Abstract
Abnormal dendritic cell (DC) differentiation and accumulation of immunosuppressive myeloid cells in cancer is one of the major factors of tumor non-responsiveness. We have previously demonstrated that hyper-activation of the Jak2/STAT3 induced by tumor-derived factors (TDF) is responsible for abnormal DC differentiation. Here, using a novel selective inhibitor of Jak2/STAT3 JSI-124, we investigated the possibility of pharmacological regulation of DC differentiation in cancer. Our experiments in vitro have demonstrated that JSI-124 overcomes the differentiation block induced by TDF and promotes the differentiation of mature DCs and macrophages. JSI-124 significantly reduced the presence of immature myeloid cells in vivo and promoted accumulation of mature DCs. In addition to a direct antitumor effect in several animal models, JSI-124 significantly enhanced the effect of cancer immunotherapy. This indicates that pharmacological inhibition of Jak2/STAT3 pathway can be an important new therapeutic strategy to enhance antitumor activity of cancer immunotherapy.
Keywords: TDF-tumor-derived factors, BM - bone marrow, LN - lymph nodes, ImC - immature myeloid cells
Introduction
DCs are specialized antigen presenting cells (APCs) that recognize, acquire, process, and present antigens to naïve resting T cells for the induction of an antigen-specific immune response (1-3). DCs are critically important for the induction and maintenance of antitumor immune responses both spontaneously developed and induced as a result of immunotherapy. Inadequate function of the host immune system may render all attempts to use immunotherapy ineffective. Data from different laboratories obtained during the past few years indicate that defects in the DC system is one of the main factors responsible for tumor escape. Recently accumulated evidence suggests that DC defects in cancer are systemic and are based on their abnormal differentiation. This abnormal differentiation produces at least three main results (rev. in (4)): 1) decreased production of functionally competent mature DCs; 2) accumulation of immature DCs that have characteristics of lineage committed DCs but cannot up-regulate MHC class II and co-stimulatory molecules or produce appropriate cytokines. These cells are not only inefficient in T-cell stimulation but also can be involved in induction of T-cell tolerance; and 3) increased production of immature myeloid cells (IMCs). IMC suppress antigen-specific T cells via direct cell-cell contact and contribute greatly into tumor non-responsiveness.
It is now established that abnormal DC differentiation is mediated by soluble factors produced by tumor cells including vascular endothelial growth factor (VEGF), M-CSF, GM-CSF, IL-10, IL-6, gangliosides, spermin, etc. (5-19). These tumor-derived factors (TDF) bind to different receptors on hematopoietic cells. This suggests that in order to exert similar functional effects on DC differentiation, these factors may converge at the level of signal transduction. Recent studies have identified one such pathway - Jak2/STAT3 signaling. Janus family tyrosine kinases (Jaks) and signal transducer and activator of transcription (STAT) proteins are critical components of diverse signal-transduction pathways that are actively involved in cellular survival, proliferation, differentiation and apoptosis (20). Jaks are constitutively associated with many cytokine and growth factor receptors, including those implicated in defective DC differentiation (rev.(21)). Activated Jaks eventually induce phosphorylation of STATs, followed by their translocation into the nucleus, where they modulate expression of target genes. Constitutive activation of one member of the STAT family, STAT3, has been demonstrated in many different tumors. This activation usually results in anti-apoptotic effect and promotes cell proliferation (rev in (22)). Recently, we have reported that TDF-inducible activation of Jak2/STAT3 is directly involved in the abnormal DC differentiation in cancer (23, 24). Myeloid cells maintain high levels of Jak2 and STAT3 activity, which results in the accumulation of IMCs and inhibition of DC differentiation in vitro (24). We hypothesized that inhibition of tumor-induced Jak2/STAT3 hyperactivation in myeloid cells may improve DC differentiation and function, and ultimately antitumor immune response.
To test this hypothesis we used new selective inhibitor of Jak2/STAT3 pathway, JSI-124 (cucurbitacin I). We have previously demonstrated that JSI-124 selectively inhibited the activation of Jak2 and STAT3 but not Src, Akt, Erk, and Jnk (25). JSI-124 inhibited the growth of tumors with constitutively active STAT3 but did not affect tumors without STAT3 hyperactivation (25).
This study, for the first time, demonstrates that inhibition of Jak2/STAT3 signaling dramatically improves differentiation of DC and eliminates immunosuppressive myeloid cells in cancer. Importantly JSI-124 significantly enhanced the effect of cancer vaccine.
Methods
Reagents, drug, and cell culture. RPMI 1640, DMEM, fetal bovine serum (FBS) and antibiotics were obtained from Gibco BRL (Grand Island, NY), recombinant murine GM-CSF and IL-4 from RDI (Flanders, NJ), lipopolysaccharides (LPS) and Conconovalin A (ConA) from Sigma (St.Louis, MO). The following antibodies were obtained from BD Pharmingen (San Diego, CA): anti-Gr-1 (anti-Ly-6G), anti-CD11b, anti-CD11c, anti-I-Ab, anti-I-Ad, anti-CD86, anti-CD40, anti-I-A/I-E, and anti-CD3, TCR Vα2. Anti-F4/80 antibody was from Serotec Inc (Raleigh, NC). Anti-clonotypic TCR (clone 6.5) was obtained from Caltag, (Burlingame, CA), JSI-124 (cucurbitacin I) was obtained from NCI and for in vivo experiments Cucurbitacin I was obtained from Indofine Chemicals Inc (Hillsborough, NJ). It was dissolved in DMSO.
Murine NIH-3T3 fibroblasts and CT26 colon carcinoma cell line were obtained from ATCC (Manassas, VA). NIH-3T3 cells stably transfected with v-Src were kindly provided by Dr.Richard Jove. MethA (methylcholantrene-induced) sarcoma cell line was obtained from Dr. Lloyd J. Old. MethA tumor was developed in BALB/c mice and passaged in vivo as an ascitic tumor. C3 fibrosarcoma was made by transformation of B6 mouse embryonic cells with human papillomavirus type 16 (26) and kindly provided by Dr. W. M. Kast (Loyola University of Chicago, Maywood, IL). To generate conditioned medium (CM) cells were kept in medium with reduced (3%) FBS concentration. After 48 hr supernatants were collected, filtered and used in experiments.
All peptides were purchased from SynPep Corporation, Dublin, CA. They include: H2-Kd restricted mutant p53-derived peptide (KYICNSSCM), H2-Kd restricted HA-derived peptide (IYSTVASSL), H-2Kb restricted HPV-16-derived peptide (RAHYNIVTF), H-2Kb-restricted OVA-derived peptide (SIINFEKL), I-Ad restricted HA-derived peptide (SFERFEIFPKE). A recombinant vaccinia virus encoding hemagglutinin (HA) from the 1934 PR8 strain of influenza was a gift from F. Guarneri (John Hopkins Institute, Baltimore, MD). A recombinant adenovirus encoding full open reading frame of wild-type p53 gene was described elsewhere (27).
Generation of DCs and isolation of cells. Bone marrow (BM) cells were obtained from the femurs and tibias of mice, and red cells were eliminated using ACK buffer. Cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 20 ng/ml GM-CSF, 10 ng/ml IL-4, and 50 μM 2-mercaptoethanol alone or in the presence of control (from 3T3 fibroblasts) or tumor cell (from CT26) CM. Half of the medium was replaced every 2 days. Gr-1 or CD11c positive cells were isolated from in vitro cultures or spleens of tumor-bearing or control mice using magnetic beads separation technique according to the manufacturer's protocol (Miltenyi Biotec, Auburn, CA). Purity of Gr-1-positive or CD11c-positive populations was more than 95% as determined by flow cytometry. Infection and activation of DCs as well as the description of p53-adenovirus (Ad-p53) were reported previously (27).
Animals and tumor models. Female BALB/c and C57BL/6 mice age 6-8 weeks were obtained from the National Cancer Institute (Frederick, MD). B6.SJL-PtrcaPep3b/BoyJ mice (CD45.1+) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and Swiss mice from Charles River Lab (Wilmington, MA). TCR-transgenic mice expressing an α/β TCR specific for MHC class II-restricted SFERFEIFPKE peptide, derived from influenza HA, were originally obtained from Harold von Boehmer (Basel Institute for Immunology, Basel, Switzerland) and then were crossed to a BALB/c background for >10 generations. Mice were kindly provided by Dr. E. Sotomayor. OT-1 TCR-transgenic mice (C57Bl/6-Tg(TCRαTCRβ)1100mjb) were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were kept in pathogen-free conditions and handled in accordance with the Guidelines for Animal Experiments requirements. C3 sarcoma was established in C57BL/6 mice by s.c. inoculation of 5 × 105 tumor cells. CT26 colon carcinoma and MethA sarcoma were established in BALB/c mice by s.c. inoculation of 5 × 105 tumor cells, 3T3-v-Src tumor in Swiss mice by s.c. inoculation of 2.5 × 106 cells.
Adoptive transfer experiments to evaluate ImC differentiation. C3 tumor was established in C57BL/6 (CD45.2+) and B6.SJL-PtrcaPep3b/BoyJ (CD45.1+) mice by s.c. inoculation of 5 × 105 tumor cells. After 2 weeks (tumor size 1-1.2 cm in diameter) Gr-1-positive cells were isolated from spleens of tumor-bearing C57BL/6 mice followed by transfer of 4×106 of them into congenic tumor-bearing 45.1+ mice. Mice were then treated i.p. with JSI-124 at dose 1 mg/kg/day or vehicle control (DMSO) for 3 days (3 mice per group). On day 4 mice were sacrificed and spleens were collected. The phenotype of the cells was evaluated in the population of donor's CD45.2+ by flow cytometry.
Adoptive cell transfer and immunization. 3-5 ×106 of purified T cells from OT-1 or HA-TCR transgenic mice were injected i.v. into naive C57BL/6 or BALB/c mice. Two days later these mice were immunized s.c. with 100 μg of OVA-derived peptide SIINFEKL or with 1×107 pfu of recombinant vaccinia encoding HA in 0.1 ml PBS. Ten days later cells from lymph nodes and spleens were isolated, re-stimulated in vitro with specific or control peptide and analyzed.
Evaluation of T cell proliferation and cytokine production. Murine CD11c DCs were used as stimulators of allogeneic T cells isolated from spleens of allogeneic mice using T cell enrichment columns (R&D systems, Minneapolis, MN). Cells were mixed at different ratios and incubated in triplicates in U-bottom 96-well plates for 4 days. 1 μCi of [3H]-Thymidine (Amersham, Arlington Heights, IL) was added per well 18h prior to cell harvest. [3H]-Thymidine incorporation was measured using liquid scintillation counter. In some experiments splenocytes were cultured for 4 days in the presence of 1 or 5 μg/ml of ConA, or 0.5 μg/ml anti-CD3 antibody. Antigen-specific T cell response was evaluated using MHC class II -restricted HA-derived peptide. Lymph node cells were cultured in the presence of 12.5 μg/mL of control or HA-derived peptide, supernatants were collected after 2 days of incubation and the level of IL-2 and IFN-γ was measured using ELISA (Endogen).
ELISPOT assay was performed as described previously (28). Briefly, MultiScreen-HA plates (Millipore, Berford, MA) were precoated with anti-mouse IFN-γ antibody (BD PharMingen) by overnight incubation at 4°C. Two hundred thousand LN cells were plated in quadruplicates in each well and cultured for 24 h at 37°C in the presence of the control (RAHYNIVTF) or specific (SIINFEKL) peptides (10 μg/ml). Cells were then washed with PBS containing 0.1% Tween, and plates were incubated overnight at 4°C with biotinylated anti-IFN-γ antibody (BD PharMingen). Results were visualized using avidin-alkaline phosphatase and 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate (Sigma-Aldrich). The number of spots were calculated on a CTL analyzer (CTL Analyzers, Cleveland, OH) using ImmunoSpot 2.8 version software (Cellular Technology, Ltd). Results are presented as number of spots per 1 × 106 cells.
Colony formation assay. Bone marrow cells isolated from control or JSI-124 treated mice were plated in triplicates in a 6-well plate at density 2×104 cells per well in semi-solid 1% methylcellulose medium supplemented with recombinant cytokines supporting the optimal growth of myeloid and erythroid colony-forming units (MethoCult GF M3434, StemCell Technologies, Vancouver Canada). Number of colonies were evaluated and counted under microscope on day 10.
Statistics. Statistical analysis was performed using JMP software (SAS Institute, Cary, NC).
Results
Inhibition of Jak-2/STAT3 pathway improves differentiation of DCs from ImCs
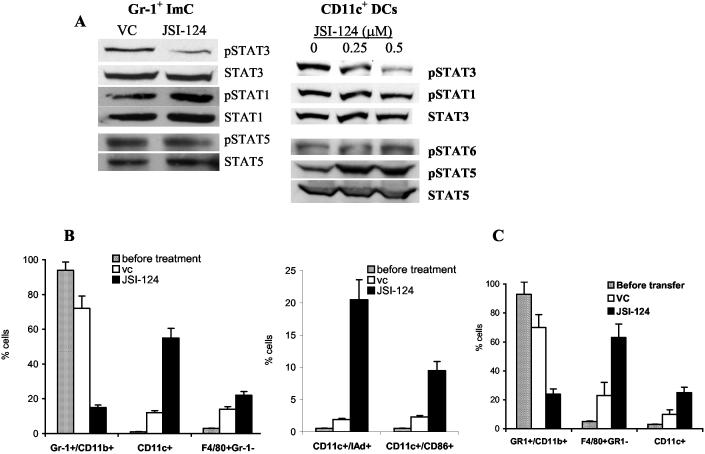
Previous studies have determined that JSI-124 inhibits STAT3 activation in tumor cells (25). Here, we have investigated whether this compound exerts similar effects on STAT3 activation in ImCs and DCs. CD11c+ DCs were generated from bone marrow precursors in the presence of conditioned medium from CT-26 tumor cells and Gr-1+ ImCs were isolated from spleens of CT26 tumor-bearing mice. Cells were treated for 24 hr with different concentrations of JSI-124 in the presence of CT26 tumor cell conditioned medium (CM) and GM-CSF. JSI-124 (0.5 μM) significantly reduced the level of phospho-STAT3 without affecting the level of total STAT3 in both ImCs and DCs (Fig. 1A). JSI-124 did not affect the level of phospho-STAT1, phospho-STAT6, and phospho-STAT5 (Fig. 1A). Phospho-STAT6 was not detectable in ImC. More than 85% of cells were viable at that concentration (data not shown).
Figure 1.
JSI-124 effects on ImC and DC differentiation in vitro. (A) Gr-1+ ImCs were isolated from spleens of CT26 tumor-bearing mice. DCs were generated from bone marrow progenitors using GM-CSF and IL-4 in the presence of CT26 CM as described in details previously (24) and CD11c+ DCs were isolated using magnetic beads. DCs and ImCs were treated with 0.5 μM JSI-124 or vehicle control (DMSO, vc) for 24 hrs in the presence of CT26 CM. Cells were then collected and Western blotting with antibodies against different members of STAT family was performed. (B) Gr-1+ ImCs were isolated from spleens of tumor-bearing mice, then treated with 0.5 μM JSI-124 or vc and cultured for 7 days in the presence of GM-CSF and CT26 CM. Cell phenotype was evaluated by flow cytometry. Cumulative results from three performed experiments are shown. (C) Four millions Gr-1+ ImCs were isolated from spleens of C3 tumor-bearing C57BL/6 (CD45.2+) mice and transferred into congenic C3 tumor-bearing B6.SJL-PtrcaPep3b/BoyJ (CD45.1+) mice (tumor size 1 cm in diameter). Mice were then treated with JSI-124 or DMSO for 3 days. On day 4 mice were sacrificed and phenotype of donor's CD45.2+ splenocytes was evaluated by flow cytometry. Each group included 4 mice.
Accumulation of ImC and their inability to differentiate into mature myeloid cells is one of the hallmarks of hematological and immunological abnormalities in tumor-bearing mice. We asked whether the inhibition of STAT3 signaling might affect differentiation of these cells. Gr-1+ ImCs were isolated from spleens of CT26 tumor-bearing mice and were cultured for 7 days with GM-CSF in the presence of CT26 tumor cell CM. JSI-124 or DMSO were added on day 0 and cell phenotype was evaluated by flow cytometry. ImC obtained from tumor-bearing mice retained immature phenotype (Gr-1+CD11b+) in the presence of TDF (Fig. 1B). The total number of cells collected after 7-day treatment with JSI-124 was reduced by 50%, comparing with the number of cells treated with DMSO (data not shown). JSI-124 had a dramatic effect on the cell phenotype. The proportion of Gr-1+CD11b+ ImC decreased almost 5-fold, whereas the proportion of DCs increased more than 5-fold (Fig. 1B).
To verify these findings in vivo we used a previously described experimental system with adoptive transfer of Gr-1+ ImC into tumor-bearing congenic hosts (29). Gr-1+ ImCs (4×106 cells) isolated from spleens of C3 tumor-bearing C57BL/6 (CD45.2+) mice were transferred i.v. into congenic C3 tumor-bearing B6.SJL-PtrcaPep3b/BoyJ (CD45.1+) mice. Treatment with JSI-124 (i.p. 1 mg/kg/day for 3 days) or DMSO began 3 hr after the transfer. On day 4 mice were sacrificed and phenotype of donor's CD45.2+ splenocytes was evaluated by flow cytometry. In mice treated with DMSO, donor's cells (CD45.2+) represented 3.4±0.6% of the nucleated cells in spleens, whereas in mice treated with JSI-124 1.8±0.5% (p<0.05). In control mice the vast majority of donor's cells remained Gr-1+CD11b+ ImCs. However, in mice treated with JSI-124 the proportion of these cells not only decreased more than 3-fold but instead a significant increase in the proportion of F4/80+Gr-1- macrophages and CD11c+ DCs was observed (Fig. 1C). These data were similar to that obtained during differentiation of ImC in vitro and suggested that inhibition of Jak2/STAT3 signaling allowed ImCs to differentiate towards DCs.
Effect of JSI-124 on ImC and DC differentiation in vivo
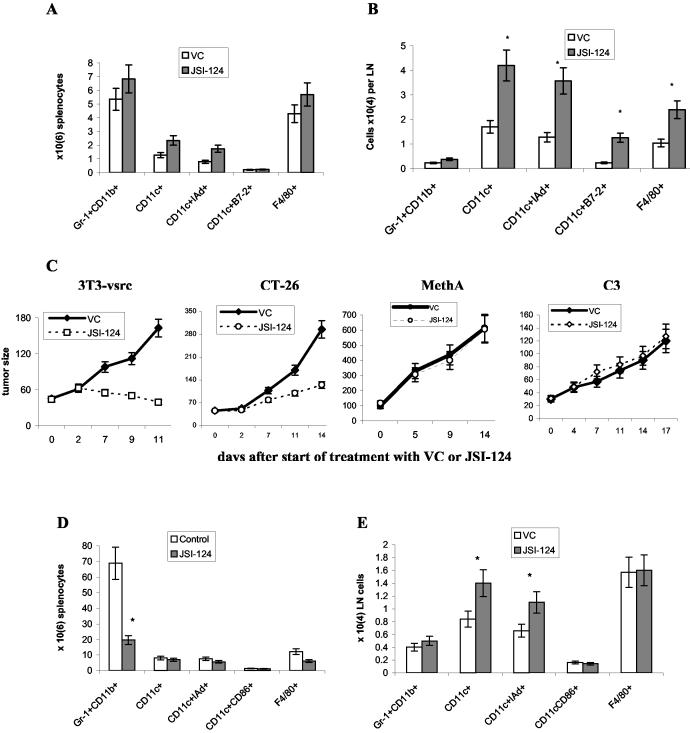
We investigated the effect of Jak2/STAT3 inhibitor in vivo in tumor-free naïve mice. JSI-124 was delivered via daily i.p. injections at dose 1 mg/kg/day. This dose was selected after preliminary experiments and was similar to the dose used in our previous study of antitumor activity of this compound (25). Mice were sacrificed 15-16 days after start of the treatment, spleens and lymph nodes were collected and the presence of different cell populations was evaluated using multicolor flow cytometry. Effect of JSI-124 on splenocytes of naïve tumor-free mice was rather modest. Only slight increase in the number of CD11c+ and CD11c+IAd+ DCs was observed. No statistically significant differences were seen in any tested populations of DCs, macrophages (F4/80+ and CD11c-CD11b+ cells), ImC (Gr-1+CD11b+), or granulocytes (Gr-1+CD11b-) (Fig. 2A and data not shown). In contrast, a significant increase in the number of DCs and macrophages was observed in lymph nodes of these mice (Fig. 2B).
Figure 2.
In vivo effect of JSI-124 on DC differentiation. Tumor-free BALB/c mice were treated with JSI-124 (i.p. 1mg/kg/day) for 14 days. Mice were sacrificed on day 15-16 and phenotype of cells in spleens (A) and lymph nodes (B) was evaluated using flow cytometry. Absolute number of cells was calculated. Cumulative results of two experiments (three mice per group in each experiment) are shown. * - statistically significant differences between control (vc) and treated groups (p<0.05). (C) 3T3-v-Src cells (2.5 × 106) were implanted s.c. into Swiss mice CT-26 carcinoma was established in BALB/c mice by s.c. inoculation of 5 × 105 cells. MethA sarcoma was established in BALB/c mice by s.c. administration of 5×105 tumor cells. C3 tumor was established in C57BL/6 mice by s.c. inoculation of 5×105 cells. Injections of JSI-124 (1 mg/kg/day, once a day, i.p.) or VC (DMSO) were started in 7-10 days when tumor reached ∼30-100 mm2. Tumor size in mice was continuously monitored during treatment. (D,E) - MethA sarcoma mice (6 mice per group) were sacrificed on day 15-16 and phenotype of cells in spleens (D) and lymph nodes (E) was evaluated using flow cytometry. Absolute number of cells was calculated. Cumulative results of two experiments (three mice per group in each experiment) are shown. * - statistically significant differences between control (vc) and treated groups (p<0.05).
Next, we studied the effect of JSI-124 in tumor-bearing mice. Several tumor models were selected. 3T3 fibroblasts transformed with v-src have high level of STAT3 activity, CT-26 colon adenocarcinoma has moderate level of STAT3 activity, C3 and MethA sarcomas don't have hyper-activated STAT3 (23, 30). Tumors were established s.c. and reatment with JSI-124 (i.p., 1 mg/kg/day) was started when tumor size reached 30-100 mm2. JSI-124 had different effect in 4 tested models. Tumor growth in 3T3-v-src tumor-bearing mice was completely blocked and by day 11, tumor size in JSI-124-treated mice was more than 4-fold lower than in DMSO-treated mice (Fig. 2C). Effect of JSI-124 on CT-26 tumor-bearing mice was less pronounced. However, tumor growth was also significantly inhibited. No effect of the compound was evident in C3 and MethA sarcoma-bearing mice (Fig. 2C). These results are consistent with our previous data (25) and the mechanism of JSI-124 action on tumor cells. If tumor cells have hyper-activated STAT3 and their growth is dependent on STAT3 activity (3T3-v-src or CT-26), than Jak2/STAT3 inhibition blocks or slows tumor progression. If tumor cells don't have STAT3 hyper-activation then JSI-124 did not affect tumor growth.
The question remains how Jak2/STAT3 inhibition may affect antitumor immune response. It is well known that tumor burden has direct impact on immune system (31, 32). Therefore, interpretation of the effect of JSI-124 on the function of DC and T cells will be very difficult in the models where this compound significantly reduced tumor burden. We focused on MethA sarcoma model where JSI-124 did not affect tumor growth. Mice were sacrificed 1-2 days after finish of the treatment, spleens and lymph nodes were collected and the presence of different cell populations was evaluated using multicolor flow cytometry. Spleens of tumor-bearing mice had a large number of Gr-1+CD11b+ ImC (7×107 comparing with 5×106 in tumor-free mice). JSI-124 dramatically reduced the absolute number of these cells in spleens (Fig. 2D). In lymph nodes the presence of ImC was only moderately increased and JSI-124 did not affect it. However, the number of CD11c+IAd+ DCs was significantly increased in JSI-treated mice similar to the results in tumor-free mice (Fig. 2E).
Effect of JSI-124 on the function of DCs and T cells in tumor-bearing mice
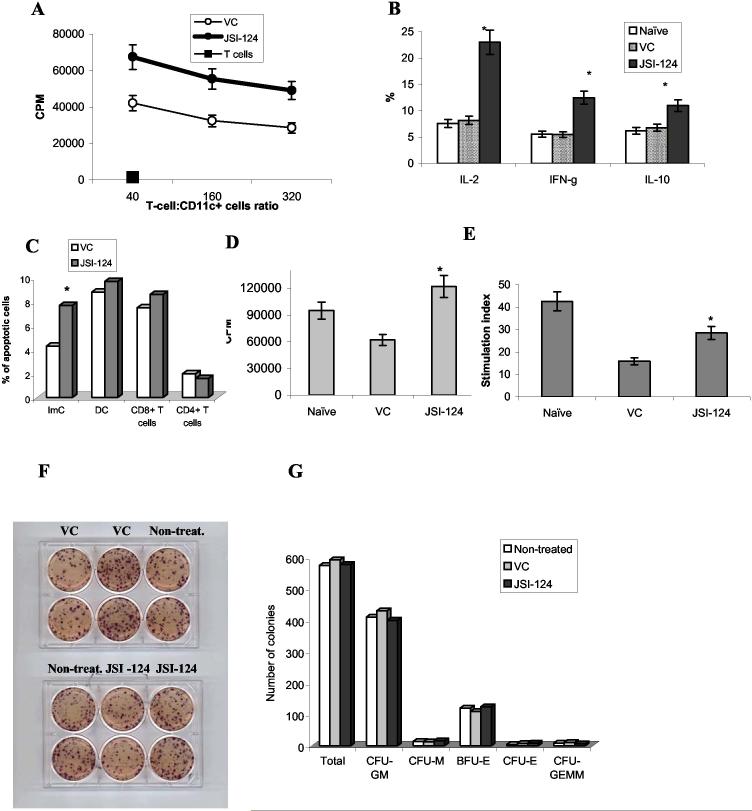
Next, we asked whether JSI-124 affected the function of DCs in tumor-bearing mice. CD11c+ were isolated from lymph nodes of MethA sarcoma-bearing mice treated with either DMSO or JSI-124 and used as stimulators of allogeneic T cells. Allostimulatory activity of DCs isolated from JSI-124 treated mice was significantly higher than that of DCs isolated from DMSO treated mice (Fig. 3A). CD11c+DCs isolated from JSI-124 treated mice induced dramatic increase in the proportion of allogeneic CD4+ T cells producing IL-2, IFN-γ and less prominent but nevertheless significant increase in the production of IL-10 (Fig. 3B). This pattern of cytokine production is typical for activated T cells without preferential shift towards Th1 or Th2 cells.
Figure 3.
In vivo effect of JSI-124 on DC function. (A, B) - CD11c+ DCs were isolated from lymph nodes (A) or spleens (B) of MethA sarcoma-bearing mice treated with either VC or JSI-124, irradiated and mixed together with T cells isolated from allogeneic C57BL/6 mice using T-cell enrichment columns (R&D Systems, Minneapolis, MN). (A) Cell proliferation was measured in triplicates after 4-day incubation using uptake of 3H-thymidine as described in Material and Methods. Two experiments with similar results were performed. (B) - after 48 hr incubation of T cells with DCs at 50:1 ratio, cells were fixed, permeabilized and stained with anti-IL-2, IFN-γ, and IL-10 antibody in combination with anti-CD4 antibody. The proportion of cytokine producing cells was evaluated within the population of CD4+ cells by flow cytometry. Each group included 3 mice. * - statistically significant differences between control (vc) and treated groups (p<0.05). (C) - MethA sarcoma-bearing mice were injected with DMSO (VC) or JSI-124 and 6 hr later splenocytes were collected and stained with Annexin V-PE/7-AAD and antibodies against different cell populations: CD11c-APC and IAd-FITC for DCs, Gr-1-APC and CD11b-FITC for ImC, CD4-FITC and CD8-APC for T cells. Apoptosis was measured within separate cell populations. Three mice formed each treated group. * - statistically significant differences between control (VC) and treated groups (p<0.05). (D, E) splenocytes were isolated from MethA sarcoma-bearing mice treated with DMSO or JSI-124. As a control splenocytes from naïve, tumor-free mice were also used in these experiments. 2×105 splenocytes were stimulated for 4 days with either (D) 0.5 μg/ml anti-CD3 antibody or (E) 5 μg/ml ConA and proliferation was measured in triplicates using uptake of 3H-thymidine. Cumulative results of two performed experiments are shown. * - statistically significant differences between control (vc) and treated groups (p<0.05). In (E) stimulation index = cell proliferation in response to ConA / cell proliferation in non-stimulated cells. (F, G) - Bone marrow cells were collected from MethA sarcoma-bearing mice after 14 days of treatment with JSI-124 or DMSO (vehicle control, VC). In parallel, bone marrow cells were collected from non-treated MethA sarcoma-bearing mice. Colony-formation assay was performed in semi-solid media as described in Methods. 2×104 bone marrow cells were plated per well. (F) - For the demonstration of overall number of colonies, cells were stained with MTT dye. Please note that only large colonies could be visible. (G) Unstained colonies were scored using inverted microscope. The numbers of colonies per 2×104 bone marrow cells are shown. Experiment was performed in duplicates and included two mice per group. Colony-forming unites (CFU): GM - granulocyte/macrophage, M - macrophage, E - erythrocyte, GEMM - mixed. BFU-E - burst forming units erythroid.
We studied the possibility that inhibition of Jak2/STAT3 pathway may induce apoptosis of T cells. Mice were injected with DMSO or JSI-124, splenocytes were collected 6 hr later and analyzed using Annexin-V/7-AAD staining and multicolor flow cytometry. JSI-124 induced significant increase in the level of early apoptosis (Annexin-V+ 7-AAD- cells) in the population of Gr-1+CD11b+ ImC. The effect of the compound on the populations of CD11c+ DCs, CD4+ and CD8+ T cells was not significant (Fig. 3C). In the next set of experiments apoptosis was measured 24 hr after the injection. No increase of apoptosis was observed in any of the tested population (data not shown). We also investigated the effect of JSI-124 on T-cell activity in tumor-bearing mice. Splenocytes were isolated from naïve tumor-free mice or tumor-bearing mice treated with DMSO or JSI-124. As shown in Fig. 3D, tumor-bearing mice had decreased T-cell response to anti-CD3 stimulation. Treatment of the mice with JSI-124 significantly up-regulated this response. Similar results were obtained after stimulation with ConA (Fig. 3E). Taken together this data indicate that JSI-124 improves DC function and overall T-cell response in tumor-bearing mice.
Two-week treatment with JSI-124 was well tolerated. A complete necropsy was performed in mice with careful gross examination of organs and cavities. No significant alterations were found. Microscopic examination failed to reveal abnormalities in any of the animals in the following organs: brain, heart, lung, kidney, adrenal gland, gastrointestinal tract, lymph nodes, thymus, skin and adipose tissue (data not shown).
Jak2/STAT3 signaling is very important for hematopoiesis. To investigate possible hematological toxicity of JSI-124, BM cells isolated from control or treated mice were placed in semisolid methylcellulose medium supplemented with cytokines supporting the growth of myeloid and erythroid colonies (MethoCult™ GF M3434, Stem Cell Technologies). No differences in the number of colonies have been found (Fig. 3F,G).
Effect of Jak2/STAT3 inhibition on the development of antigen-specific CD4+ and CD8+ T-cell responses
Improvement of DC function by itself was not sufficient to affect tumor growth of MethA sarcoma-bearing mice. It is known that in order to control tumor growth immune system should be stimulated with tumor-specific antigen. MethA sarcoma contains two point mutations in p53 gene and responds to immunization with wild-type p53 (27, 33). However, one of the main limitations of cancer immunotherapy in tumor-bearing host is that antitumor effect is short-lived and tumor growth resumed in about a week after termination of the treatment. We hypothesized that positive effect of JSI-124 could be translated into antitumor activity in combination with cancer vaccine.
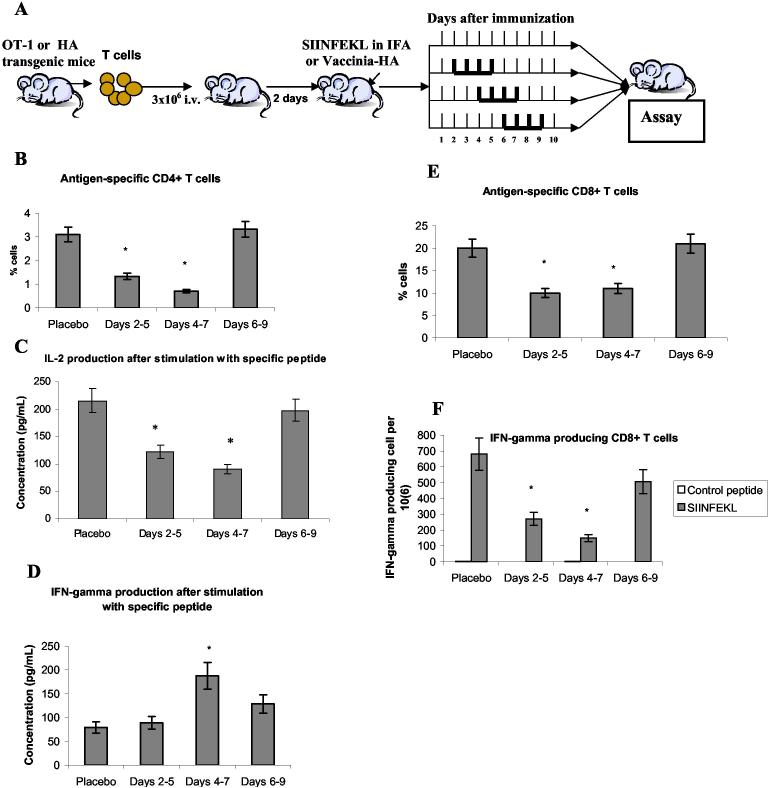
Development of antitumor immune response requires adequate function of T cells. We asked what effect inhibition of Jak2/STAT3 signaling could have on T cells during induction of antigen-specific immune response and initial expansion of antigen-specific T cells. It is especially important for combination of this treatment with immunotherapy. To address this question we used two different experimental systems. Effect of JSI-124 on induction of antigen-specific CD4+ T cells was evaluated using adoptive transfer of transgenic T cells specific of I-Ad matched HA-derived peptide to syngeneic control recipient. Two days after the transfer mice were immunized with Vaccinia-HA. Our previous studies demonstrated that this protocol results in significant accumulation of antigen-specific CD4+ T cells and increased production of IL-2 and IFN-γ (28, 34). JSI-124 was injected (i.p. 1 mg/kg/day) for 4 consecutive days. One group of mice was treated on days 2-5 after immunization, second group on days 4-7, and the third group - on days 6-9 (Fig. 4A). Not treated mice were used as control. All mice were sacrificed on day 10 after immunization, lymph node cells were isolated and re-stimulated with either control or HA-specific MHC class II restricted peptide. JSI-124 treatment during days 2-5 and 4-7 after immunization resulted in significant decrease in the presence of antigen specific CD4+ T cells and specific antigen-inducible production of IL-2 (Fig. 4 B,C). No effect was observed when JSI-124 was injected on days 6-9. In contrast, no decrease in IFN-γ production was seen at any time point. Moreover, JSI-124 increased it when delivered on days 4-7 after immunization (Fig. 4D).
Figure 4.
Effect of JSI-124 on induction of antigen-specific T-cell response. (A) - Description of experimental model. (B, C, D) - Experimental model with adoptive transfer of HA TCR transgenic T cells specific for MHC class II restricted HA-derived peptide (SFERFEIFPKE). (B) - Lymph node cells were labeled with APC-conjugated anti-CD4 antibody and FITC conjugated anti-clonotypic (clone 6.5) antibody. The proportion of clonotypic positive cells within the population of CD4+ T cells was calculated. Each group included 3 mice. * - statistically significant difference from control mice (placebo group) (p<0.05). (C, D) - lymph node cells (106/ml) were stimulated with 12.5 μg/ml of control or specific peptide for 48 hr, supernatants were collected and the levels of IL-2 (C) or INF-γ (D) were measured by ELISA. * - statistically significant difference from control mice (placebo group) (p<0.05). Only the results of stimulation with the specific peptide are shown. After the stimulation with control peptide the levels of IL-2 production was less than 50 pg/ml and IFN-γ less than 15 pg/ml. (E, F) - Experimental model with adoptive transfer of OT-1 transgenic T cells specific for MHC class I restricted OVA-derived peptide (SIINFEKL). (E) - splenocytes were labeled with APC conjugated anti-CD8 antibody and PE conjugated anti-TCRVα2 antibody. The proportion of Vα2 positive cells within the population of CD8+ T cells was evaluated. Each group included 3 mice. * - statistically significant difference from control mice (placebo group) (p<0.05). (F) - splenocytes were stimulated for 24 hr with specific (SIINFEKL) or control (RAHYNIVTF) peptides and the number of IFN-γ producing cells was evaluated in ELISPOT assay as described in Methods. Each group included 3 mice. * - statistically significant difference from control mice (placebo group) (p<0.05).
In a different model we tested the effect of Jak2/STAT3 inhibition on CD8+ T cells. OT-1 TCR transgenic T cells specific to H2Kb matched OVA-derived peptide were adoptively transferred into naïve C57BL/6 mice. After two days these mice were immunized s.c. with this peptide in incomplete Freund's adjuvant (IFA). JSI-124 was injected exactly as described above (Fig. 4A). Cells from spleens were collected and re-stimulated in vitro with cognate or irrelevant control peptides. As in case of CD4+ T cells JSI-124 significantly inhibited presence of antigen-specific CD8+ T cells when administered at early time points after immunization. No inhibition was seen when the compound was administered on days 6-9 (Fig. 4E). IFN-γ production by CD8+ T cells in response to the specific peptide demonstrated the same pattern (Fig. 4F). Thus, inhibition of Jak2/STAT3 signaling significantly reduced the level of antigen specific CD4+ or CD8+ T cells. However, that inhibition occurred only during early phases of T-cell stimulation. Six days after immunization the negative effect of JSI-124 on T-cell response was not detected suggesting that this time frame may be used in cancer immunotherapy.
Effect of JSI-124 on the effect of immunotherapy in tumor-bearing mice
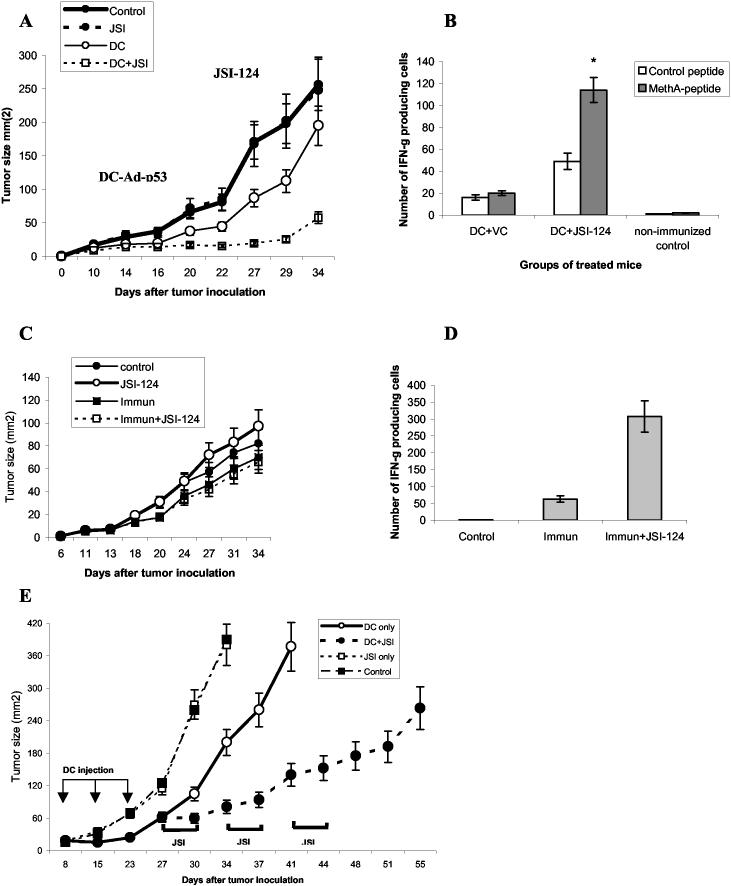
To test the hypothesis that JSI-124 can be useful in cancer immunotherapy we used MethA sarcoma tumor model. MethA sarcoma was established in BALB/c mice. When tumor became palpable mice were immunized with DCs transduced with wild-type p53 gene using adenoviral construct described earlier (Ad-p53) (27). Immunization was repeated 6 days later. Treatment with JSI-124 (i.p. 1 mg/kg/daily) or DMSO was started 4 days after second immunization and continued for 14 days. Immunization with Ad-p53 DCs substantially decreased tumor growth. However, as expected, it resumed soon after second immunization (Fig. 5A). Treatment of immunized mice with JSI-124 dramatically reduced tumor growth. By the end of 5th week after tumor inoculation tumor size in mice treated with combination of JSI-124 and DC-Ad-p53 vaccine was more than 4-fold smaller than in mice treated with the vaccine alone (Fig. 5A). To evaluate tumor-specific immune response in treated mice LN were collected at the end of the study and response of CD8+ T cells to MHC class I bound MethA sarcoma specific peptide was measured in ELISPOT assay. Only mice treated with the vaccine and JSI-124 demonstrated significant IFN-γ production by CD8+ T cells in response to the specific peptide (Fig. 5B). This data indicates that Jak2/STAT3 inhibitor enhances the immunological and antitumor effects of cancer immunotherapy. Combined treatment resulted in a significant delay in tumor growth. It is still possible that immune response was observed not because of a direct effect of JSI-124 on immune system but because of lower tumor burden. We addressed this question using different tumor model. During previous studies we have developed a sub-line of C3 cells (C3R), which in contrast to original C3 tumor cells were not recognized by CTLs specific for H2Kb restricted peptide RAHYNIVTF. Immunization of C3R tumor-bearing mice with this peptide did not affect tumor growth. This provides us with an opportunity to evaluate the direct effect of JSI-124 on the development and maintenance of immune response. C3R tumor-bearing mice were either immunized three times with s.c. injection of RAHYNIVTF in incomplete Freund's adjuvant (IFA), treated with JSI-124 alone, or with combination of these regimens. As expected tumor growth was not affected by any of these treatments (Fig. 5C). Mice were sacrificed 5 weeks after tumor inoculation and LN cells were re-stimulated with specific or control peptides. Mice treated with combination of JSI-124 and immunization had substantially higher level of CD8+T-cell response in IFN-γ ELISPOT assay to the specific peptide than mice treated with immunization alone (Fig. 5D).
Figure 5.
Combination of JSI-124 treatment and immunotherapy. (A) MethA sarcoma was established in BALB/c mice by sc inoculation of 5×105 cells. When tumor became palpable mice were split into 4 groups (5 mice per group) with equal size of the tumors. Immunization with DC-Adp-53 and treatment with JSI-124 or DMSO (VC) was performed as described in Materials and Methods. Tumor size was monitored constantly during mice treatment (B) Mice were sacrificed 5 weeks after tumor inoculation. Lymph node cells were isolated from DC-Ad-p53 immunized mice treated with JSI-124 or VC, or from tumor-bearing non-immunized mice and used in ELISPOT assay. Cells were stimulated with MHC class I bound control or MethA-specific peptides. Number of spots was measured using CTL reader. Data are presented as average number of INF-γ producing cells per 1×106 cells per group. * - statistically significant difference between cells stimulated with control and MethA-specific peptides (p<0.05). (C) C3R tumor was established by s.c. inoculation of 5×105 cells. Mice were immunized in opposite flank with 100 μg of RAHYNIVTF peptide in IFA (Immun). In control mice were injected with IFA alone. Immunizations were performed on days 6, 11, and 14. JSI-124 (Immun + JSI-124, JSI-124) or DMSO (Control, Immun) were administered i.p. from day 18 till day 30. Each group included 5 mice. (D) Mice described above were sacrificed 34 days after tumor inoculation, lymph node cells were stimulated in quadruplicates with specific (RAHYNIVTF) or irrelevant control peptide (SIINFEKL). Both peptides bind H2Kb. The number of IFN-γ producing cells was evaluated in ELISPOT assay. The number of spots in response to control peptide was subtracted from the number of spots in response to the specific peptide and calculated per 106 cells. Each group included 3 mice. Mean ± st. error are shown. Immun. - mice immunized with RAHYNIVTF, Immun. + JSI-124 - mice immunized with the peptide and treated with JSI-124. (E). Treatment of MethA sarcoma-bearing mice was performed essentially as described in Fig. 5A. Mice were treated with DC-control or with DC-Ad-p53 vaccine as indicated. JIS-124 was administered in three cycles, 4 days each with 3 days break. Tumor size was monitored. Each group included 6 mice and Mean ± st. error are shown.
To investigate the possibility of prolonged treatment of tumor-bearing mice with JSI-124 we used intermittent schedule of administration. Mice were treated 4 days in the row with 3-day interval. This cycle was repeated twice (total 3 cycles). The goal was to decrease potential toxicity, since prolonged (more than 3 weeks) continuous administration of JSI-124 resulted in the development of ascites. Gross and microscopic examination of the different tissues from these mice demonstrated lack of any abnormalities (data not shown). Mice were immunized with DC-Ap-53 vaccine three times followed by administration of JSI-124. JSI-124 significantly improved the effect of immunotherapy (Fig. 5E). By day 34, all mice from ”control” and “JSI-124 alone” groups had to be euthanatized because of a bulky tumor and mice from immunized group were euthanatized 10 days later. However, mice in the group with combined treatment retained relatively small tumor size by day 51 (Fig. 5E). Tumor growth resumed 7 days after last administration of JSI-124, which probably reflect continues accumulation of ImC. Thus, these data indicate that at least in MethA sarcoma model, the treatment with JSI-124 can markedly augment immunotherapy during relatively long period of time (8 weeks after tumor inoculation).
Discussion
Previous studies have demonstrated a critical role of Jak2/STAT3 hyperactivation in abnormalities observed in DC differentiation in cancer (23, 24). Signaling from different TDF may converge on STAT3 and hyper-activation of STAT3 results in accumulation of ImCs, and decreased production of mature DCs, which contribute greatly into tumor non-responsiveness (4). Therefore, inhibition of Jak/STAT3 pathway may be an attractive therapeutic approach to improve the differentiation and function of DCs in cancer. To test this hypothesis we used JSI-124 a recently discovered selective JAK/STAT3 signaling pathway inhibitor with potent antitumor activity against human tumors in immune deficient as well as immune competent mouse models (25). JSI-124 (cucurbitacin I) is a member of the cucurbitacin family of compounds that are isolated from various plant families such as the Cucurbitaceae and Cruciferae. Previous study has demonstrated that JSI-124 inhibits the cellular levels of phosphotyrosine-STAT3 and phospho-Jak2, but not phospho-ERK1/2, phospho-JNK, and phospho-Akt (25). Importantly, although JSI-124 is very effective at suppressing the levels of tyrosine-phosphorylated STAT3 and JAK2, it is unable to directly inhibit Src or Jak2 kinase activities in vitro, whereas as AG-490 (known inhibitor of Jak kinases) inhibited kinase activity of both Jak1 and Jak2 (25). JSI-124 could down-regulate phosphotyrosine-STAT3 levels by promoting the protein phosphatase activities of SHP-1 and SHP-2 (35, 36). Alternatively, JSI-124 could also activate physiological inhibitors that are known to directly or indirectly down-regulate STAT3 activation (37). Current efforts are geared at identifying the actual biochemical target of JSI-124. However, regardless of the precise molecular mechanism of action it is clear that JSI-124 provides a significant selective inhibition of Jak2/STAT3 pathway in tumor cells. JSI-124 dramatically reduced the presence of Gr-1+CD11b+ ImCs in vitro and after adoptive transfer in vivo. It appears that inhibition of Jak2/STAT3 pathway has two major effects on ImCs. The total number of cells was reduced almost by half suggesting that substantial proportion of these cells were killed by this drug. This was directly confirmed by the increased level of apoptosis in ImCs 2-3 days after exposure to JSI-124 (data not shown) and 6 hr after JSI-124 administration in vivo. These findings were not surprising since anti-apoptotic effects of STAT3 are well established (37). Furthermore, STAT3 inhibition also promoted differentiation of ImCs. In our in vitro experiments before start of the treatment the population of ImCs contained less than 1% of CD11c+ DCs and less than 2% Gr-1-F4/80+ macrophages. After the treatment with JSI-124, DCs represented more than 50% of cells and macrophages almost 20% of cells. Absolute number of DCs in culture had increased more than 30-fold and macrophages more than 10-fold. These dramatic changes could not be explained by simple re-distribution of the cells populations caused by loss of cells. These results suggest that inhibition of Jak2/STAT3 signaling in ImCs promotes differentiation of these cells towards mature myeloid cells.
Recent study from Laouar et al.(38) has demonstrated using conditional knockout mice that STAT3 is necessary for normal DC differentiation. In other studies this group reported accumulation of myeloid cells in STAT3-deficient mice (39). We believe that there is no contradiction between our results. In conditional knockout mice STAT3 was targeted on early stages of myeloid cell differentiation. STAT3 activity in early progenitors is critically important for the development of DCs. In our experiments STAT3 inhibitor predominantly targeted population of ImC, which is represented by a mixed group of myeloid cells primarily in the late stages of myeloid cell differentiation. It is likely that the effect of STAT3 on myeloid cells depends on the stage of cell development. At present, the molecular mechanisms of the effect of STAT3 inhibition on myeloid cell differentiation are under investigation.
One of the main advantages of JSI-124 is that this compound can be potentially used in clinical trials. We tested its activity in vivo on several tumor models. As expected, JSI-124 inhibited growth of tumors with hyperactivated STAT3 (v-src transformed fibrosarcoma, CT26 colon adenocarcinoma) and did not affect the tumor without STAT3 hyperactivation (MethA sarcoma, C3R tumor). It is known that elimination of tumor (surgical resection) improves DC differentiation (4), which will obscure ( OR bring about a very difficult interpretation of) the direct effect of JSI-124 on DCs in vivo. Therefore, we only focused on tumor models where JSI-124 did not directly affect tumor growth. JSI-124 induced dramatic decrease in the presence of immunosuppressive ImC in MethA-bearing mice. It was associated with substantial increase in the presence and functional activity of DCs in lymph nodes. However, simple improvement of DC function in cancer is not sufficient to reject established tumors. It is evident that inhibition of Jak2/STAT3 pathway needs to be combined with adequate immunization strategy. Jak2/STAT3 inhibition may cause significant decrease in T-cell proliferation, which may blunt any potential benefit of improvement of DC function. Our experiments demonstrated that it happens only during early expansion of antigen-specific T-cells. It suggested that JSI-124 could be combined with immunotherapy. To address this question we used our previously developed approach, which utilizes immunization of MethA sarcoma-bearing mice with DCs transduced with wild-type p53. MethA sarcoma responds to such therapy (27, 40). Importantly, as it is the case with many other tumors, the vaccination results in only temporary decrease in tumor growth. Combination of JSI-124 with immunotherapy dramatically reduced tumor growth. The effect was observed for more than four weeks after last immunization and was associated with tumor antigen-specific CD8+ T-cell response. It appears that the level of Jak2/STAT3 inhibition in vivo provided by JSI-124 was not sufficient to cause any detectable toxicity in mice, which suggest that it can be potentially used in clinical settings. Our experiments demonstrated that intermittent administration of the compound provide the same if not a better effect than continuous treatment, which suggests another approach for reducing potential toxicity.
In recent years several other strategies were developed to block IL-10 induced STAT3 signaling, which may also have potential therapeutic implications. Tellurium compound AS101 was shown to inhibit IL-10 in several tumor cell lines, which resulted in dephosphorylation of STAT3. AS101 sensitized tumor cells to chemotherapeutic drugs, resulting in their increased apoptosis (41). Immunosuppressant rapamycin was shown to inhibit of IL-10 secretion by B-cell lymphomas. The reduced IL-10 production was accompanied by corresponding decreases in the constitutive activation of STAT1 and STAT3 (42).
Thus, this has demonstrated that selective inhibition of Jak2/STAT3 pathway with novel pharmacological agent JSI-124 significantly improved DC differentiation and decreased presence of immunosuppressive immature myeloid cells in tumor-bearing hosts and suggested that pharmacological inhibition of the Jak2/STAT3 pathway may be potentially useful in cancer immunotherapy.
Acknowledgement
We thank Dr. Sotomayor and Dr. Cheng for providing us with mice and for their help in performing experiments with vaccinia-HA. We thank Dr. Jove for providing us with 3T3 v-src cell line. This work was supported by NIH grants CA 84488 and CA 100062 to DG and 5P01 CA 78038 to SMS. YN was supported by NIH fellowship F32 CA103393. This work has been supported in part by the Flow Cytometry Core and Molecular Imaging Core at H. Lee Moffitt Cancer Center.
References
- 1.Steinman RM. Some interfaces of dendritic cell biology. Apmis. 2003;111:675–97. doi: 10.1034/j.1600-0463.2003.11107802.x. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–812. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 4.Gabrilovich D. The mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–52. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 5.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nature Medicine. 1996;2:1096–103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 6.Gabrilovich D, Ishida T, Oyama T, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–66. [PubMed] [Google Scholar]
- 7.Menetrier-Caux C, Montmain G, Dieu M, et al. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage-colony-stimulating factor. Blood. 1998;92:4778–83. [PubMed] [Google Scholar]
- 8.Takahashi A, Kono K, Ichihara F, et al. Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not by proinflammatory cytokines. Cancer Immunol Immunother. 2004;53:543–50. doi: 10.1007/s00262-003-0466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan XH, Han BH, Dong QG, et al. [Vascular endothelial growth factor inhibits dendritic cells from patients with non-small cell lung carcinoma] Zhonghua Jie He He Hu Xi Za Zhi. 2003;26:539–43. [PubMed] [Google Scholar]
- 10.Menetrier-Caux C, Thomachot MC, Alberti L, Montmain G, Blay JY. IL-4 prevents the blockade of dendritic cell differentiation induced by tumor cells. Cancer Res. 2001;61:3096–104. [PubMed] [Google Scholar]
- 11.Ratta M, Fagnoni F, Curti A, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230–7. doi: 10.1182/blood.v100.1.230. [DOI] [PubMed] [Google Scholar]
- 12.Bronte V, Chappell DB, Apolloni E, et al. Unopposed Production of Granulocyte-Macrophage Colony-Stimulating Factor by Tumors Inhibits CD8+ T Cell Responses by Dysregulating Antigen-Presenting Cell Maturation. J Immunol. 1999;162:5728–37. [PMC free article] [PubMed] [Google Scholar]
- 13.Serafini P, Carbley R, Noonan KA, et al. High-Dose GM-CSF-Producing Vaccines Impair The Immune Response Through The Recruitment Of Myeloid Suppressor Cells. Cancer Res. 2004;64:6337–43. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 14.Caux C, Massacrier C, Vanbervliet B, et al. Interleukin 10 inhibits T cell alloreaction induced by human dendritic cells. Intern Immunol. 1994;6:1177–85. doi: 10.1093/intimm/6.8.1177. [DOI] [PubMed] [Google Scholar]
- 15.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 16.Allavena P, Piemonti L, Longoni D, et al. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. 1998;28:359–63. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Buelens C, Verhasselt V, De Groote D, et al. Human dendritic cell responses to lipopolysaccharide and CD40 ligation are differentially regulated by interleukin-10. Eur J Immunol. 1997;27:1848–52. doi: 10.1002/eji.1830270805. [DOI] [PubMed] [Google Scholar]
- 18.Shurin GV, Shurin MR, Bykovskaia S, et al. Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001;61:363–9. [PubMed] [Google Scholar]
- 19.Bella SD, Gennaro M, Vaccari M, et al. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer. 2003;89:1463–72. doi: 10.1038/sj.bjc.6601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rane SG, Reddy ES. Janus kinases:components of multiple signaling pathways. Oncogene. 2000;19:5662–79. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- 21.Imada K, Leonard WJ. The JAK-STAT pathway. Mol Immunol. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 23.Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 24.Nefedova Y, Huang M, Kusmartsev S, et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464–74. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 25.Blaskovich MA, Sun J, Cantor A, et al. Discovery of JSI-124 (Cucurbitacin I), a Selective Janus Kinase/Signal Transducer and Activator of Transcription 3 Signaling Pathway Inhibitor with Potent Antitumor Activity against Human and Murine Cancer Cells in Mice. Cancer Res. 2003;63:1270–9. [PubMed] [Google Scholar]
- 26.Feltkamp MCW, Smits HL, Vierboom MPM, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–9. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 27.Nikitina EY, Chada S, Muro-Cacho C, et al. An effective immunization and cancer treatment with activated dendritic cells transduced with full-length wild-type p53. Gene Therapy. 2002;9:345–52. doi: 10.1038/sj.gt.3301670. [DOI] [PubMed] [Google Scholar]
- 28.Kusmartsev S, Cheng F, Yu B, et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441–9. [PubMed] [Google Scholar]
- 29.Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: The role of reactive oxygen species. J Leukoc Biol. 2003;74:186–96. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 30.Niu G, Heller R, Catlett-Falcone R, et al. Gene Therapy with Dominant-negative Stat3 Suppresses Growth of the Murine Melanoma B16 Tumor in Vivo. Cancer Res. 1999;59:5059–63. [PubMed] [Google Scholar]
- 31.Gabrilovich D, Pisarev V. Tumor escape from immune response: mechanisms and targets of activity. Curr Drug Targets. 2003;4:525–36. doi: 10.2174/1389450033490849. [DOI] [PubMed] [Google Scholar]
- 32.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–39. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi Y, Richards EC, Chen YT, Old LJ. Influence of interleukin 12 on p53 peptide vaccination against established Meth A sarcoma. Proc Natl Acad Sci U S A. 1995;92:2219–23. doi: 10.1073/pnas.92.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu B, Kusmartsev S, Cheng F, et al. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clin Cancer Res. 2003;9:285–94. [PubMed] [Google Scholar]
- 35.Stofega MR, Wang H, Ullrich ACarter-Su C. Growth hormone regulation of SIRP and SHP-2 tyrosyl phosphorylation and association. J Biol Chem. 1998;273:7112–7. doi: 10.1074/jbc.273.12.7112. [DOI] [PubMed] [Google Scholar]
- 36.Schaper F, Gendo C, Eck M, et al. Activation of the protein tyrosine phosphatase SHP2 via the interleukin-6 signal transducing receptor protein gp130 requires tyrosine kinase Jak1 and limits acute-phase protein expression. Biochem J. 1998;335(Pt 3):557–65. doi: 10.1042/bj3350557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turkson J, Jove R. STAT proteins: novel molecular targets for cancer drug discovery. Oncogene. 2000;19:6613–26. doi: 10.1038/sj.onc.1204086. [DOI] [PubMed] [Google Scholar]
- 38.Laouar Y, Welte T, Fu X-Y, Flavell RA. STAT3 Is Required for Flt3L-Dependent Dendritic Cell Differentiation. Immunity. 2003;19:903–12. doi: 10.1016/s1074-7613(03)00332-7. [DOI] [PubMed] [Google Scholar]
- 39.Welte T, Zhang SS, Wang T, et al. STAT3 deletion during hematopoiesis causes Crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci U S A. 2003;100:1879–84. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishida T, Chada S, Stipanov M, et al. Dendritic cells transduced with wild type p53 gene elicit potent antitumor immune responses. Clinic. Exper. Immunol. 1999;117:244–51. doi: 10.1046/j.1365-2249.1999.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sredni B, Weil M, Khomenok G, et al. Ammonium trichloro(dioxoethylene-o,o′)tellurate (AS101) sensitizes tumors to chemotherapy by inhibiting the tumor interleukin 10 autocrine loop. Cancer Res. 2004;64:1843–52. doi: 10.1158/0008-5472.can-03-3179. [DOI] [PubMed] [Google Scholar]
- 42.Nepomuceno RR, Balatoni CE, Natkunam Y, et al. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer Res. 2003;63:4472–80. [PubMed] [Google Scholar]