Abstract
We have examined the gene expression profiles of young, old and cataractous human lenses in order to differentiate those gene expression changes specific for cataract from those also associated with lens aging. Differentially expressed transcripts were identified by oligonucleotide microarray analysis and clustered according to their known functions. Four hundred and twelve transcripts that are increased and 919 transcripts that are decreased were identified at the 2-fold or greater level between epithelia isolated from cataract relative to clear lenses while 182 transcripts that are increased and 547 transcripts that are decreased were identified at the 2-fold or greater level between young and old lens epithelia. Comparison of the cataract gene expression changes with those detected in lens aging revealed that only 3 transcripts exhibited similar trends in gene expression. These data suggest that cataract- and age-specific changes in gene expression do not overlap and provide evidence for multiple cataract- and age-specific gene expression changes in the human lens.
Keywords: age-related cataract, clear human lenses, gene expression
1. Introduction
Age-related cataract is a multi-factorial disease contributed by aging, genetics and environmental factors that, among others, include UV-light, X-irradiation, toxins, metals, steroids, drugs and diseases including diabetes (Phelps Brown, 1996). These combined factors result in numerous lens changes that culminate to produce lens opacity including increased proteolysis, alterations in the cell cycle, altered growth and differentiation of lens epithelial cells, altered ion transport and osmotic balance as well as DNA damage (Phelps Brown, 1996).
An important step in understanding cataractogenesis is to identify those metabolic and biochemical pathways altered between cataract and clear lenses. In the present survey, we have sought to identify those gene expression differences between clear human lenses relative to age-related cataracts and we have focused on the lens epithelium since this monolayer of cells is essential for the growth, differentiation and homeostasis of the entire organ (Bloemendal, 1981; Piatigorsky, 1981), contains the highest levels of enzymes and transport systems in the lens (Reddy, 1971; Reddan, 1982; Spector, 1982) and is the first part of the lens exposed to environmental insults (Reddan, 1982; Spector, 1982). Multiple studies suggest that the lens epithelium is capable of communicating with the underlying fiber cells (Rae et al., 1996) and direct damage to the lens epithelium and its enzyme systems is known to result in cataract formation (Harding and Crabbe, 1984; Hightower, 1995; Spector, 1995; Phelps Brown, 1996). Importantly, the majority of transcription occurs in the epithelial cells of the lens, and therefore these cells make up the majority of lens cells capable of responding to environmental insults and/or the presence of cataract through altered gene expression. Since the lens epithelium is composed of a single cell-type it represents an ideal model for differential gene expression studies.
Considerable evidence suggests that gene expression in the lens epithelium is altered by the presence of cataract. For instance, metallothionein IIa (Kantorow et al., 1998b) osteonectin, also known as SPARC (Kantorow et al., 1998a) and adhesion-related kinase (Sheets et al., 2002) are up-regulated in cataract relative to clear lenses while multiple ribosomal proteins (Zhang et al., 2002) and protein phosphatase 2A (Kantorow et al., 1998b) are down-regulated in cataract relative to clear lenses. Many of these genes have functions consistent with processes associated with cataract formation. Metallothionein IIa is involved in metal binding and detoxification (Kagi and Schaffer, 1988) and heavy metals such as cadmium are known to be associated with cataract (Ramakrishnan et al., 1995). Osteonectin, a calcium-binding protein that functions in the regulation of cell growth (Sage et al., 1995), when deleted in mice, results in cataract formation (Gilmour et al., 1998). Decreased expression of ribosomal proteins results in decreased protein synthesis, a phenomenon that has been linked to cataract formation (Haloui et al., 1990).
2. Gene expression profiles of human age-related cataracts
Although these individual changes in gene expression are informative, further gene identification is needed to define those functional gene clusters that could elucidate major pathways associated with cataract. Here, we have used oligonucleotide microarrays to compare the global gene expression profiles between pooled, approximately age-matched, human lens epithelia isolated from cataract (average age 71 years) and clear lenses (average age 64 years). This technology allows us to examine the expression levels of over half of the genes comprising the human genome. The cataracts used in this study were approximately 2–3 mm2 central lens epithelial tags and represent the entire population of patients undergoing cataract surgery at the Jules Stein Eye Institute, UCLA School of Medicine. These cataracts were classified according to a modified version of the Lens Opacities Classification Scale (LOCS)-III grading system and are represented by approximately 70% mixed, 20% nuclear, 5% cortical and 2% posterior subcapsular cataracts. The clear lenses were obtained from organ donors within 24 hr post-mortem by the West Virginia Eye Bank and the Lions Eye Bank of Oregon. The clear lenses were micro-dissected for central epithelium (6–8 mm2) and contaminating fiber cells were removed. Any lens exhibiting opacity was excluded from the present study.
This analysis revealed 412 transcripts whose expression levels are increased by 2-fold or greater in human age-related cataract relative to clear lenses and an additional 919 transcripts whose expression levels are decreased by 2-fold or greater (Hawse et al., 2003). Of these genes, 74 are increased by 5-fold or greater and 241 are decreased by 5-fold or greater in cataract (Hawse et al., 2003). Semi-quantitative RT-PCR confirmations indicate that the microarray data is approximately 84% accurate (Hawse et al., 2003).
Functional clustering and over-representation analysis of the identified genes using the EASE bioinformatics software package revealed that multiple biological pathways, represented by functional gene clusters, are significantly altered upon cataract formation. Of the genes increased in cataract by 2-fold or greater, the following categories were identified as being significantly altered; chromosome organization, nuclear organization, transcription/DNA-dependent, transcription, nucleic acid metabolism, nucleic acid binding, ligand binding or carrier and DNA binding (Hawse et al., 2003). Of the genes decreased in cataract by 2-fold or greater, the following categories were identified as being significantly altered; RNA splicing, protein biosynthesis, protein synthesis elongation, protein synthesis initiation, macromolecule biosynthesis, amine biosynthesis, peroxidase reaction, microtubule-based process, organelle organization, cytoskeleton organization, temperature response, heat-shock response, vision, response to external stimulus, U6 snRNA binding, pre-mRNA splicing factor, mRNA binding, proteasome endopeptidase, translation factor, selenium binding, alcohol dehydrogenase, heat-shock protein, oxidoreductase, glutathione peroxidase, chaperone, structural constituents of lens and structural molecules (Hawse et al., 2003).
Although it is extremely difficult to summarize this large amount of data, a few groups of genes that may play important roles in cataract formation are worthy of noting. The relative expression differences of these genes and other genes not discussed here are available (Hawse et al., 2003). Of the genes increased in cataract, many are associated with ionic transport. In particular, a PQ type voltage gated calcium channel was detected to be increased in cataract by nearly 5-fold. Calcium is likely to be an important factor in cataract formation since the activity of calcium-ATPase is reduced by 50% in the membranes of lens epithelia isolated from cataractous lenses compared to clear human lenses (Paterson et al., 1997). Oxidative stress has also been demonstrated to have an effect on the activity of calcium transporters in the lens. For example, hydrogen peroxide decreases the activity of calcium transporters in rabbit lenses (Borchman et al., 1989). These phenomenons are closely associated with our results demonstrating an increase in calcium transporters, possibly in an attempt to overcome their decreased activity in cataractous lenses.
Adducin, another member of the ligand binding or carrier group, was detected to be increased in cataracts by 6-fold. Adducin is a cytoskeletal protein involved in signal transduction mechanisms through modulation of the actin cytoskeleton at cell–cell contact sites (Kuhlman et al., 1996). The actin-based cytoskeleton has been shown to interact with epithelial sodium channels, sodium/potassium/ chloride co-transporters and sodium/potassium ATPase and is therefore likely to be involved in alterations in ionic transporters. Copine III is another gene involved in membrane trafficking processes (Creutz et al., 1998) upon calcium binding. We have detected that Copine III is increased in cataracts relative to clear lenses by 7-fold. Other genes known to be involved in ligand binding or transport that exhibited high levels of increased expression in cataracts were sodium/potassium ATPase beta 1 polypeptide, chloride channel 3, pleiotrophin and sodium/hydrogen exchanger isoform 2.
Another major group of genes that exhibit increased expression in cataractous epithelia compared to clear lens epithelia are extracellular matrix proteins. Specific examples include adducin, pleiotrophin, an extracellular matrix protein that binds heparin (Fath et al., 1999) and is induced during wound repair (Deuel et al., 2002). Another gene included in this category is claudin, a component of tight junction filaments capable of interacting adhesively with complementary molecules on adjacent epithelial cells (Gonzalez-Mariscal et al., 2003). Recent studies have found that over-expression of claudin-2 induces cation-selective channels in tight junctions of epithelial cells resulting in increased ion permeability (Amasheh et al., 2002). Other genes include supervillin, an F-actin bundling plasma membrane protein that contains functional nuclear localization signals (Wulfkuhle et al., 1999), bamacan, a chondroitin sulphate proteoglycan that abounds in basement membranes and is thought to be involved in the control of cell growth and transformation (Ghiselli et al., 1999) and osteonectin which has previously been demonstrated to be increased in human age-related cataracts (Kantorow et al., 2000).
The majority of genes whose expression levels are altered between cataracts and clear lenses exhibit decreased expression. These genes function in diverse processes including protein synthesis, oxidative stress, structural proteins, chaperones and cell cycle control proteins. Many of these processes represent metabolic systems designed to preserve lens homeostasis and their decreased expression may reflect the inability of the lens to maintain its internal environment in the presence of stress and/or cataract. Specific examples of these genes include multiple ribosomal protein subunits involved in protein synthesis including large subunits 21, 15, 13a and 7a which were previously shown to be decreased in cataract relative to clear human lenses (Zhang et al., 2002), selenoprotein W1, a glutathione-dependent antioxidant known to protect lung cells against H2O2 cytotoxicity (Jeong et al., 2002) which could play a role in defending the lens against oxidative stress, glutathione peroxidases 1, 3 and 4, important oxidative stress enzymes that are likely to play major roles in lens protection and maintenance (Reddy et al., 2001), multiple crystallins and other lens structural components, Hsp70, a key ATPase activated chaperone (Haslbeck, 2002), Hsp27-1, a small heat-shock protein likely to be important for lens protection (Ganea, 2001), Hsp27-2, a small heat-shock protein closely related to αB-crystallin (Iwaki et al., 1997) which may also be important for lens protection, and αA-crystallin that, in addition to its structural role in the lens, is also a small heat-shock protein that can prevent protein aggregation in the lens (Horwitz, 1992).
It is important to note that some of the same genes, and their corresponding magnitude changes detected in the present study, correlate almost exactly with the gene expression differences and magnitude changes detected between cataract epithelia and clear lens epithelia using an entirely different population of human subjects as well as a different type of hybridization screening (Ruotolo et al., 2003). Previous studies employing RT-PCR differential display technology have also identified similar trends in the expression of specific genes between human age-related cataract and clear lenses (Zhang et al., 2002). These complementary studies provide confidence that the gene expression differences detected in the present survey are truly accurate.
3. Gene expression profiles of aged human lenses
One intriguing question concerning these gene expression changes is whether they would be specific for cataract or would also be detected in young verses old lenses. Therefore, we have also conducted oligonucleotide microarray studies on old and young lens epithelia to identify gene expression changes that occur in the lens with age. For this experiment, we used 10 pooled young lens epithelia, average age 32·3 years, and 10 pooled old lens epithelia, average age 64 years. The chip representing the pooled old lens epithelia sample is the exact same chip that represents the clear lens sample used in the cataract comparison. This analysis revealed that 182 transcripts are increased in old lenses compared to young lenses while 547 transcripts are decreased at the 2-fold or greater level. Of these, only 4 transcripts are increased with age at the 5-fold or greater level while 74 transcripts are decreased with age at the 5-fold or greater level.
Functional clustering of the identified gene expression differences between young and old lenses revealed that biological processes such as regulation of translation, protein synthesis, intracellular transport, cell growth and/or maintenance, and response to stress, among others, are increased with age (Fig. 1) while Biological Processes such as double-strand break repair, telomere maintenance, transcription, chromosome segregation, extracellular matrix organization and DNA unwinding, among others, are decreased with age (Fig. 2).
Fig. 1.
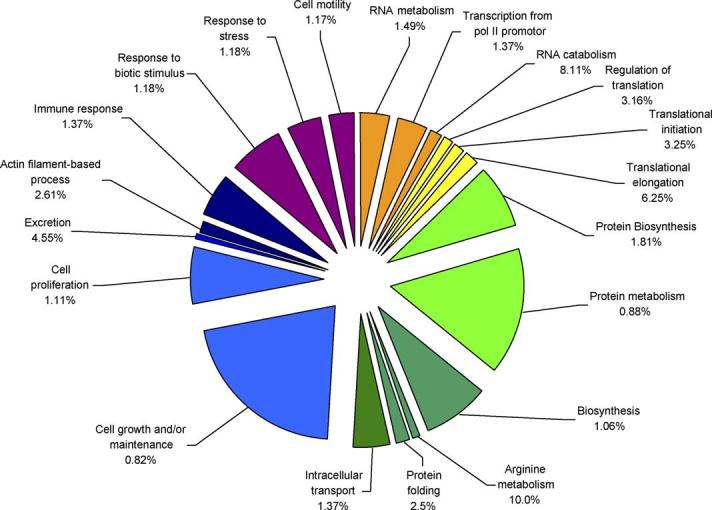
Functional clusters of genes involved in biological processes which have increased expression levels in old lens epithelium compared to young lens epithelium. The specific sub-categories of genes determined to be significantly altered using the statistical clustering program, EASE, are indicated. Percentages indicate the number of altered genes in each sub-category relative to their total representation on the microarray. Colours denote the approximate relative cellular location for which the genes in each sub-category function ranging from the nucleus to the plasma membrane (red to violet). Pie piece size approximates the number of changed genes in each sub-category.
Fig. 2.
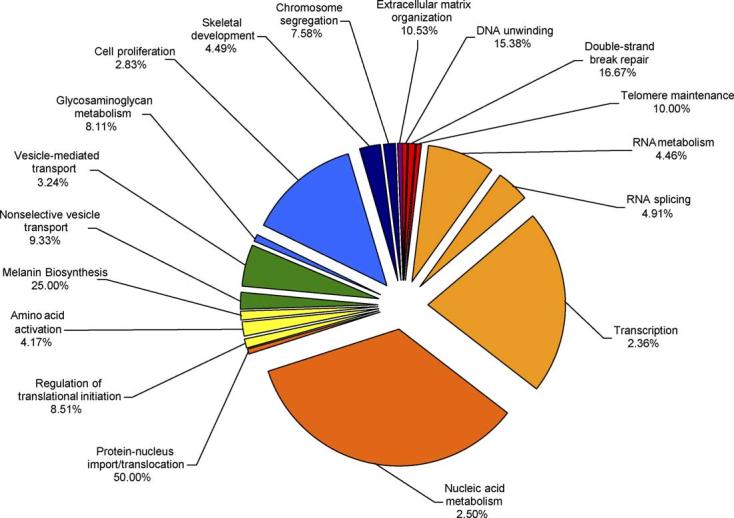
Functional clusters of genes involved in biological processes which have decreased expression levels in old lens epithelium compared to young lens epithelium. The specific sub-categories of genes determined to be significantly altered using the statistical clustering program, EASE, are indicated. Percentages indicate the number of altered genes in each sub-category relative to their total representation on the microarray. Colours denote the approximate relative cellular location for which the genes in each sub-category function ranging from the nucleus to the plasma membrane (red to violet). Pie piece size approximates the number of changed genes in each sub-category.
Specific genes whose transcript levels were detected to be increased in old lens epithelia relative to young lens epithelia included multiple small and large ribosomal protein subunits as well as several translation initiation, elongation and termination factors. MIP, tubulin and cyclin D1 are examples of genes involved in cell growth and/or maintenance that were increased in aged lens epithelia. Another category of interest, which also exhibited increased expression with age, is response to stress, which includes growth arrest and DNA damage-inducible alpha gene, TNF receptor member 17, beta 1 integrin and chemokine ligand 2. It must be noted that, as mentioned above, the majority of these genes have increased expression levels of only 2–3-fold.
There were many more genes with much larger fold changes that exhibited decreased expression with age. Major categories of these genes included double-strand break repair and telomere maintenance. Specifically, we detected decreased expression levels of telomeric repeat-binding factor 1, the deletion of which causes growth defects and chromosomal instability in mouse embryonic stem cells (Iwano et al., 2004), dyskerin, which is believed to function in maintaining cell proliferation and/or function (Heiss et al., 1999) and Nijmegen breakage syndrome 1 which functions in double-strand break repair and cell cycle checkpoints (Carney et al., 1998).
Another major category, that exhibited decreased expression with age, was composed of genes associated with transcription, including numerous zinc finger proteins and other transcription factors. It is well documented that many genes involved in transcriptional processes are down regulated with age in multiple tissues and organisms including rats (Blalock et al., 2003), mice (Frasca et al., 2003) and humans (Roy et al., 2002) and their down regulation is a central hypothesis as to why cells age and eventually die.
4. Comparison of gene expression differences between cataractous and aged human lenses
In order to identify those gene expression differences that are likely to be specific for cataract and not aging of the lens, we compared the aging data with the cataract data. In comparing these two sets of data, only 3 transcripts were identified to be common between the detected cataract-specific gene expression differences and the age-specific gene expression differences even at the 2-fold or greater level. These three transcripts, hevin, opioid-binding protein/cell adhesion molecule and FXYD domain containing ion transport regulator 6, were detected to be decreased in cataracts relative to clear lenses and were also decreased with age. There were no genes identified to be increased in cataract and simultaneously increased with age. A total of 126 transcripts exhibit decreased expression in cataracts and increased expression with age while 171 transcripts were detected to be increased in cataract and decreased with age. There were 1031 transcripts detected to be altered between cataract and clear lenses but unchanged with age and another 429 transcripts that were detected to be altered with age but unchanged in cataract. It is interesting to note that many of the functional categories that are increased in cataracts are actually decreased with age and those that are decreased in cataracts are increased with age.
5. Summary
In this presentation summary, we highlight those transcripts, and their associated functional categories, that were detected to be altered in cataract relative to clear lenses. We acknowledge that this data only reflects alterations in the mRNA levels of the identified genes and that some of these changes may not be reflected at the protein level and further studies will be needed to determine this relationship. Since the gene expression changes associated with aging of the human lens are different than those identified in cataracts, our data may indicate that the majority of gene expression changes present in cataractous human lenses reflect responses of the lens to the presence of cataract. Although this work is descriptive, and does not distinguish consequential gene expression differences from true responses of the lens epithelium to the presence of cataract, it nevertheless reveals many functional processes likely to be altered in cataract whose further study will provide significant insight into this disease.
References
- Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J. Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H. Molecular Biology of the Eye Lens. Willey; New York: 1981. [Google Scholar]
- Borchman D, Paterson CA, Delamere NA. Oxidative inhibition of Ca2 + -ATPase in the rabbit lens. Invest. Ophthalmol. Vis. Sci. 1989;30:1633–1637. [PubMed] [Google Scholar]
- Carney JP, Maser RS, Olivares H, Davis EM, Le Beau M, Yates JR, III, Hays L, Morgan WF, Petrini JH. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell. 1998;93:477–486. doi: 10.1016/s0092-8674(00)81175-7. [DOI] [PubMed] [Google Scholar]
- Creutz CE, Tomsig JL, Snyder SL, Gautier MC, Skouri F, Beisson J, Cohen J. The copines, a novel class of C2 domain-containing, calcium-dependent, phospholipid-binding proteins conserved from Paramecium to humans. J. Biol. Chem. 1998;273:1393–1402. doi: 10.1074/jbc.273.3.1393. [DOI] [PubMed] [Google Scholar]
- Deuel TF, Zhang N, Yeh HJ, Silos-Santiago I, Wang ZY. Pleiotrophin: a cytokine with diverse functions and a novel signaling pathway. Arch. Biochem. Biophys. 2002;397:162–171. doi: 10.1006/abbi.2001.2705. [DOI] [PubMed] [Google Scholar]
- Fath M, VanderNoot V, Kilpelainen I, Kinnunen T, Rauvala H, Linhardt RJ. Interaction of soluble and surface-bound heparin binding growth-associated molecule with heparin. FEBS Lett. 1999;454:105–108. doi: 10.1016/s0014-5793(99)00785-1. [DOI] [PubMed] [Google Scholar]
- Frasca D, Nguyen D, Riley RL, Blomberg BB. Effects of aging on proliferation and E47 transcription factor activity induced by different stimuli in murine splenic B cells. Mech. Ageing Dev. 2003;124:361–369. doi: 10.1016/s0047-6374(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Ganea E. Chaperone-like activity of alpha-crystallin and other small heat-shock proteins. Curr. Protein Pept. Sci. 2001;2:205–225. doi: 10.2174/1389203013381107. [DOI] [PubMed] [Google Scholar]
- Ghiselli G, Siracusa LD, Iozzo RV. Complete cDNA cloning, genomic organization, chromosomal assignment, functional characterization of the promoter, and expression of the murine Bamacan gene. J. Biol. Chem. 1999;274:17384–17393. doi: 10.1074/jbc.274.24.17384. [DOI] [PubMed] [Google Scholar]
- Gilmour DT, Lyon GJ, Carlton MB, Sanes JR, Cunningham JM, Anderson JR, Hogan BL, Evans MJ, Colledge WH. Mice deficient for the secreted glycoprotein SPARC/osteonectin/BM40 develop normally but show severe age-onset cataract formation and disruption of the lens. EMBO J. 1998;17:1860–1870. doi: 10.1093/emboj/17.7.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Haloui Z, Pujol JP, Galera P, Courtois Y, Laurent M. Analysis of lens protein synthesis in a cataractous mutant mouse: the Cat Fraser. Exp. Eye Res. 1990;51:487–494. doi: 10.1016/0014-4835(90)90078-9. [DOI] [PubMed] [Google Scholar]
- Harding JJ, Crabbe MJ. The lens: development, proteins, metabolism and cataract. In: Davson H, editor. The Eye. 1B. Academic Press; Orlando, FL: 1984. [Google Scholar]
- Haslbeck M. sHsps and their role in the chaperone network. Cell Mol. Life Sci. 2002;59:1649–1657. doi: 10.1007/PL00012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawse JR, Hejtmancik JF, Huang Q, Sheets NL, Hosack DA, Lempicki RA, Horwitz J, Kantorow M. Identification and functional clustering of global gene expression differences between human age-related cataract and clear lenses. Mol. Vis. 2003;9:515–537. [PMC free article] [PubMed] [Google Scholar]
- Heiss NS, Girod A, Salowsky R, Wiemann S, Pepperkok R, Poustka A. Dyskerin localizes to the nucleolus and its mislocalization is unlikely to play a role in the pathogenesis of dyskeratosis congenita. Hum. Mol. Genet. 1999;8:2515–2524. doi: 10.1093/hmg/8.13.2515. [DOI] [PubMed] [Google Scholar]
- Hightower KR. The role of the lens epithelium in development of UV cataract. Curr. Eye Res. 1995;14:71–78. doi: 10.3109/02713689508999916. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc. Nat. Acad. Sci. USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki A, Nagano T, Nakagawa M, Iwaki T, Fukumaki Y. Identification and characterization of the gene encoding a new member of the alpha-crystallin/small hsp family, closely linked to the alphaB-crystallin gene in a head-to-head manner. Genomics. 1997;45:386–394. doi: 10.1006/geno.1997.4956. [DOI] [PubMed] [Google Scholar]
- Iwano T, Tachibana M, Reth M, Shinkai Y. Importance of TRF1 for functional telomere structure. J. Biol. Chem. 2004;279:1442–1448. doi: 10.1074/jbc.M309138200. [DOI] [PubMed] [Google Scholar]
- Jeong D, Kim TS, Chung YW, Lee BJ, Kim IY. Selenoprotein W is a glutathione-dependent antioxidant in vivo. FEBS Lett. 2002;517:225–228. doi: 10.1016/s0014-5793(02)02628-5. [DOI] [PubMed] [Google Scholar]
- Kagi JH, Schaffer A. Biochemistry of metallothionein. Biochemistry. 1988;27:8509–8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- Kantorow M, Horwitz J, Carper D. Up-regulation of osteonectin/SPARC in age-related cataractous human lens epithelia. Mol. Vis. 1998a;4:17. [PubMed] [Google Scholar]
- Kantorow M, Kays T, Horwitz J, Huang Q, Sun J, Piatigorsky J, Carper D. Differential display detects altered gene expression between cataractous and normal human lenses. Invest. Ophthalmol. Vis. Sci. 1998b;39:2344–2354. [PubMed] [Google Scholar]
- Kantorow M, Huang Q, Yang XJ, Sage EH, Magabo KS, Miller KM, Horwitz J. Increased expression of osteonectin/SPARC mRNA and protein in age-related human cataracts and spatial expression in the normal human lens. Mol. Vis. 2000;6:24–29. [PMC free article] [PubMed] [Google Scholar]
- Kuhlman PA, Hughes CA, Bennett V, Fowler VM. A new function for adducin. Calcium/calmodulin-regulated capping of the barbed ends of actin filaments. J. Biol. Chem. 1996;271:7986–7991. doi: 10.1074/jbc.271.14.7986. [DOI] [PubMed] [Google Scholar]
- Paterson CA, Zeng J, Husseini Z, Borchman D, Delamere NA, Garland D, Jimenez-Asensio J. Calcium ATPase activity and membrane structure in clear and cataractous human lenses. Curr. Eye Res. 1997;16:333–338. doi: 10.1076/ceyr.16.4.333.10689. [DOI] [PubMed] [Google Scholar]
- Phelps Brown B. Lens disorders: a clinical manual of cataract diagnosis. 1996:1–135. [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Rae JL, Bartling C, Rae J, Mathias RT. Dye transfer between cells of the lens. J. Membr. Biol. 1996;150:89–103. doi: 10.1007/s002329900033. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan S, Sulochana KN, Selvaraj T, Abdul RA, Lakshmi M, Arunagiri K. Smoking of beedies and cataract: cadmium and vitamin C in the lens and blood. Br. J. Ophthalmol. 1995;79:202–206. doi: 10.1136/bjo.79.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddan JR. Control of cell division in the ocular lens, retina and vitreous humour. In: McDevitt DS, editor. Cell Biology of the Eye. Academic Press; New York: 1982. pp. 299–375. [Google Scholar]
- Reddy VN. Metabolism of glutathione in the lens. Exp. Eye Res. 1971;11:310–328. doi: 10.1016/s0014-4835(71)80043-x. [DOI] [PubMed] [Google Scholar]
- Reddy VN, Giblin FJ, Lin LR, Dang L, Unakar NJ, Musch DC, Boyle DL, Takemoto LJ, Ho YS, Knoernschild T, Juenemann A, Lutjen-Drecoll E. Glutathione peroxidase-1 deficiency leads to increased nuclear light scattering, membrane damage, and cataract formation in gene-knockout mice. Invest. Ophthalmol. Vis. Sci. 2001;42:3247–3255. [PubMed] [Google Scholar]
- Roy AK, Oh T, Rivera O, Mubiru J, Song CS, Chatterjee B. Impacts of transcriptional regulation on aging and senescence. Ageing Res. Rev. 2002;1:367–380. doi: 10.1016/s1568-1637(02)00006-5. [DOI] [PubMed] [Google Scholar]
- Ruotolo R, Grassi F, Percudani R, Rivetti C, Martorana D, Maraini G, Ottonello S. Gene expression profiling in human age-related nuclear cataract. Mol. Vis. 2003;9:538–548. [PubMed] [Google Scholar]
- Sage EH, Bassuk JA, Yost JC, Folkman MJ, Lane TF. Inhibition of endothelial cell proliferation by SPARC is mediated through a Ca(2 + )-binding EF-hand sequence. J. Cell Biochem. 1995;57:127–140. doi: 10.1002/jcb.240570113. [DOI] [PubMed] [Google Scholar]
- Sheets NL, Chauhan BK, Wawrousek E, Hejtmancik JF, Cvekl A, Kantorow M. Cataract- and lens-specific upregulation of ARK receptor tyrosine kinase in Emory mouse cataract. Invest. Ophthalmol. Vis. Sci. 2002;43:1870–1875. [PMC free article] [PubMed] [Google Scholar]
- Spector A. Aging of the lens and cataract formation. In: Sekuler R, Kline D, Dismukes K, editors. Aging and Human Visual Function. Alan Liss; New York: 1982. [Google Scholar]
- Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995;9:1173–1182. [PubMed] [Google Scholar]
- Wulfkuhle JD, Donina IE, Stark NH, Pope RK, Pestonjamasp KN, Niswonger ML, Luna EJ. Domain analysis of supervillin, an F-actin bundling plasma membrane protein with functional nuclear localization signals. J. Cell Sci. 1999;112:2125–2136. doi: 10.1242/jcs.112.13.2125. [DOI] [PubMed] [Google Scholar]
- Zhang W, Hawse J, Huang Q, Sheets N, Miller KM, Horwitz J, Kantorow M. Decreased expression of ribosomal proteins in human age-related cataract. Invest. Ophthalmol. Vis. Sci. 2002;43:198–204. [PMC free article] [PubMed] [Google Scholar]


