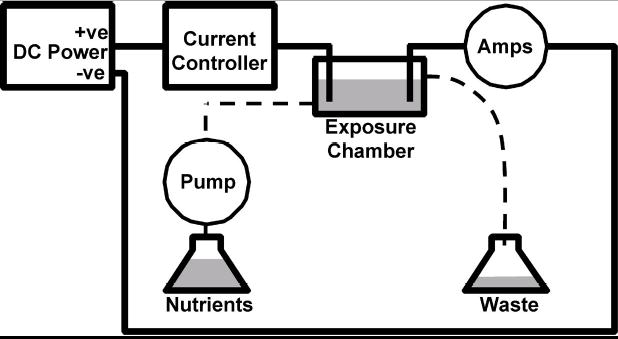
A schematic diagram shows the main components for in vitro testing of the bioelectric effect. The biofilm is grown or positioned in the exposure chamber. Antibiotics can be pumped into the chamber with nutrients and a DC current can be applied through an anode and cathode. +ve = positive electrode, −ve = negative electode
Caubet et al6 have evaluated the use of DC and a 10 megahertz (10 × 10^6 hertz) time-varying AC in an exposure chamber similar to that of McLeod et al.18 These authors used Escherichia coli biofilms treated with either gentamicin or with oxytetracycline and studied the effect of adding either a DC or the ten megahertz AC to the antibiotic in the support medium. The current for the DC and AC work was delivered through the support medium flowing in the exposure chamber through electrodes extending into the medium at either end of the exposure chamber (ie, the AC was not induced in the chamber). The DC plus antibiotic treatment produced a four to five log10 reduction in colony forming units (CFU), achieving very similar results to those of McLeod et al,18 whereas a slightly more modest reduction in CFU of 2.5 to 3.5 logs was achieved with the AC plus the antibiotic. This study supports the hypothesis that enhanced efficacy can be achieved for an antibiotic with the addition of an AC, although the magnitude of the improvement may be less than that obtained with direct current.
Pickering et al24 expand the field even further by examining the effect of an induced current on antibiotic efficacy against biofilm bacteria. In this work, a pulsed electromagnetic field (PEMF) was applied to S. epidermidis biofilm bacteria which induced a current within the biofilm. This induced current reduced the minimum inhibitory concentration of gentamicin by at least 50%, but did not show any significant effect with vancomycin.
Although there is an increasing body of evidence in support of a bioelectric effect against biofilms, the mechanism by which these electrical phenomena exert their actions is unknown, and indeed may well vary with the type of electrical current applied. In the 10 MHz AC system of Caubet et al,6 for example, the authors point out that “there is no transport of ions between the electrodes, no creation of new ions, and no electrolysis” as would occur in a DC system, but a bioelectric effect still appears. Stewart et al29 investigated the mechanisms by which a DC bioelectric effect may operate. Their work discounted the suggestions that reduced pH, increased temperature, or generation of inhibitory ions or reactive oxygen intermediates were the relevant means by which a bioelectric effect manifests. They did find that electrolytic generation of oxygen (potentially increasing the local oxygen concentration in the biofilm microenvironment) appeared to partially explain the augmentation of antibiotic efficiency. Based on this study, we hypothesize that the bioelectric effect results from increased metabolic and replicative activity associated with increased O2 tensions within the biofilm bacteria which make them more susceptible to antibiotic-induced killing. It is well established that antibiotics are much more effective against rapidly metabolizing and dividing bacteria than they are to metabolically quiescent bacteria, and one of the limiting nutrients within the core of the biofilm is O2. We have shown that the provision of O2 deep within the biofilm results in greatly increased metabolism.10
The majority of work evaluating the bioelectric effect has to date been carried out in in vitro systems and has focused mainly on aminoglycoside antibiotics. Only Pickering et al24 failed to find any significant bioelectric effect with vancomycin but they did not evaluate it in a directly applied DC system. In clinical terms for orthopaedics, the bioelectric effect could become important if it can be shown in in vivo conditions and with antibiotics routinely used against the staphylococci, especially vancomycin. We therefore are establishing protocols in which direct or induced currents can be delivered within the joint capsule of an infected knee prosthesis in small animal and large animal models. If the bioelectric effect can be achieved in vivo, the likelihood of developing a human-use device to supply adjuvant electrical therapy to patients with infected implants will be increased substantially.
Discussion
Infected artificial joints require extensive and expensive surgical and medical interventions, the latter modalities often require months of treatment. These adminstrations are required because chronic bacterial infections are associated with the formation of bacterial biofilms on host tissues and implant surfaces. Biofilm bacteria are highly resistant to both host defenses, including innate and adaptive immune responses, and to pharmaceutical antimicrobial agents. These multi-fold resistances are attributable to fundamental difference in the metabolism of biofilm bacteria compared with their planktonic counterparts (4, 5).
The best strategy to combat orthopedic implant biofilm infections is to prevent their occurrence in the first place. This should be possible as bacterial infections are always intially planktonic in nature, and only after an acute phase do the bacteria attach and elaborate a biofilm matrix. To realize such a preemptive strategy will require a marriage of engineering, surgical, and microbiological strategies. Towards this end a multidisciplinary team of scientists, surgeons, and engineers has been assembled to develop strategies and devices to either: A) continuously treat the impant using the bioelectric effect; or B) utlize and intelligent implant that can, 1) continuously monitor implant sites for the presence of bacteria; 2) release a cocktail of antimicrobial compounds upon the detection of bacteria; 3) monitor the release of the antimicrobial agents; and 4) report all bacterial detections and treatments via telemetry to the patient and physician.
The technical challenges to realizing an “intelligent implant” are nontrivial and will require a concerted effort by a large multidisciplinary team over an extended period of time. Whereas the development of a device to deliver a bioelectric effect is much more straightforward as such devices already exist for other orthopedic applications, notably in the realm of inducing bone growth in the case of nonunion fractures. The most complicated aspect of the intelligent implant project will likely be the construction of a chimeric protein switch that upon binding a bacterial signaling ligand will undergo a conformational change that activates an enzymatic function. However, the most difficult challenge may be the no technical issues associated with finding a manufacturer willing to underwrite the development of such a device and then shepard it through the regulatory process. Finally, assuming such a device was available for implant, it would require a major educational campaign to get the rank and file reconstructive orthopedic surgeon to adopt such an instrument.
Footnotes
One or more of the authors received funding from the Allegheny Orthopedic Research Foundation, the Allegheny Singer Research Institute, and NIH grants 3R01 DC02418 (GDE), 2R01 DC04173 (GDE) and R01 DC05659 (JCP).
References
- 1.Aaron RK, Ciombor DM, Simon BJ. Treatment of nonunions with electric and electromagnetic fields. Clin Orthop. 2004;419:21–29. doi: 10.1097/00003086-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Balaban N, Goldkorn T, Gov Y, et al. Regulation of Staphylococcus aureus pathogenesis via target of RNAIII-activating Protein (TRAP) Biol Chem. 2001;276:2658–2667. doi: 10.1074/jbc.m005446200. [DOI] [PubMed] [Google Scholar]
- 3.Balaban N, Stoodley P, Fux CA, et al. Prevention of Staphylococcal biofilms-associated infections by the quorum sensing inhibitor RIP. Clin Orthop. 2005;437:XXX–XXX. doi: 10.1097/01.blo.0000175889.82865.67. [DOI] [PubMed] [Google Scholar]
- 4.Borriello G, Werner E, Roe F, et al. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother. 2004;48:2659–2664. doi: 10.1128/AAC.48.7.2659-2664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borriello, G., Richards, L., Ehrlich, G.D., Stewart, P.S. Arginine Enhances Antibiotic Susceptibility of Pseudomonas aeruginosa in Biofilms (In Press: Antimicrobial Agents and Chemotherapy) [DOI] [PMC free article] [PubMed]
- 6.Caubet R, Pedarros-Caubet F, Chu M, et al. A radio frequency electric current enhances antibiotic efficacy against bacterial biofilms. Antimicrob Agents Chemother. 2004;48:4662–4664. doi: 10.1128/AAC.48.12.4662-4664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costerton JW, Ellis B, Lam K, Johnson F, Khoury E. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob Agents Chemother. 1994;38:2803–2809. doi: 10.1128/aac.38.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costerton W, Veeh R, Shirtliff M, et al. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest. 2003;112:1466–1477. doi: 10.1172/JCI20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrlich GD, Hu FZ, Lin Q, Costerton JW, Post JC. Intelligent Implants to Battle Biofilms. ASM News. 2004;70:127–133. [Google Scholar]
- 10.Ehrlich GD, Hu FZ, Post JC. Role for Biofilms in Infectious Disease. In Ghannoum MA, O’Toole G (eds). Microbial Biofilms. Washington, ASM Press 332–358, 2004.
- 11.Ehrlich GD, Hu FZ, Shen K, Stoodley P, Post JC. Bacterial plurality as a general mechanism driving persistence in chronic infections. Clin Orthop. 2005;437:XXX–XXX. doi: 10.1097/00003086-200508000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerkov HH, Lohmann CH, Liu Y, et al. Pulsed electromagnetic fields increase growth factor release by nonunion cells. Clin Orthop. 2001;384:265–279. doi: 10.1097/00003086-200103000-00031. [DOI] [PubMed] [Google Scholar]
- 13.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 14.Jeckelmann J, Seibold A: GlucOnline™ - a new approach to continuous glucose monitoring. Diabetesprofile 2/02. www.disetronic.com/download/02_Seib.pdf 10–10-2003
- 15.Khoury AE, Lam K, Ellis B, Costerton JW. Prevention and control of bacterial infections associated with medical devices. ASAIO J. 1992;38:M172–M178. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Korem M, Sheoran AS, Gov Y, et al. Characterization of RAP, a quorum sensing activator of Staphylococcus aureus. FEMS Microbiol Lett. 2003;223:167–175. doi: 10.1016/S0378-1097(03)00326-4. [DOI] [PubMed] [Google Scholar]
- 17.Mayville P, Ji G, Beavis R, et al. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci U S A. 1999;96:1218–1223. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLeod BR, Fortun S, Costerton JW, Stewart PS. Enhanced bacterial biofilm control using electromagnetic fields in combination with antibiotics. Methods Enzymol. 1999;310:656–670. doi: 10.1016/s0076-6879(99)10051-x. [DOI] [PubMed] [Google Scholar]
- 19.MDowell P, Affas Z, Reynolds C, et al. Structure, activity and evolution of the group I thiolactone peptide quorum-sensing system of Staphylococcus aureus. Mol Microbiol. 2001;41:503–512. doi: 10.1046/j.1365-2958.2001.02539.x. [DOI] [PubMed] [Google Scholar]
- 20.Muschik M, Luck W, Schlenzka D. Implant removal for late-developing infection after instrumented posterior spinal fusion for scoliosis: reinstrumentation reduces loss of correction. A retrospective analysis of 45 cases. Eur Spine J. 2004;13:645–51. doi: 10.1007/s00586-004-0694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson FR, Brighton CT, Ryaby J, et al. Use of physical forces in bone healing. J Am Acad Orthop Surg. 2003;11:344–354. doi: 10.5435/00124635-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Otter MW, McLeod KJ, Rubin CT. Effects of electromagnetic fields in experimental fracture repair. Clin Orthop. 1998;355(Suppl):S90–S104. doi: 10.1097/00003086-199810001-00011. [DOI] [PubMed] [Google Scholar]
- 23.Parsek MR, Greenberg EP. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Pickering SA, Bayston R, Scammell BE. Electromagnetic augmentation of antibiotic efficacy in infection of orthopaedic implants. J Bone Joint Surg. 2003;85B:588–593. doi: 10.1302/0301-620x.85b4.12644. [DOI] [PubMed] [Google Scholar]
- 25.Redfield RJ. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 2003;10:365–370. doi: 10.1016/s0966-842x(02)02400-9. [DOI] [PubMed] [Google Scholar]
- 26.Roberts ME, Stewart PS. Modeling protection from antimicrobial agents in biofilms through the formation of persister cells. Microbiology. 2005;151:75–80. doi: 10.1099/mic.0.27385-0. [DOI] [PubMed] [Google Scholar]
- 27.Ryaby JT. Clinical effects of electromagnetic and electric fields on fracture healing. Clin Orthop. 1998;355(Suppl):S205–S215. doi: 10.1097/00003086-199810001-00021. [DOI] [PubMed] [Google Scholar]
- 28.Shen K, Wang X, Post JC, Ehrlich GD: Molecular and Translational Research Approaches for the study of Bacterial Pathogenesis in Otitis Media. In Rosenfeld R, Bluestone CD (eds). Evidence-based Otitis Media. Ed 2. Hamilton, B.C. Decker Inc. pp99–119X, 2003.
- 29.Stewart PS, Wattanakaroon W, Goodrum L, Fortun SM, McLeod BR. Electrolytic generation of oxygen partially explains electrical enhancement of tobramycin efficacy against Pseudomonas aeruginosa biofilm. Antimicrob Agents Chemother. 1999;43:292–296. doi: 10.1128/aac.43.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoodley P, Kathju S, Hu FZ, et al. Molecular and imaging techniques for bacterial biofilms in arthroplastic joint infections. Clin Orthop. 2005;437:XXX–XXX. doi: 10.1097/01.blo.0000175129.83084.d5. [DOI] [PubMed] [Google Scholar]
- 31.Thomas C, Cadwallader HL, Riley TV. Surgical-site infections after orthopaedic surgery: statewide surveillance using linked administrative databases. J Hosp Infect. 2004;57(1):25–30. doi: 10.1016/j.jhin.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 32.Wattanakaroon W, Stewart PS. Electrical enhancement of Streptococcus gordonii biofilm killing by gentamicin. Arch Oral Biol. 2000;45:167–171. doi: 10.1016/s0003-9969(99)00132-6. [DOI] [PubMed] [Google Scholar]
- 33.Wellman N, Fortun SM, McLeod BR. Bacterial biofilms and the bioelectric effect. Antimicrob Agents Chemother. 1996;40:2012–2014. doi: 10.1128/aac.40.9.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


