Abstract
Background & Aims
Intestinal adaptation in short bowel syndrome (SBS) consists of increased epithelial cell (EC) proliferation as well as apoptosis. Previous microarray analyses of intraepithelial lymphocytes (IEL) gene expression after SBS showed an increased expression of angiotensin converting enzyme (ACE). Because ACE has been shown to promote alveolar EC apoptosis, we examined if IEL-derived ACE plays a role in intestinal EC apoptosis.
Methods
Mice underwent either a 70% mid-intestinal resection (SBS group) or a transection (Sham group) and were studied at 7 days. ACE expression was measured, and ACE inhibition (ACE-I, enalaprilat) was used to assess ACE function.
Results
IEL-derived ACE was significantly elevated in SBS mice. The addition of an ACE-I to SBS mice resulted in a significant decline in EC apoptosis. To address a possible mechanism, tumor necrosis factor alpha (TNF-α) mRNA expression was measured. TNF-α was significantly increased in SBS mice, and decreased with ACE-I. Interestingly, ACE-I was not able to decrease EC apoptosis in TNF- α knockout mice.
Conclusions
This study shows a previously undescribed expression of ACE by IEL. SBS was associated with an increase in IEL-derived ACE. ACE appears to be associated with an up-regulation of intestinal EC apoptosis. ACE-I significantly decreased EC apoptosis.
Keywords: angiotensin converting enzyme, intraepithelial lymphocytes, apoptosis, tumor necrosis factor alpha, epithelial cells
INTRODUCTION
Short bowel syndrome (SBS) after a massive small bowel resection results in a series of adaptive processes 1–3. A number of growth factors and nutrients may help to mediate these changes 1, 4–7. In addition to an increase in functional absorption, adaptation also consists of an increase in villus length and crypt depth, and an increased number of microvilli. This adaptation requires a remodeling of the cryptvillus complex and includes significant increases in both epithelial cell (EC) proliferation as well as EC apoptosis 8–10. The regulation of gastrointestinal adaptation is complex and the precise factors which guide this adaptive process remain unclear 11. A number of local and distant trophic factors contribute to this adaptation 5, 12–15. Intraepithelial lymphocytes (IEL) also have a role in this adaptive process. The gamma delta T-cell receptor sub-population of the IEL, as an example, express the trophic molecule keratinocyte growth factor (KGF), which is up-regulated during intestinal mucosal injury 16, and following massive small bowel resection 17. To further elucidate the relationship between IEL and the homeostasis of the intestinal epithelium, and to identify IEL-derived factors contributing to structural intestinal adaptation in SBS, IEL-gene analysis (microarray assays) were performed 1 week after massive small bowel resection using a mouse model. The focus of analysis was on genes which have a role in affecting EC proliferation or apoptosis pathways. A detailed analysis of these findings has been previously reported 18. In this previous report, IEL-derived expression of angiotensin converting enzyme (ACE) was examined, as it was found to be significantly elevated after SBS creation. Interestingly, ACE has been shown to have a significant role in promoting apoptosis in a number of organs including pulmonary alveolar epithelial cells19 , renal epithelial cells20 and cardiac myocytes 21. ACE has been previously detected in the mucosal epithelium in human, swine, rat, as well as mouse intestine 18, 22–27; however, whether ACE has a functional role in mediating EC apoptosis in the gastrointestinal mucosa has not been evaluated. This study was designed to confirm the expression of ACE and understand its role in small bowel during EC turnover.
METHODS
Animals
Male, 2 month old, C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were maintained in a 12-hour day-night rhythm at 23° C and a relative humidity of 40%–60%. 24 hours before surgery chow was exchanged to micro-stabilized rodent liquid diet (TestDiet, Richmond, IN). Anesthesia was achieved using an injection of ketamine (87 mg/kg) and rompun (13 mg/kg) given together intramuscularly. For some experiments B6;129S6-Tnftm1Gkl (TNF-α-knockout mice, Jackson Labs) were used. Experiments were approved by the University Committee on Use and Care of Animals at the University of Michigan.
Animal Model
Short Bowel Syndrome (SBS) Model
A 70% mid-small bowel resection was performed similar to that previously described 28. This consisted of a resection of the bowel between 3.5cm distal to the ligament of Treitz and 3.5cm proximal to the ileocecal valve, and was followed by an end-to-end jejunoileal anastomosis with 8-0 absorbable suture (Vicryl, Ethicon Corporation, USA),.
Sham operation
Mid-small bowel was transected and re-anastomosed without bowel resection.
Post-Surgical Care
Postoperatively a subcutaneous bolus of 3 ml 0.9% saline solution was given to both groups in order to maintain hydration status. Mice were allowed ad libitum water and liquid diet after surgery 28. Mice were sacrificed using CO2 at 7 days post-surgery, and the intestine was harvested.
Treatment with ACE inhibitor
The ACE inhibitor (ACE-I) enalaprilat, (0.6 mg/kg/day i.p. Abbott Laboratories, North Chicago, IL) was given to a separate group of Sham and SBS mice; starting one day prior to the surgery. In some experiments, mice were matched to a control group of non-operated mice; and were studied after a 7 day period.
Histology
A 0.5 cm segment of mid-small bowel (at least 2 cm away from the anastomosis) was fixed in 10% formaldehyde, and processed for hematoxylin and eosin staining. Villus height and crypt depth were measured using a calibrated micrometer. Each measurement consisted of a mean of 16 different low power fields.
Epithelial cell apoptosis assays
TUNEL staining was used for EC apoptosis assays. Briefly, paraffin-embedded tissue was assayed with TUNEL (Terminal deoxynucleotidyl transferase Biotin-dUTP Nick End Labeling) staining, according to manufacturer’s instructions (ApopTag InSitu Apoptosis Detection Kit, Serological Corporation, Norcross, GA), with slight modification. Slides were incubated with only one-third of the recommended concentration of TdT enzyme, in order to avoid over-staining. Apoptosis rate (percent of EC showing apoptosis) was assessed with TUNEL staining and by morphological criteria (nuclear margination, chromatin and cytoplasmic condensation, shrinkage from neighboring cells, and formation of apoptotic bodies with nuclear and cytoplasmic fragmentation) 29. Each assessment of apoptosis consisted of the mean of a minimum of 16 different crypt-villus-complexes per animal, and a minimum of 5 animals per experimental set. Scoring of apoptosis was similar to that described by Marshman, et 30. A Nikon TS-100 microscope was used at 400X power, and images were digitally recorded with an Evolution MP 5.1 CCD camera. Sections were selected that were completely longitudinal along the crypt/villus axis. In general, expression of EC apoptosis is referred to as the total number of apoptotic cells along this axis per 100 EC. In some cases a more detailed analysis of the location of apoptosis was performed using previously established techniques 30. For this, apoptosis along the villi were differentiated between the lower one-third of the villi and upper one-third of the villi. Apoptosis was recorded as the number of apoptotic cells per 100 EC. For apoptosis in the crypts, cells in each crypt were numbered starting at the base of the crypt column, and the number of apoptotic cells at each position was recorded as a ratio of all counted cells at this position 30.
Epithelial cell proliferation
Mice were injected intraperitoneally with 5-bromo-2-deoxyuridine (BrdU, 50 mg/kg, Roche Diagnostic Corporation, IN) 1 h before mice were sacrificed. Paraffin-embedded sections of 5 μm thickness were deparaffinized with xylene. Immunohistochemistry was done by using a BrdU In-Situ detection Kit according manufacturer’s guidlines (BD PharMingen, San Diego, CA). Briefly, endogenous peroxidase was quenched with 3% H2O2. Slides were then incubated with biotinylated anti-BrdU antibody in a 1:10 dilution, washed and then incubated with Streptavidin-horse radish peroxidase. Slides were then exposed to DBA substrate and counter-stained with hematoxylin. An index of the crypt cell proliferation rate was calculated by the ratio of the number of crypt cells incorporating BrdU to the total number of crypt cells. The total number of proliferating cells per crypt was defined as a mean of proliferating cells in 10 crypts (counted at 45X magnification).
Mucosal cell isolation
Isolation of mucosal cells was performed using a previously described protocol 31. This included isolation of cells with an extraction buffer (l mM EDTA, lmM dithiotheritol in phosphate buffer saline), and purification in 30% isotonic Percoll (Pharmacia, Piscataway, NJ). Viability exceeded 95% using trypan blue exclusion staining.
IEL purification
Isolation of purified IEL from mucosal cell isolates was performed by direct magnetic separation. This allowed the detection of mRNA changes in a purified IEL population. Magnetic beads conjugated with antibody to CD45 (pan-lymphoid marker) were used to segregate IEL from EC (BioMag SelectaPure Anti-Mouse CD 45R Antibody Particles, Polyscience Inc., Warrington, PA). Magnetic separation was performed twice to further deplete EC. Final IEL purity was greater than 99% by flow cytometry.
IEL staining and sorting
IEL were stained with antibodies to T-cell receptor (TCR)-α β(H57, Invitrogen Corporation Life Technologies, Carlsbad, CA), TCR-γδ (GL3, Invitrogen), CD4 (RM4-5, BD PharMingen, San Diego, CA) or CD8 α (53-6.7, PharMingen). Isotype control antibodies were used to adjust gating. IEL sub-populations were sorted using an EPICS Elite (Coulter, Miami, Florida) flow cytometer. Sorted IEL were kept at 4ºC until RNA isolation.
Isolation of total RNA
A guanidine isothiocyanate/chloroform extraction method was performed using Trizol (Gibco BRL, Gaithersburg, MD) according to manufacturer’s directions.
Reverse transcriptase polymerase chain reaction (RT-PCR)
mRNA (poly-A positive) was reversed transcribed into cDNA following a standard protocol 31. Specific primers for selected gene sequences were designed using proprietary software (LaserGene, DNAStar, Inc, Madison, WI). PCR and gel were run under standard conditions 32. To ensure that DNA product was generated at the exponential portion of the product curve 28 cycles were used for somatic ACE, 32 cycles for TNF-α; and 34 cycles for angiotensin II receptors. Gel bands were analyzed by DNA sequencing to ensure the correct product. Kodak EDAS System (Rochester, NY) was used for imaging and quantification. Results were expressed as the ratio of the investigated mRNA over β-actin mRNA expression.
Real-time PCR
Real-time PCR was run to better quantify actual changes in ACE expression. cDNA was also used for real time PCR. The same primers for somatic ACE and β-actin were used as for conventional PCR, SYBR Green I was utilized for fluorescence. PCR was run with the following steps: 94º C for 15 seconds, 66º C for 15 seconds, and 72 º C for 25 seconds. A cDNA standard curve was created with defined concentrations of cDNA. This allowed for an extrapolation of the numbers of copies using the following formula: cDNA copies/ml = DNA concentration (mg/ml) x (106 pg/mg) x (pmol/660 pg) x (1/sequence size [bp]) x (1012 mol/103 ml) x 6.023 x 1023. Ct- values of ACE and β-actin were converted to numbers of cDNA copies, and results expressed as the ratio of ACE over β-actin expression.
Immunoblot analysis
Protein was extracted from isolated IEL, and immunoblots performed using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA) with standard methods 33. Purified biotinylated anti-mouse ACE (R&D Systems, Inc., Mineapolis, MN) (at a concentration of 0.1 μg/ml diluted in blocking solution (Zymed Laboratories, Inc., San Francisco, CA) was used for ACE detection. This was followed by an application of horse radish peroxidasestreptavidin conjugate (1:5000 dilution; Zymed). Expression for β-actin was determined in the same fashion by re-probing membranes with purified anti-mouse β-actin (1:8000, Sigma-Aldrich, St. Louis, MO). The peroxidase-conjugated second antibody is goat anti-mouse IgG (1:5000 in blocking solution) from Santa Cruz Biotechology (Santa Cruz, CA). Results are expressed as the ratio of ACE over β-actin protein expression.
Statistical analysis
Data are expressed as mean ± standard deviation. These results were analyzed using ANOVA with least significant difference testing for post-hoc analysis of significance. For assessment of changes in apoptosis between crypt cells using an apoptotic index, a Fisher Exact test was used. Statistical significance was set at P<0.05.
RESULTS
Changes in ACE expression after bowel resection
Increased ACE expression after bowel resection
To confirm previous microarray observations, IEL-derived ACE expression was measured after bowel resection using both real-time PCR and Western blot. Real-time PCR showed a significantly increased expression of IEL-derived ACE mRNA in the SBS group when compared to the Sham group (P<0.05, N=5) (Figure 1). Western immunoblotting showed a 24% increase in the SBS (2.07 ± 0.59) group, and was significantly (P<0.05) higher than the Sham (1.69 ± 0.14) group (Figure 2).
Figure 1.
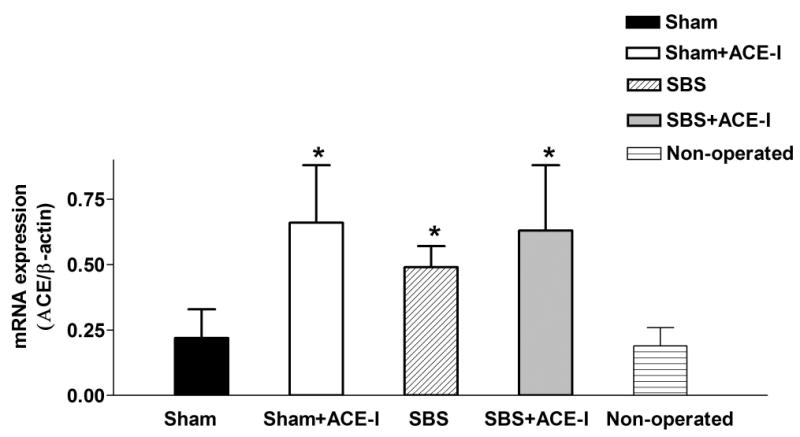
ACE mRNA expression: mRNA expression of angiotensin converting enzyme (ACE) by real-time polymerase chain reaction techniques. Results are the number of ACE-cDNA copies over β-actin-cDNA copies (mean ±SD, data from at least 5 mice per group). Abbreviations: IEL, intraepithelial lymphocytes; ACE-I, angiotensin converting enzyme inhibitor, SBS, short bowel syndrome. *P<0.05 Sham vs. SBS.
Figure 2.
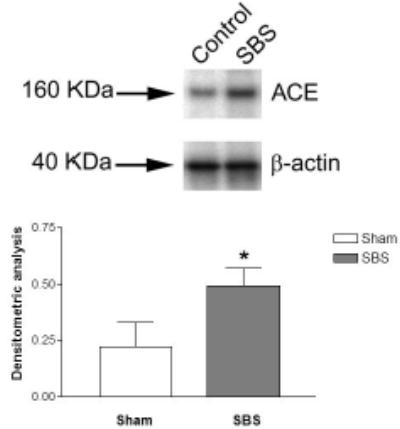
ACE protein expression: Western immunoblots from a representative mouse in the Sham (Control) and SBS groups. Both ACE (at 160 KDa) and beta actin (β-actin, 40 KDa), used as a control, are shown.
Changes of ACE expression in IEL sub-populations
Because of the unique phenotype of the IEL, cells were sorted by phenotype markers. This allowed a detailed examination of the specific subpopulations of the IEL that expressed or did not express ACE, and the differential expression of ACE in IEL sub-population after bowel resection. mRNA expression of ACE was detected in all sorted IEL sub-populations (Table 1). Although a greater expression of ACE was noted in the CD8α sub-population, no statistical difference was noted among groups.
Table 1.
ACE mRNA expression in IEL sub-populations.
| IEL Sub-population | ACE mRNA expression |
|---|---|
| αβ– TCR | 0.63 ±0.01 |
| γδ–TCR | 0.57 ±0.15 |
| CD4 | 0.77 ±0.13 |
| CD8α | 0.86 ±0.13 |
IEL were derived from adult, untreated mice, and were purified using flow cytometric sorting. Note the fairly uniform expression of ACE in each sub-population. Abbreviations: TCR, T-cell receptor. Data is derived from semi-quantitative PCR based on the relative expression of beta actin, and is a mean (± SD) of IEL derived from 3 individual sortings.
Effects of ACE inhibition on small intestine
To understand the significance of increased ACE expression on SBS adaptive changes, the ACE inhibitor (ACE-I) enalaprilat was given to a separate group of Sham and SBS mice. These mice were matched to a control group of untreated mice, and were studied after a 7 day period.
Weight changes in the SBS model
All groups of mice showed some degree of weight loss following surgery; however, the loss in each of these plateaued by the latter two days of the study period. Pre-operative weights in the Sham, SBS and SBS+ACE-I groups were (grams): 25.5±1.3, 25.0±1.5 and 24.1±1.2, respectively. One week following surgery, weights were (grams): 24.2±2.6, 20.8±1.7, and 21.6±1.7, respectively; and represented a −4.7%, −16.5% and a −10.7% weight loss, respectively. The loss of weight in the SBS group was significantly greater than the Sham or SBS+ACE-I groups (p=0.001 and P=0.01, respectively); however, the loss of weight in the SBS+ACE-I group was not significantly different than the Sham operated group (P=0.07).
ACE-I administration significantly altered mucosal structural adaptation
Bowel adaptation in the SBS model: Bowel resection (i.e., the SBS model) led to significant intestinal hypertrophy (Table 2). The villus height was noted to significantly increase in the SBS group when compared with the Sham group. The crypt depth increased significantly after bowel resection (P=).
Table 2.
Epithelial cell apoptosis and crypt-villus dimensions
| Group | EC Apoptosisa (%) | Villus height (μm) | Crypt depth (μm) |
|---|---|---|---|
| Non-operated Control | 1.6 ± 0.7 | 310 ± 42 | 93 ± 18 |
| Sham | 2.7 ± 1.0 | 353 ± 40 | 77 ± 10 |
| Sham+ACE-I | 1.9 ± 0.4† | 340 ± 20 | 87 ± 6 |
| SBS | 4.3 ± 1.3* | 556 ± 66* | 113 ± 14* |
| SBS+ACE-I | 1.8 ± 0.5‡ | 552 ± 56* | 145 ± 22‡ |
Summary (mean ±SD, from at least 6 mice per group) of results of ACE-inhibitor (ACE-I) studies. Sham+ACE-I was used as an additional control to assess the effects of ACE-I in the absence of a SBS.
Results of TUNEL analysis (number of apoptotic cells per 100 epithelial cells).
Abbreviations: IEL, intraepithelial lymphocytes; ACE-I angiotensin converting enzyme inhibitor; EC, epithelial cells.
P<0.05 sham vs. SBS
P<0.05 Sham vs. Sham + ACE-I
P<0.05 SBS vs. SBS+ACE-I.
Alteration of adaptation with ACE-I: ACE-I administration led to a further increase in this intestinal hypertrophy (Table 2). ACE-I administration to SBS mice (SBS+ACE-I) resulted in a significant (P=0.02), additional, increase in crypt depth beyond that seen in untreated SBS mice. Villus height was unchanged compared to the untreated SBS group. Those mice undergoing a sham resection and treated with ACE-I (Sham+ACE-I) showed no significant alteration in histological features in either crypt depth or villus height when compared with the Sham group.
ACE-I significantly decreased SBS-associated apoptosis
Changes in EC apoptosis with surgical manipulation: Rates of EC apoptosis significantly increased in both the Sham and SBS groups compared to non-operated mice (Figures 3 and 4, and Table 2). Rates of apoptosis were highest in the SBS group (Table 2), however, both forms of surgical manipulation (Sham and SBS groups) led to increases in EC apoptosis compared to non-operated mice. Apoptosis was increased both in the crypt (Figure 3) as well as the villi (Figure 4).
Figure 3.
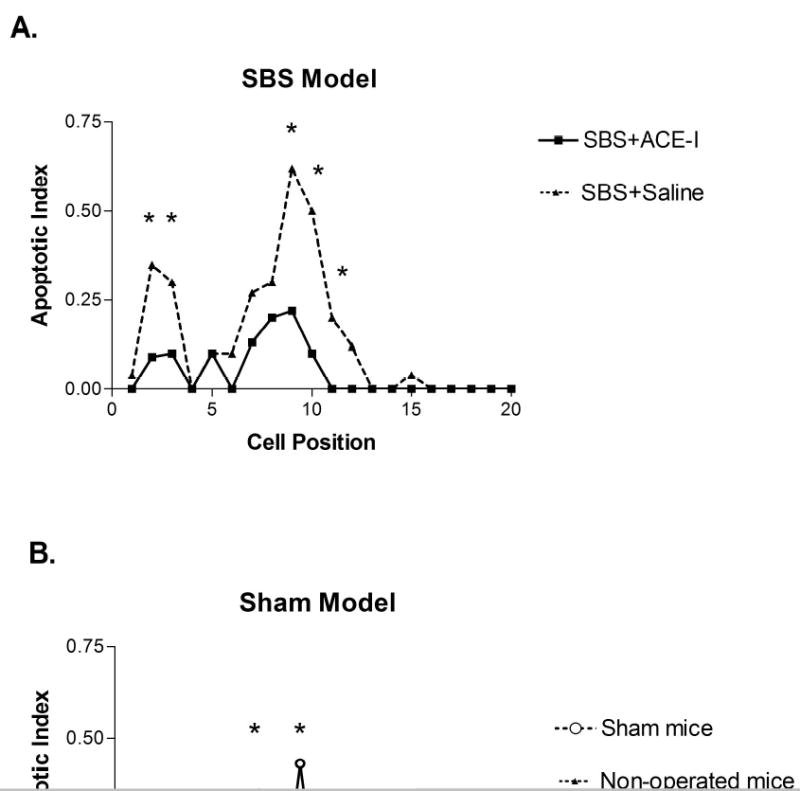
Crypt cell apoptosis: Cell positional distribution of apoptosis in small intestinal crypts from mice in each treatment group. The first cell position is at the base of the crypt, and apoptosis is reported as an index of apoptotic cells to non-apoptotic cells found in that cell position. A minimum of 10 half-crypts (one side of a crypt) were counted per animal, and at least 5 animals for each study group were assessed. * P<0.05 comparing the two groups of mice.
A) Apoptotic index is shown for the short bowel syndrome (SBS) group with or without angiotensin converting enzyme (ACE-I) treatment.
B) Apoptotic index is shown for the Sham treated (transection without resection) and non-operated mouse groups.
Figure 4.
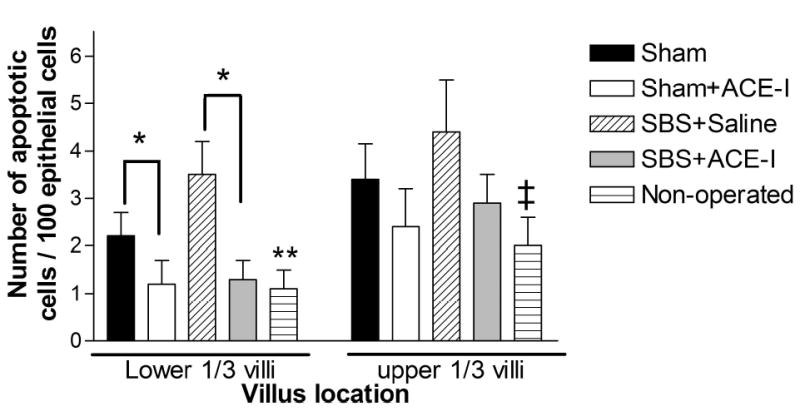
Villus apoptosis: Apoptotic rate (number of apoptotic cells per 100 epithelial cells) is shown for the experimental groups. Data is from a minimum of 5 mice in each group, and at least 10 villi from each mouse.
Left portion of the graph: Data from the lower one-third of the villi, *P<0.05 Sham vs. Sham + ACE-I, and SBS group vs. SBS+ACE-I. **P<0.01 Non-operated mice compared to Sham operated and SBS mice.
Right portion of the graph: Data from the upper one-third of the villi. ‡P<0.05 Non-operated mice vs. SBS and Sham groups. ACE-I resulted in a reduction in apoptosis for the SBS and Sham groups, but this was not significant (P=0.15).
Alteration of EC apoptosis with ACE-I: Because of the known action of ACE-I on apoptosis in the cardiac and pulmonary systems, intestinal EC apoptosis was examined by using ACE-I. ACE-I resulted in a significant decline in EC apoptosis rates was observed in both the Sham and SBS groups. EC apoptosis decreased 2.4-fold when compared with the untreated SBS group (Table 2). These levels declined to those similar to that observed in non-operated mice (Table 2). The action of ACE-I on lowering the rate of EC apoptosis varied with the location along the crypt-villus axis. A significant decline in the rate of apoptosis was noted in both the crypt and lower one-third of the intestinal villi (Figures 3 and 4). Whereas, only a slight decline in EC apoptosis was noted with ACE-I in the distal one-third of the intestinal villi (Figures 3 and 4).
ACE-I administration significantly increased epithelial cell proliferation
To better understand the implications of ACE-I administration on the adaptation of ECs during the early stages of SBS, EC proliferation was assessed with BrdU incorporation. EC proliferation was much greater (P<0.01) in SBS mice (0.24 ± 0.07) compared to the Sham group (0.12 ± 0.02 (number of crypt cells incorporating BrdU to the total number of crypt cells)). Administration of ACE-I led to a significant (P<0.001) increase in EC proliferation in SBS mice (0.39 ± 0.06) compared to SBS mice which did not receive ACE-I (0.24 ± 0.07).
ACE-I administration significantly increased ACE expression
Administration of ACE-I led to a significant increase in both IEL-derived ACE mRNA expression in both Sham (0.66 ± 0.22) and SBS (0.63 ± 0.25) groups of treated mice as compared to untreated Sham and non-operated mice (P<0.05, P<0.01, respectively, see Figure 1). A 24% increase in ACE mRNA expression between SBS and SBS+ACE-I groups, but the difference was not significant.
Mechanism of ACE-mediated EC apoptosis
Alteration in angiotensin II receptor expression
ACE is mediated via angiotensin II (ANG II) via type 1 (subtypes 1a and 1b) and type 2 receptors 21. To address an ANG II-mediated mechanism of ACE and/or ACE-I on EC apoptosis, EC mRNA expression of type 1a, type 1b and type 2 ANG II receptors were measured (Table 3). For this purpose 3 groups of mice were investigated, Sham, SBS and SBS+ACE-I. ANG II receptor type 2 expression decreased significantly with administration of ACE-I (P<0.05 SBS vs. SBS+ACE-I). No significant difference in mRNA expression was found between untreated Sham and SBS mice. ANG II receptor type 1a mRNA expression showed a decrease in the SBS+ACE-I group compared to the other groups; however, this difference did not approach significance. Type 1b receptor significantly decreased (P=0.01) with ACE-I treatment in SBS mice compared to untreated SBS mice. No significant difference in mRNA expression was found between untreated Sham and SBS mice.
Table 3.
Angiotensin II (ANG II) receptor type 1 and type 2 mRNA expression
| Group | ANG II receptor, type 1a | ANG II receptor, type 1b | ANG II receptor, type 2 |
|---|---|---|---|
| Sham | 0.13 ±0.14 | 0.14 ±0.17 | 0.41 ± 0.19 |
| SBS | 0.05 ± 0.05 | 0.14 ± 0.03 | 0.44 ± 0.09 |
| SBS+ACE-I | 0.03 ± 0.03 | 0.03 ± 0.03† | 0.30 ± 0.20* |
Expression of the ANGII receptor type 1a and 2 were significantly decreased with administration of ACE-I (P<0.05 for SBS vs. SBS + ACE-I). Data are from semi-quantitative RT-PCR based on the relative expression of the target gene to beta actin expression (mean ±SD, N=6).
P<0.05 for SBS+ACE-I group vs. SBS group.
P=0.001 for SBS+ACE-I group vs. SBS group.
Alteration of pro-inflammatory cytokine gene expression
Because ACE-I may decrease apoptosis in the cardio-pulmonary system via a down-regulation of pro-inflammatory cytokine expression 34, 35, a similar mechanism was examined in the intestinal mucosa. To address this, expression of mRNA from a number of potential pro-inflammatory cytokines were examined: IL-1a, IL-6, IL-12a and 12b, tumor necrosis factor alpha (TNF-α) and interferon gamma (Figure 5). Three groups were studied: Sham, SBS and SBS+ACE-I. Of note, TNF-α showed a significant rise in mRNA expression in the SBS group (0.70 ± 0.19) compared to Sham levels (0.41 ± 0.13, P<0.01). SBS mice treated with ACE-I showed a significant decline in TNF-α mRNA (0.51 ± 0.07, P <0.05) compared to untreated SBS mice; and levels were not significantly different than Sham levels. A small decline in IL-1a was also noted, but this was not significant.
Figure 5.
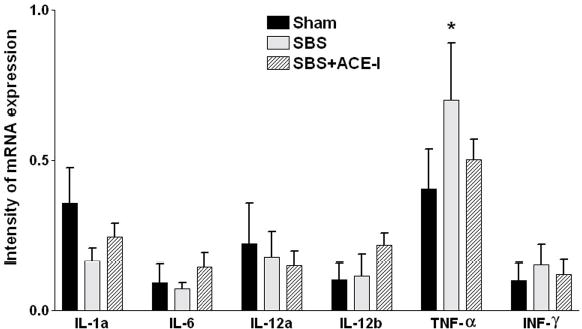
IEL Cytokine expression: Cytokine modulation of intraepithelial lymphocytes (IEL) 1 week postoperatively in Sham, SBS and SBS+ACE-I groups. Intensity of mRNA expression is given as the relative value compared to beta actin expression. Of note, TNF-α showed a significant rise in mRNA expression in the SBS group compared to Sham levels. SBS mice treated with ACE-I showed a decline in TNF-α mRNA to Sham levels. A small decline in IL-1a was also noted, but this was not significant. *P<0.01 Sham vs. SBS, and P<0.05 for SBS vs. SBS+ACE-I.
TNF-α and ACE-mediated EC apoptosis
The increase in TNF- α in SBS mice, and the observed decline in TNF- α expression with ACE-I treatment suggested this pathway may be needed for ACE-I to mediate its blockade of EC apoptosis. To further evaluate the mechanism by which ACE-I treatment blocks EC apoptosis, TNF-α-knockout (TNF-α−/−) mice were used. For this, a series of Sham operated mice (intestinal transection and re-anastomosis of the mid-small bowel) were studied in this section. This model was used as TNF-α −/−mice were less tolerant of major bowel resections, yet increased levels of EC apoptosis were still observed in Sham mice. Two groups of mice were investigated: 1) Sham operated TNF-α −/− mice; and 2) Sham operated TNF-α −/−mice treated with ACE-I. Rates of apoptosis in all TNF-α −/−mice were consistently lower that wild-type mice (compare to Table 2). Interestingly, we found that ACE-I administration had no effect on the EC apoptosis in TNF-α −/−mice. Rates of apoptosis were determined in each group. Sham TNF-α −/− mice treated with ACE-I showed an EC apoptotic rate of 1.7 ± 0.3% with TUNEL assay. Sham TNF-α −/−mice which did not receive ACE-I had an EC apoptotic rate of 1.8 ± 0.3% with TUNEL staining (P>0.05). This suggests that the presence of TNF-α was essential for ACE-mediation of EC apoptosis.
Alteration of Fas and Fas ligand expression
Based on cardio-pulmonary literature, Fas and Fas ligand (FasL) have been suggested to mediate ACE apoptotic actions 35. EC mRNA expression of Fas and IEL mRNA expression of FasL were measured (using magnetic bead isolation, Table 4). EC-derived Fas-expression increased slightly (but not significantly) in SBS mice (1.12 ± 0.13) compared to Sham mice (0.96 ± 0.08). The expression of Fas decreased with ACE-I treatment in SBS mice (Table 4), but the decline was also not significant. IEL-derived FasL did not significantly change between Sham; SBS; and SBS +ACE-I groups.
Table 4.
Fas and Fas ligand mRNA expression
| Group | Fas | FasL |
|---|---|---|
| Sham | 0.96 ± 0.08 | 0.36 ± 0.25 |
| SBS | 1.12 ± 0.13 | 0.24 ± 0.20 |
| SBS+ACE-I | 1.00 ± 0.17 | 0.24 ± 0.11 |
Data shown are Fas and Fas ligand mRNA expression. Fas is expressed by epithelial cells and FasL is expressed by intraepithelial lymphocytes. Note there was a slight increase in Fas in the SBS group, and a decline with ACE-I treatment. This approached, but did not reach significance (P=0.07). FasL expression did not appreciably change in any of the groups. Data (mean ± SD, N=6 per group) are from semi-quantitative RT-PCR analysis based on the relative expression of beta actin
DISCUSSION
In this study, several novel findings are reported (Summarized in Table 5). First, the increase in IEL-derived ACE expression after formation of SBS was confirmed with both real-time PCR and Western immunoblots. Additionally, ACE expression was found in all four major T-lymphocyte IEL sub-populations: αβ–TCR+ and γδ–TCR+, as well as both CD4+ and CD8+ IEL. This increase in IEL-derived ACE may be associated with an up-regulation of intestinal EC apoptosis after bowel resection. ACE-I was used to assess the functional role of ACE in the mucosa. We observed that the administration of ACE-I significantly decreased EC apoptosis. Additionally, the finding that ACE-I was ineffective in reducing EC apoptosis in TNF-α knockout mice suggests that TNF-α may be involved in this ACE-mediated EC apoptosis.
Table 5.
Summary of the major findings of ACE-I action after SBS formation
| Parameter studied after formation of SBS | Action of ACE-I |
|---|---|
| Crypt-villus architecture | Increased crypt depth |
| Epithelial cell apoptosis | Decreased |
| Epithelial cell apoptosis in TNF-α knockout mice | No effect |
| Epithelial cell proliferation | Increased |
| ACE expression | Increased |
ACE has been identified as a membrane-bound enzyme (peptidyl dipeptidase) in several immunologic cells, including lymphocytes and macrophages. T-lymphocytes have been shown to contain high levels of ACE, approximately 28 times more per cell than monocytes 36. High levels of ACE have also been found along the intestinal mucosa in human, swine, rat, as well as mouse intestine 22, 23 24–27. Immunohistochemical studies 23, 25 have shown that there is strong expression of ACE identified in non-vascular sites of human and mouse intestinal mucosa; however, ACE expression has not been previously described in the IEL. ACE protein is located on the microvillus surface of the epithelium, and notably on the luminal surface of the small intestine. Despite this well-appreciated presence of ACE within the gastrointestinal mucosa – the functional role of the RAS in the intestinal mucosa has not been well defined. ACE has been recently found to mediate the induction of alveolar EC apoptosis 19, 37. Administration of ACE-I has led to reduced apoptotic rates in alveolar EC, renal EC and cardiac myocytes 20, 35, 38. Such action may have great importance in preservation of the function of these organs in a number of disease processes. Based on our finding of increased ACE mRNA and protein expression within the IEL, we then hypothesized that ACE may also have a relevant role in the modulation of EC apoptosis within the gastrointestinal mucosa. Administration of the ACE-I enalaprilat to SBS mice reversed the increase in EC apoptosis observed with SBS alone, bringing levels to that of non-operated mice. Interestingly, this observed ACE-I- associated decline in EC apoptosis was predominately in the crypt region and lower one-third of the villi. Morphologically, this decline in EC apoptosis was reflected in a significant increase in crypt depth; whereas there was relatively little change in total villus length. Crypt cell apoptosis has been shown to progress quite differently compared to apoptosis along the villi 30. This also suggests that ACE may play a key role in the remodeling of the crypt during the early phases of SBS adaptation. This location of EC apoptosis was different from the finding of apoptosis in the villus tips, as seen with the normal process of villus growth and in some models of intestinal ischemia 39. Importantly, however, EC apoptosis in other mouse models of intestinal injury as well as in other studies of SBS have been shown to predominate in the crypt region 30, 40. Thus it appears that different processes will greatly affect the location of apoptosis along the crypt-villus axis. The observed changes in weight loss – and particularly the lower degree of weight loss in the SBS+ACE-I group may suggest an improvement in SBS adaptation in the ACE-I treated group, although specific absorption studies would need to be performed in future studies to prove this. It was also interesting to note that EC proliferation was also augmented in the SBS+ACE-I group compared to the SBS group. Interestingly, ACE may also demonstrate effects on proliferation, as well as apoptosis 20. This ACE-I associated augmentation of EC proliferation, may help to augment SBS adaptation, although this will need future work to confirm.
The mechanism by which ACE-I affects apoptosis is not fully understood. Several studies have shown a change in lymphocyte cytokine expression with the use of ACE-I. Schindler, et al. investigated the in vitro synthesis of TNF-α from human peripheral blood mononuclear cells in the presence of the ACE-I captopril. These investigators showed a dose-dependent suppression of the synthesis of TNF-α41. Our group has previously showed that IEL-derived TNF-α is increased after the creation of a SBS model in mice 32. Our current study confirmed this finding. Interestingly, we further observed a significant decline in TNF-α with ACE-I. To further address the possibility of a TNF-α-dependent mechanism for the reduction of apoptosis with ACE-I, TNF-α −/− mice were used. TNF-α −/− mice subjected to a Sham (transection) operation demonstrated no change in EC apoptotic rates with or without the administration of the ACE-I enalaprilat.. This strongly suggests that ACE-mediated EC apoptosis within the gastrointestinal tract is dependent on TNF-α. It also suggests that the observed down-regulation in TNF-α expression by enalaprilat could be a major mechanism by which ACE-I mediates its anti-apoptotic action. This effect of ACE-I on TNF-α expression has also been shown by Fukuzawa, et al using blood mononuclear cells. In their study the administration of captopril, delapril or cilazapril was shown to inhibit the expression of TNF-α42. Ortiz, et al studied modulation of TNF-α expression in a mouse model of pulmonary hypertension induced by bleomycin. In this model there was an enhanced expression of TNF-α expression in the lung. Administration of enalaprilat inhibited lung-TNF-α expression 43.
ACE may work via the renin-angiotensin-system by producing angiotensin II (ANG II). Wang, et al 44 found that ANG II was a potent inducer of apoptosis in alveolar EC. It has been shown that ANG II acts through ANG II type 1 and type 2 receptors 21. Although variable responses may be seen in different tissues, type 1 receptors can mediate the effects of smooth muscle cell proliferation and growth 20, 45. Others have also associated type 1 receptors are also important mediators for apoptosis, although the type of mediation may vary with different tissues. In vascular smooth muscles cells, apoptosis was significantly reduced in ANG II type 1a knockout mice 46. Conversely, others have shown that the type 1a receptor is needed for the mediation of alveolar EC apoptosis 47. Type 2 receptors may enhance cell differentiation 48 as well as stimulate apoptosis 49. In our study we found that administration of enalaprilat to SBS mice had led to a significant decline in type 1b and type 2 receptors, as well as a large, but non-significant decline in the type 1a receptor. This may imply that ACE mediation of EC apoptosis, and its prevention with enalaprilat, is via a modulation of the expression of ANG II receptors. Further assessment of this pathway will be needed in the future to confirm this observation. ACE may be expressed by both IEL (as observed in our current study) as well as by EC, themselves 50. Although there was a marked increase in ACE expression by IEL in our SBS model, future studies will need to be done to better define the amount of apoptosis mediated by IEL-derived versus EC-derived ACE in the modulation of EC apoptosis following SBS formation.
Another signaling pathway for EC apoptosis is via the Fas/Fas ligand (FasL) system 51. Wang, et al showed that activation of Fas stimulates alveolar EC to synthesize ANG II de novo 44. Moreover, the autocrine synthesis of ANG II was found to be required for the induction of apoptosis by Fas. Apoptosis could be abrogated by ANG receptor antagonists or other blockers of ANG II function. In our study, we did not find any significant changes in Fas or FasL mRNA expression after bowel resection. Additionally, ACE-I administration after bowel resection did not lead to any significant change in either Fas, or FasL expression. These results are supported by work from Knott et al 52 and a study from Tang et al 53. Both of these investigators reported that there were no significant changes in Fas and FasL after bowel resection. These results may suggest that Fas/FasL extrinsic pathway is not involved in this SBS associated EC apoptosis.
The intrinsic pathway via the Bcl-2 family of proteins have been well studied and found to play an important role in the SBS related EC apoptosis 52–55. An increase in intestinal bax (pro-apoptotic gene) expression and a decrease in bcl-2 (anti-apoptotic gene) were found in a mouse SBS model 55, 56. Interestingly, the bcl-2 family of proteins appears to be another mediator of apoptosis by the RAS 46, 57–60. Studies have identified a link between ANGII signaling and apoptosis through this family of proteins 57–61, including a decrease in the bcl-2 : bax protein ratio in other systems. Whether IEL-derived ACE mediates apoptosis via the intrinsic pathway, including changes in the bcl-2 family, will be important areas for future investigations
Conclusion
In conclusion, SBS was associated with a previously undescribed expression of ACE by intestinal IEL. Another novel finding was that administration of the ACE-I enalaprilat reversed the increase in SBS-associated EC apoptosis; suggesting that, as observed in cardiac and pulmonary tissue, ACE-I may have a similar action on intestinal epithelial cells. Possible anti-apoptotic mechanisms of the ACE-I enalaprilat in the intestinal tract may be through RAS via suppression of TNF-α expression. It is potentially possible that SBS patients may benefit from the use of ACE-I, via a reduction of the apoptotic rate – thus facilitating the degree of adaptation. Future investigation will hopefully offer greater insight into the action of ACE on the gastrointestinal mucosa.
Acknowledgments
Supported by National Institute of Health, USA (AI44076-06), Novartis Stiftung Schweiz, Swiss National Foundation, and Swiss Society of Pediatric Surgery. This research was also supported (in part) by the National Institutes of Health through the University of Michigan’s Cancer Center Support Grant (5 P30 CA46592); with the use of the Flow Cytometry Facility,
References
- 1.Ray EC, Avissar NE, Sax HC. Growth factor regulation of enterocyte nutrient transport during intestinal adaptation. Am J Surg. 2002;183:361–71. doi: 10.1016/s0002-9610(02)00805-x. [DOI] [PubMed] [Google Scholar]
- 2.Westergaard H. Short bowel syndrome. Seminars in Gastrointestinal Disease. 2002;13:210–20. [PubMed] [Google Scholar]
- 3.Vanderhoof JA, Langnas AN, Pinch LW, Thompson JS, Kaufman SS. Short bowel syndrome. Journal of Pediatric Gastroenterology & Nutrition. 1992;14:359–70. doi: 10.1097/00005176-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Petersen Y, Burrin D, Sangild P. GLP-2 has differential effects on small intestine growth and function in fetal and neonatal pigs. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1986–93. doi: 10.1152/ajpregu.2001.281.6.R1986. [DOI] [PubMed] [Google Scholar]
- 5.Stern LE, Erwin CR, O’Brien DP, Huang F, Warner BW. Epidermal growth factor is critical for intestinal adaptation following small bowel resection. Microsc Res Tech. 2000;51:138–48. doi: 10.1002/1097-0029(20001015)51:2<138::AID-JEMT5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 6.Thiesen A, Wild G, Tappenden K, Drozdowski L, Keelan M, Thomson B, McBurney M, Clandinin M, Thomson A. The locally acting glucocorticosteroid budesonide enhances intestinal sugar uptake following intestinal resection in rats. Gut. 2003;52:252–9. doi: 10.1136/gut.52.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sukhotnik I, Lerner A, Sabo E, Krausz M, Siplovich L, Coran A, Mogilner J, Shiloni E. Effects of enteral arginine supplementation on the structural intestinal adaptation in a rat model of short bowel syndrome. Dig Dis Sci. 2003;48:1346–51. doi: 10.1023/a:1024167428092. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien DP, Nelson LA, Kemp CJ, Williams JL, Wang Q, Erwin CR, Hasselgren PO, Warner BW. Intestinal permeability and bacterial translocation are uncoupled after small bowel resection. J Pediatr Surg. 2002;37:390–4. doi: 10.1053/jpsu.2002.30807. [DOI] [PubMed] [Google Scholar]
- 9.Welters CF, Dejong CH, Deutz NE, Heineman E. Intestinal adaptation in short bowel syndrome. ANZ J Surg. 2002;72:229–36. doi: 10.1046/j.1445-2197.2002.02357.x. [DOI] [PubMed] [Google Scholar]
- 10.Falcone RA, Jr, Shin CE, Erwin CR, Warner B. The adaptive intestinal response to massive enterectomy is preserved in c-SRC-deficient mice. Journal of Pediatric Surgery. 1999;34:800–4. doi: 10.1016/s0022-3468(99)90376-7. [DOI] [PubMed] [Google Scholar]
- 11.Klein RM, McKenzie JC. The role of cell renewal in the ontogeny of the intestine. II. Regulation of cell proliferation in adult, fetal, and neonatal intestine. J Pediatr Gastroenterol Nutr. 1983;2:204–228. [PubMed] [Google Scholar]
- 12.Sham J, Martin G, Meddings JB, Sigalet DL. Epidermal growth factor improves nutritional outcome in a rat model of short bowel syndrome. Journal of Pediatric Surgery. 2002;37:765–9. doi: 10.1053/jpsu.2002.32273. [DOI] [PubMed] [Google Scholar]
- 13.Gillingham MB, Dahly EM, Carey HV, Clark MD, Kritsch KR, Ney DM. Differential jejunal and colonic adaptation due to resection and IGF-I in parenterally fed rats. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2000;278:G700–9. doi: 10.1152/ajpgi.2000.278.5.G700. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Wildhaber BE, Teitelbaum DH. 2003 Harry M. Vars Research Award. Keratinocyte growth factor improves epithelial function after massive small bowel resection. JPEN J Parenter Enteral Nutr. 2003;27:198–206. doi: 10.1177/0148607103027003198. discussion 206–7. [DOI] [PubMed] [Google Scholar]
- 15.Tappenden K, Albin D, Bartholome A, Mangian H. Glucagon-like peptide-2 and short-chain fatty acids: a new twist to an old story. J Nutr. 2003;133:3717–7320. doi: 10.1093/jn/133.11.3717. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Chou K, Fuchs E, Havran W, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Aacd Sci USA. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gammadelta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol. 2004;172:4151–8. doi: 10.4049/jimmunol.172.7.4151. [DOI] [PubMed] [Google Scholar]
- 18.Wildhaber B, Yang H, Coran A, Teitelbaum D. Gene alteration of intestinal intraepithelial lymphocytes in response to massive small bowel resection. Ped Surg Int. 2003;19:310–315. doi: 10.1007/s00383-003-1001-x. [DOI] [PubMed] [Google Scholar]
- 19.Wang R, Zagariya A, Ibarra-Sunga O, Gidea C, Ang E, Deshmukh S, Chaudhary G, Baraboutis J, Filippatos G, Uhal BD. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Physiol. 1999;276:L885–9. doi: 10.1152/ajplung.1999.276.5.L885. [DOI] [PubMed] [Google Scholar]
- 20.Siragy HM. AT(1) and AT(2) receptors in the kidney: role in disease and treatment. Am J Kidney Dis. 2000;36:S4–9. doi: 10.1053/ajkd.2000.9684. [DOI] [PubMed] [Google Scholar]
- 21.Schiffrin EL. Vascular and cardiac benefits of angiotensin receptor blockers. Am J Med. 2002;113:409–18. doi: 10.1016/s0002-9343(02)01241-x. [DOI] [PubMed] [Google Scholar]
- 22.Ward PE, Sheridan MA, Hammon KJ, Erdos EG. Angiotensin I converting enzyme (kininase II) of the brush border of human and swine intestine. Biochem Pharmacol. 1980;29:1525–9. doi: 10.1016/0006-2952(80)90603-6. [DOI] [PubMed] [Google Scholar]
- 23.Defendini R, Zimmerman EA, Weare JA, Alhenc-Gelas F, Erdos EG. Angiotensin-converting enzyme in epithelial and neuroepithelial cells. Neuroendocrinology. 1983;37:32–40. doi: 10.1159/000123512. [DOI] [PubMed] [Google Scholar]
- 24.Erickson RH, Suzuki Y, Sedlmayer A, Song IS, Kim YS. Rat intestinal angiotensin-converting enzyme: purification, properties, expression, and function. Am J Physiol. 1992;263:G466–73. doi: 10.1152/ajpgi.1992.263.4.G466. [DOI] [PubMed] [Google Scholar]
- 25.Danilov SM, Faerman AI, Printseva O, Martynov AV, Sakharov I, Trakht IN. Immunohistochemical study of angiotensin-converting enzyme in human tissues using monoclonal antibodies. Histochemistry. 1987;87:487–90. doi: 10.1007/BF00496822. [DOI] [PubMed] [Google Scholar]
- 26.Tamura K, Yokoyama N, Sumida Y, Fujita T, Chiba E, Tamura N, Kobayashi S, Kihara M, Murakami K, Horiuchi M, Umemura S. Tissue-specific changes of type 1 angiotensin II receptor and angiotensin-converting enzyme mRNA in angiotensinogen gene-knockout mice. J Endocrinol. 1999;160:401–8. doi: 10.1677/joe.0.1600401. [DOI] [PubMed] [Google Scholar]
- 27.Skidgel RA, Erdos EG. Angiotensin I converting enzyme. Adv Exp Med Biol. 1989;247A:25–8. doi: 10.1007/978-1-4615-9543-4_4. [DOI] [PubMed] [Google Scholar]
- 28.Helmrath MA, VanderKolk WE, Can G, Erwin CR, Warner BW. Intestinal adaptation following massive small bowel resection in the mouse. Journal of the American College of Surgeons. 1996;183:441–9. [PubMed] [Google Scholar]
- 29.Wilkins HR, Ohneda K, Keku TO, D’ercole AJ, Fuller CR, Williams KL, Lund PK. Reduction of spontaneous and irradiation-induced apoptosis in small intestine of IGF-I transgenic mice. American Journal of Physiology Gastrointestinal and Liver Physiology. 2002;283:G457–G464. doi: 10.1152/ajpgi.00019.2002. [DOI] [PubMed] [Google Scholar]
- 30.Marshman E, Ottewell P, Potten C, Watson A. Caspase activation during spontaneous and radiationinduced apoptosis in the murine intestine. J Pathol. 2001;195:285–292. doi: 10.1002/path.967. [DOI] [PubMed] [Google Scholar]
- 31.Kiristioglu I, Teitelbaum DH. Alteration of the intestinal intraepithelial lymphocytes during total parenteral nutrition. Journal of Surgical Research. 1998;79:91–96. doi: 10.1006/jsre.1998.5408. [DOI] [PubMed] [Google Scholar]
- 32.Yang H, Fan Y, Finaly R, Teitelbaum DH. Alteration of intestinal intraepithelial lymphocytes after massive small bowel resection. J Surg Res. 2003;110:276–86. doi: 10.1016/s0022-4804(03)00032-5. [DOI] [PubMed] [Google Scholar]
- 33.Yang H, Wildhaber B, Tazuke Y, Teitelbaum DH. 2002 Harry M. Vars Research Award. Keratinocyte growth factor stimulates the recovery of epithelial structure and function in a mouse model of total parenteral nutrition. JPEN J Parenter Enteral Nutr. 2002;26:333–40. doi: 10.1177/0148607102026006333. discussion 340–1. [DOI] [PubMed] [Google Scholar]
- 34.Akbar AN. Life (and death) in the Fas lane [comment] Gut. 1998;43:5–6. doi: 10.1136/gut.43.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odaka C, Mizuochi T. Angiotensin-converting enzyme inhibitor captopril prevents activation-induced apoptosis by interfering with T cell activation signals. Clin Exp Immunol. 2000;121:515–22. doi: 10.1046/j.1365-2249.2000.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costerousse O, Allegrini J, Lopez M, Alhenc-Gelas F. Angiotensin I-converting enzyme in human circulating mononuclear cells: genetic polymorphism of expression in T-lymphocytes. Biochem J. 1993;290:33–40. doi: 10.1042/bj2900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang R, Ramos C, Joshi I, Zagariya A, Pardo A, Selman M, Uhal BD. Human lung myofibroblast-derived inducers of alveolar epithelial apoptosis identified as angiotensin peptides. Am J Physiol. 1999;277:L1158–64. doi: 10.1152/ajplung.1999.277.6.L1158. [DOI] [PubMed] [Google Scholar]
- 38.Yu G, Liang X, Xie X, Su M, Zhao S. Diverse effects of chronic treatment with losartan, fosinopril, and amlodipine on apoptosis, angiotensin II in the left ventricle of hypertensive rats. Int J Cardiol. 2001;81:123–9. doi: 10.1016/s0167-5273(01)00539-3. discussion 129–30. [DOI] [PubMed] [Google Scholar]
- 39.Coopersmith CM, D OD, Gordon JI. Bcl-2 inhibits ischemia-reperfusion-induced apoptosis in the intestinal epithelium of transgenic mice. American Journal of Physiology. 1999;276:G677–86. doi: 10.1152/ajpgi.1999.276.3.G677. [DOI] [PubMed] [Google Scholar]
- 40.Jarboe M, Juno R, Bernal N, Knott A, Zhang Y, Erwin C, Warner B. Bax deficiency rescues resection-induced enterocyte apoptosis in mice with perturbed EGF receptor function. Surgery. 2004;136:121–6. doi: 10.1016/j.surg.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Schindler R, Dinarello C, Koch K. Angiotensin-converting-enzyme inhibitors suppress synthesis of tumour necrosis factor and interleukin 1 by human peripheral blood mononuclear cells. Cytokine. 1995;7:526–533. doi: 10.1006/cyto.1995.0071. [DOI] [PubMed] [Google Scholar]
- 42.Fukuzawa M, Satoh J, Sagara M, Muto G, Muto Y, Nishimura S, Miyaguchi S, Qiang X, Sakata Y, Nakazawa T, Ikehata F, Ohta S, Toyota T. Angiotensin converting enzyme inhibitors suppress production of tumor necrosis factor-alpha in vitro and in vivo. Immunopharmacology. 1997;36:49–55. doi: 10.1016/s0162-3109(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 43.Ortiz LA, Champion HC, Lasky JA, Gambelli F, Gozal E, Hoyle GW, Beasley MB, Hyman AL, Friedman M, Kadowitz PJ. Enalapril protects mice from pulmonary hypertension by inhibiting TNF-mediated activation of NF-kappaB and AP-1. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1209–21. doi: 10.1152/ajplung.00144.2001. [DOI] [PubMed] [Google Scholar]
- 44.Wang R, Ibarra-Sunga O, Verlinski L, Pick R, Uhal B. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2000;279:L143–51. doi: 10.1152/ajplung.2000.279.1.L143. [DOI] [PubMed] [Google Scholar]
- 45.Horiuchi M, Akishita M, Dzau V. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–621. doi: 10.1161/01.hyp.33.2.613. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki J, Iwai M, Nakagami H, Wu L, Chen R, Sugaya T, Hamada M, Hiwada K, Horiuchi M. Role of angiotensin II-regulated apoptosis through distinct AT1 and AT2 receptors in neointimal formation. Circulation. 2002;106:847–852. doi: 10.1161/01.cir.0000024103.04821.86. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Rayford H, Uhal BD. Essential roles for angiotensin receptor AT1a in bleomycin-induced apoptosis and lung fibrosis in mice. Am J Pathol. 2003;163:2523–30. doi: 10.1016/S0002-9440(10)63607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabri A, Levy B, Poitevin P, Caputo L, Faggin E, Marotte F, Rappaport L, Samuel J. Differential roles of AT1 and AT2 receptor subtypes in vascular trophic and phenotypic changes in response to stimulation with angiotensin II. Arterioscler Thromb Vasc Biol. 1997;17:257–264. doi: 10.1161/01.atv.17.2.257. [DOI] [PubMed] [Google Scholar]
- 49.Schiffrin E. Reactivity of small blood vessels in hypertension: relation with structural changes. State of the art lecture. Hypertension. 1992;19:1–9. doi: 10.1161/01.hyp.19.2_suppl.ii1-a. [DOI] [PubMed] [Google Scholar]
- 50.Naim HY. Human small intestinal angiotensin-converting enzyme: intracellular transport, secretion and glycosylation. Biochem J. 1993;296 ( Pt 3):607–15. doi: 10.1042/bj2960607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abreu-Martin MT, Palladino AA, Faris M, Carramanzana NM, Nel AE, Targan SR. Fas activates the JNK pathway in human colonic epithelial cells: lack of a direct role in apoptosis. Am J Physiol. 1999;276:G599–605. doi: 10.1152/ajpgi.1999.276.3.G599. [DOI] [PubMed] [Google Scholar]
- 52.Knott AW, O’Brien DP, Juno RJ, Zhang Y, Williams JL, Erwin CR, Warner BW. Enterocyte apoptosis after enterectomy in mice is activated independent of the extrinsic death receptor pathway. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2003;285:G404–13. doi: 10.1152/ajpgi.00096.2003. [DOI] [PubMed] [Google Scholar]
- 53.Tang Y, Swartz-Basile D, Swietlicki E, Yi L, Rubin D, Levin M. Bax is required for resection-induced changes in apoptosis, proliferation, and members of the extrinsic cell death pathways. Gastroenterology. 2004;126:220–225. doi: 10.1053/j.gastro.2003.10.077. [DOI] [PubMed] [Google Scholar]
- 54.Stern LE, Falcone RA, Jr, Huang F, Kemp CJ, Erwin CR, Warner BW. Epidermal growth factor alters the bax:bcl-w ratio following massive small bowel resection. Journal of Surgical Research. 2000;91:38–42. doi: 10.1006/jsre.2000.5897. [DOI] [PubMed] [Google Scholar]
- 55.Stern LE, Huang F, Kemp CJ, Falcone RA, Jr, Erwin CR, Warner BW. Bax is required for increased enterocyte apoptosis after massive small bowel resection. Surgery. 2000;128:165–70. doi: 10.1067/msy.2000.107370. [DOI] [PubMed] [Google Scholar]
- 56.Stern LE, Falcone RA, Jr, Kemp CJ, Stuart LA, Erwin CR, Warner BW. Effect of massive small bowel resection on the Bax/Bcl-w ratio and enterocyte apoptosis. Journal of Gastrointestinal Surgery. 2000;4:93–100. doi: 10.1016/s1091-255x(00)80038-4. [DOI] [PubMed] [Google Scholar]
- 57.Leri A, Claudio P, Li Q, Wang X, Reiss K, Wang S, Malhotra A, Kajstura J, Anversa P. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J Clin Invest. 1998;101:1326–31. doi: 10.1172/JCI316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abbate A, Biondi-Zoccai G, Baldi A. Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling. J Cell Physiol. 2002;193:145–51. doi: 10.1002/jcp.10174. [DOI] [PubMed] [Google Scholar]
- 59.Kobara M, Tatsumi T, Kambayashi D, Mano A, Yamanaka S, Shiraishi J, Keira N, Matoba S, Asayama J, Fushiki J, Nakagawa M. Effects of ACE inhibition on myocardial apoptosis in an ischemia-reperfusion rat heart model. J Cardiovasc Pharmacol. 2003;41:880–885. doi: 10.1097/00005344-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Pang J, Xu R, Xu X, Cao J, Ni C, Zhu W, Asotra K, Chen M, Chen C. Hexarelin protects rat cardiomyocytes from angiotensin II-induced apoptosis in vitro. Am J Physiol Heart Circ Physiol. 2004;286:H1063–67. doi: 10.1152/ajpheart.00648.2003. [DOI] [PubMed] [Google Scholar]
- 61.Kumar D, Zimpelmann J, Robertson S, Burns K. Tubular and interstitial cell apoptosis in the streptozotocin-diabetic rat kidney. Nephron Exp Nephrol. 2004;96:e77–79. doi: 10.1159/000076749. [DOI] [PubMed] [Google Scholar]


