Abstract
Electrical excitability, which plays an important role in excitation-contraction coupling in the pulmonary vasculature, is regulated by transmembrane ion flux in pulmonary artery smooth muscle cells (PASMC). This study aimed to characterize the electrophysiological properties and molecular identities of voltage-gated Na+ channels in cultured human PASMC. We recorded tetrodotoxin-sensitive and rapidly inactivating Na+ currents with properties similar to those described in cardiac myocytes. Using RT-PCR, we detected transcripts of seven Na+ channel α genes (SCN2A, 3A, 4A, 7A, 8A, 9A, and 11A), and two β subunit genes (SCN1B and 2B). Our results demonstrate that human PASMC express TTX-sensitive voltage-gated Na+ channels. Their physiological functions remain unresolved, although our data suggest that Na+ channel activity does not directly influence membrane potential, intracellular Ca2+ release, or proliferation in normal human PASMC. Whether their expression and/or activity are heightened in the pathological state is discussed.
Keywords: membrane potential, Na+ channels, vascular smooth muscle, pulmonary
Voltage-gated Na+ channels play a major role in regulating cell excitability, particularly as it pertains to the generation of action potentials in neurons, skeletal muscle, and cardiac myocytes. Evidence has shown that, in vascular smooth muscle, action potentials are not dependent on Na+ channels since tetrodotoxin (TTX) has no effect on amplitude and duration of action potentials [18], leading to the belief that these channels might not be present or prominent in these tissues. Nonetheless, Na+ currents (INa) have been measured in visceral smooth muscles such as myometrium and uterus (pregnant), colon, esophagus, stomach, and ureter as well as in certain vascular smooth muscles such as the portal and azygos veins [2, 8, 24-26, 31, 33, 34]. The identification of functional voltage-gated Na+ channels in quiescent and proliferating vascular smooth muscle cells (VSMC), however, has not been as forthcoming. Currently, INa have been measured in smooth muscle cells isolated from the coronary artery, aorta, vena cava, and pulmonary artery of various species, including humans [5, 16, 23, 27]. On only rare occasions have INa been recorded in freshly dissociated human VSMC, although they are readily detected when the same cells are cultured [28]. In isolated cases, INa have been recorded in both freshly dispersed rabbit [27] and cultured human [16] PASMC. The relative inability to detect INa in freshly dissociated, but not cultured, cells from the same vascular bed may bear some relation to cell de-differentiation and proliferation [28]. More specifically, voltage-gated Na+ channel expression and activity may be required either to facilitate the transition or to promote the de-differentiation of cells from “contractile” to “synthetic” or “proliferative” phenotypes [20, 30]. This raises the possibility that the expression of functional voltage-gated Na+ channels in cultured cells act as a trigger for cell de-differentiation and proliferation, possibly via enhanced cytoplasmic free Ca2+ concentration ([Ca2+]cyt).
The molecular identification of the subunits that make up the functional voltage-gated Na+ channel in smooth muscles has also lagged behind. Of the 11 known pore-forming α subunit (SCN-A) genes, only five (i.e. SCN3A, SCN4A, SCN5A, SCN6A, and SCN7A) have currently been identified in smooth muscle cells, while all or most are expressed in brain tissues, skeletal muscle, or cardiac myocytes [8, 13, 15]. In vascular tissues, the list has been further narrowed down to SCN5A, SCN6A, and SCN7A, which all represent TTX-resistant Na+ channel isoforms [21]. Whether these genes form functional channels in pulmonary artery smooth muscle cells (PASMC) has not been examined. However, it is interesting to note that SCN6A and SCN7A gene expression is upregulated in uterine tissue from pregnant rats [10, 17], lending further credence to the proposal that Na+ channels are involved in cell de-differentiation and proliferation. As it concerns the pulmonary vasculature, function and expression of voltage-gated Na+ channels in PASMC may be an important factor contributing to pulmonary vascular remodeling in patients with pulmonary arterial hypertension.
The purpose of this study was to identify and characterize rapidly activating and inactivating voltage-gated Na+ channels in cultured human PASMC. We also sought to identify the voltage-gated Na+ channel genes expressed in human PASMC and to determine their role(s) in modulating human PASMC excitability. Our results clearly indicate that TTX-sensitive voltage-gated Na+ channels are functionally expressed in human PASMC. However, their inhibition does not appear to alter resting membrane potential or agonist-induced intracellular Ca2+ release.
MATERIALS AND METHODS
Cell preparation and culture. Human PASMC (Clonetics, Walkersville, MD) were plated onto cover slips and incubated in a humidified atmosphere of 5% CO2 in air at 37°C in smooth muscle growth medium (SMGM, Clonetics); SMGM is composed of smooth muscle basal medium (SMBM) supplemented with 5% fetal bovine serum (FBS), 0.5 ng/ml human epidermal growth factor (hEGF), 2 ng/ml human fibroblast growth factor (hFGF), and 5 μg/ml insulin. The medium was changed after 24 hrs followed by every 48 hrs thereafter until confluence. Cells were subcultured or plated when 70-90% confluence was achieved. The cells at passages 5-9 were used for experimentation.
Electrophysiological measurements. A cover slip plated with cells was mounted on a plexiglass perfusion chamber on a Nikon inverted microscope, and cells were bathed in physiological saline solution (PSS) containing (in mM): 141 NaCl, 4.7 KCl, 1.8 CaCl2, 1.2 MgCl2, 1 EGTA, 10 glucose, 10 HEPES (pH 7.4 with NaOH). For measuring optimal INa, CaCl2 in PSS was replaced by equimolar MgCl2 to eliminate inward Ca2+ currents. Tetrodotoxin (TTX) was dissolved into water to make a stock solution of 1 mM, which was then diluted (1:1,000 into PSS) to a final concentration of 1 μM. For some experiments, Na+ in PSS was substituted by equimolar replacement with N-methyl-D-glucamine (NMG).
Borosilicate patch pipettes (2-4 MΩ) were fabricated on a Model P-97 electrode puller (Sutter Instruments; Novato, CA) and polished with a MF-63 microforge (Narashige Scientific Instruments Laboratories; Tokyo, Japan). The pipette solution for measuring INa contained (in mM): 10 NaCl, 135 CsCl, 5 ATP-Na2, 10 EGTA, 10 HEPES (pH 7.2 with CsOH). For some experiments using K+-containing pipette solutions, CsCl was replaced by equimolar KCl, and pH was adjusted with KOH. All chemicals were obtained from Sigma Chemical Co. (St. Louis, MO).
All experiments were performed at room temperature (22-24°C). Whole-cell currents were recorded from human PASMC with an Axopatch 1D amplifier and a DigiData 1200 interface (Molecular Devices (formerly Axon Instruments), Union City, CA) using conventional voltage clamp techniques. Step-pulse protocols and data acquisition were performed using pCLAMP software (Molecular Devices). Currents were filtered at 1-2 kHz (-3 dB) and digitized at 2-4 kHz. Leak and capacitative currents were subtracted using the P/4 protocol in pCLAMP software.
RNA extraction and reverse transcriptase - polymerase chain reaction (RT-PCR). Total RNA was isolated from human PASMC using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA). Human brain total RNA was purchased from Gibco BRL. Genomic DNA was removed with RNase-free DNase as per the manufacturer's instructions. cDNA was synthesized using SuperScript™ reverse transcriptase (Invitrogen, Carlsbad, CA). PCR was performed by a GeneAmp PCR System (Perkin Elmer, Boston, MA) using a Platinum PCR Supermix (Gibco). The sequences of sense and antisense primers that were specifically designed from the coding regions of various Na+ channel genes are shown in Table 1. The fidelity and specificity of the sense and antisense primers were examined using a BLAST program. As a control for integrity of total RNA, primers specific for glyceraldehyde phosphate dehydrogenase (GAPDH) were used. Amplified cDNA products were separated on 1.5% agarose gels and visualized by ethidium bromide staining. The net intensity values of the PCR product bands measured by a Kodak Electrophoresis Documentation System (Eastman Kodak Company; Rochester, NY) were normalized to the net intensity values of the GAPDH signals; the ratios are expressed as arbitrary units for quantitative comparisons.
Table 1.
Oligonucleotide sequences of the primers used for RT-PCR
| Standard Names (Accession No.)* | Size (bp) | Predicted Sense/Antisense | Location (nt) | Gene (chromosome) |
|---|---|---|---|---|
| SCN1A (XM_114281) | 227 | 5′-CTCCCCACACCAGTCTTTGT-3′/5′-TGGTCTGACTCAGGTTGCTG-3′ | 1667-1686 | 2q24.3 |
| 1874-1893 | ||||
| SCN1B (NM_001037) | 361 | 5′-TGTCAAGATCCTGCGCTATG-3′/5′-GGCAGCGATCTTCTTGTAGC-3′ | 392-411 | 19q13.1 |
| 752-771 | ||||
| SCN2A (X65361) | 309 | 5′-GCCCTTCGAATACAGATGGA-3′/5′-ATCATACGAGGGTGGAGACG-3′ | 497-516 | 2q23-q24.3 |
| 785-804 | ||||
| SCN2B (U87555) | 331 | 5′-GCTTCAGAGAGGACCTGGTG-3′/5′-GGCCTCATCCACAGTCCTAA-3′ | 1119-1138 | 11q23 |
| 1430-1449 | ||||
| SCN3A (NM_006922) | 241 | 5′-GTAGTGGTGCATTGGCCTTT-3′/5′-GCACCGAGTTCTGAGTAGCC-3′ | 4127-4146 | 2q24 |
| 4348-4367 | ||||
| SCN3B (NM_018400) | 193 | 5′-GAGGAGGCTGGAGAGGACTT-3′/5′-CCGCAGAGTTCTCCTTGTTC-3′ | 1242-1261 | 11q24.1 |
| 1415-1434 | ||||
| SCN4A (NM_000334) | 312 | 5′-CTCTCACCTGCTTCCCAGTC-3′/5′-CTCTGCAACCTGCACACAGT-3′ | 7350-7369 | 17q23.1-q25.3 |
| 7642-7661 | ||||
| SCN5A (M77235.1) | 466 | 5'-AGAAGATGGTCCCAGAGCAATG-3′/5'-CTCGAAGCCATCTACACACGGA-3' | 1647-1669 | 3p21 |
| 2090-2112 | ||||
| SCN6A (M91556) | 507 | 5′-CAGATGAGGCCAAGACCATACA-3′/5′-ATCGAAGAAGAGCCATTCCTGC-3′ | 1422-1444 | 2q21-q23 |
| 1906-1928 | ||||
| SCN7A ?(NM_002976) | 259 | 5′-CCAGTACCTCGCCCATTAAA-3′/5′-CAAAAATGTTCCACGCAATG-3′ | 3833-3852 | 2q21-q23 |
| 4072-4091 | ||||
| SCN8A (NM_014191) | 380 | 5′-GTGGCCTGGACCAAACTAAA-3′/5′-CCTTCAGAGGAGCTGGTGTC-3′ | 3200-3219 | 12q13 |
| 3560-3579 | ||||
| SCN9A (NM_002977) | 247 | 5′-CCACTTCATCCACCACCTCT-3′/5′-ACTGCACTGCCTTCGAGAAT-3′ | 5858-5877 | 2q24 |
| 6085-6104 | ||||
| SCN10A ?(NM_006514) | 260 | 5′-CTCTTCGCAGGGAAGTTTTG-3′/5′-CACTTGGGTTGCATGTTGAC-3′ | 3904-3923 | 3p22-p21 |
| 4144-4163 | ||||
| SCN11A ?(AF188679) | 239 | 5′-CCCAGCAGCTGTTAAAGGAG-3′/5′-CTGGGACAGTCGTTTGGTTT-3′ | 1301-1320 | 3p24-p21 |
| 1520-1539 | ||||
| GAPDH | 719 | 5′-GAGCCAAAA-GGGTCATCATCTC-3′/5'-AGGGTCTCTCTCTTCCTCTT-3' |
The accession numbers in GenBank for the sequence used in designing the primers
Statistical analysis. Data are expressed as means ± SEM. Statistical analysis was performed using the unpaired Student's t test, or analysis of variance, as indicated. Differences were considered to be significant when P<0.05.
RESULTS
Biophysical properties of voltage-gated Na+ currents. Our first task was to identify Na+ currents in human PASMC. In cells dialyzed with K+-containing solution, an inward Na+ current (INa) and a sustained outward K+ current (IK) were observed in the absence of extracellular Ca2+ (Figs. 1A and 1B). INa was activated at potentials close to -60 mV (-22 ± 4 pA) and peaked at +0 mV (-343 ± 75 pA) (Fig. 1C). In contrast, IK recorded in the same cells also activated at approximately -60mV (-5 ± 3 pA), but peaked at highly positive potentials (1485 ± 186 pA at +80 mV).
Figure 1.
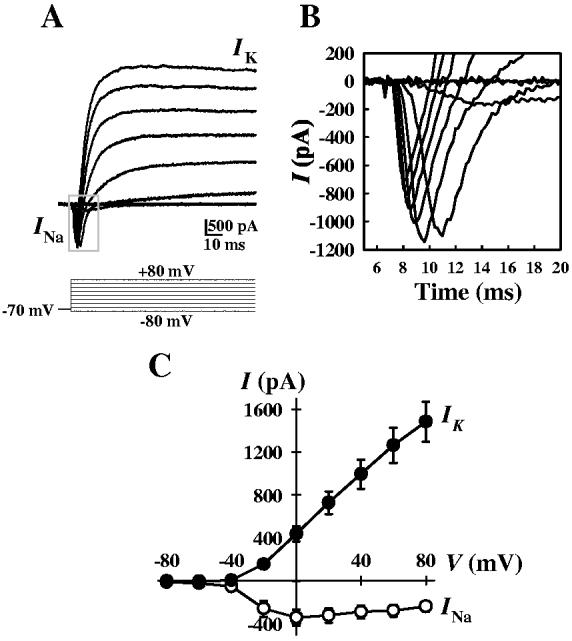
Whole-cell inward (INa) and outward (IK) currents in cultured human PASMC. A. Representative currents were elicited by depolarizing the cell from a holding potential of -70 mV to a series test potentials ranging from -80 to +80 mV in 20 mV increments. The cells were superfused with Ca2+-free PSS and dialyzed with K+-containing pipette solution. B. Enlarged view of inward currents (boxed section in panel A) triggered by step depolarization. C. Summarized current-voltage (I-V) relationship curves (means ± SE) for inward currents (INa) and outward currents (IK) in human PASMC (n = 20).
The inward INa appeared to belong to the rapidly activating and inactivating family of Na+ channels. Removal of K+ from pipette solution allowed for clearer definition of the whole-cell INa in human PASMC and for analysis of the channel gating and kinetic properties. In cells superfused with Ca2+-free solution and dialyzed with K+-free solution (Cs+ replacing K+), only inward INa were recorded (Fig. 2A). INa activated and inactivated rapidly; the time constants for activation and inactivation are 0.485 ± 0.06 ms and 2.85 ± 0.41 ms, respectively, at 0 mV (Fig. 2B). The currents did not exhibit any tendency for sustained activation (Fig. 2B), as has been previously reported for voltage-gated Na+ currents in human coronary artery smooth muscle cells [28].
Figure 2.
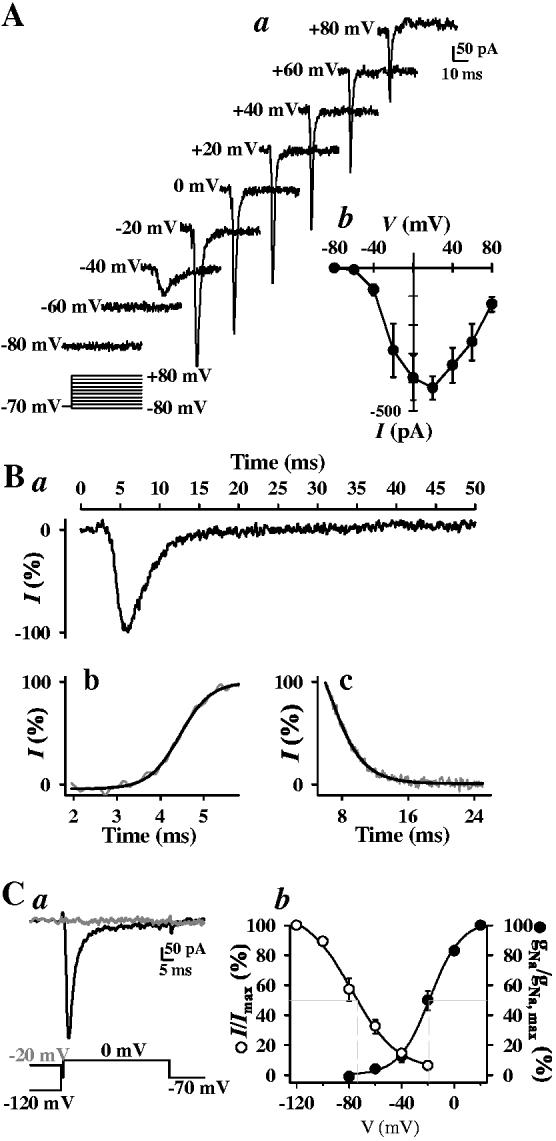
Electrophysiological properties of inward INa in human PASMC. A. Cultured human PASMC were superfused with Ca2+-free bath solution and dialyzed with a Cs+-containing, Ca2+-free pipette solution. Representative currents (a) were elicited by depolarizing the cell from a holding potential of -70 mV to test potentials between -80 and +80 mV in 20 mV increments. Summarized current-voltage (I-V) relationship curves of INa (b) are averaged from 8 cells. B. Time course (a) of normalized INa shows the rapid inactivation kinetics. Steady-state activation (b) and inactivation (c) of the currents recorded at 0 mV (peak current) occurred within < 5 ms and < 16 ms, respectively. Data are representative of 10 experiments (mean ± se τact and τinact values provided in text). C. Currents (a) were evoked by a step depolarization to 0 mV from different conditioning potentials (-120 and -20 mV) applied for 10 s prior to the test depolarization. Voltage-dependent steady-state availability (I/Imax, n = 8) and normalized conductance-voltage relationship (gNa/gNa, max, n = 6) of the peak INa are shown in panel b. The I/Imax and gNa/gNa, max curves were best fit using the Boltzman equation. Calculated V50 values for both activation and inactivation are given in the text.
Based on standard double-pulse protocols, activation (gNa/gNa.max) achieved 50% of its maximum at a conditioning potential of -18 ± 2 mV, while inactivation (I/Imax) was half-maximal at -75 ± 3 mV (Fig. 2C). The window currents determined by the overlap between the activation and inactivation curves were found in the voltage-range of -60 to -20 mV in human PASMC cultured in growth medium. Presence of this window current suggests that Na+ currents may participate in the regulation of resting membrane potential (Em) in human PASMC.
Pharmacological identification of INa. We confirmed that the channels were conducting Na+ ions by simple ion replacement. In Na+-free media (i.e., extracellular Na+ was replaced by equimolar N-methyl-d-glucamine (NMG)), rapid inward currents recorded during a 65 ms depolarization from -70 mV to 0 mV were abolished (P<0.01) in human PASMC compared to currents measured in the presence of external Na+ (Fig. 3A); this effect was reversible upon return to normal external [Na+]. These results confirm that the rapidly activating inward currents were generated by Na+ ions. Voltage-gated Na+ channels can be loosely qualified as either sensitive or not to tetrodotoxin (TTX), a selective and potent blocker of voltage-gated Na+ channels. Extracellular application of 1 μM TTX also reversibly abolished the inward currents (P<0.01) (Fig. 3B). Inhibition by TTX was concentration-dependent (Fig. 3C). The EC50 value for TTX was 17.5 nM at 0 mV (Fig. 3D), with the currently being nearly abolished at 1 μM. Therefore, the currents seem to be generated by Na+ influx through the TTX-sensitive voltage-gated Na+ channels similar to those described in neurons and cardiomyocytes [12].
Figure 3.
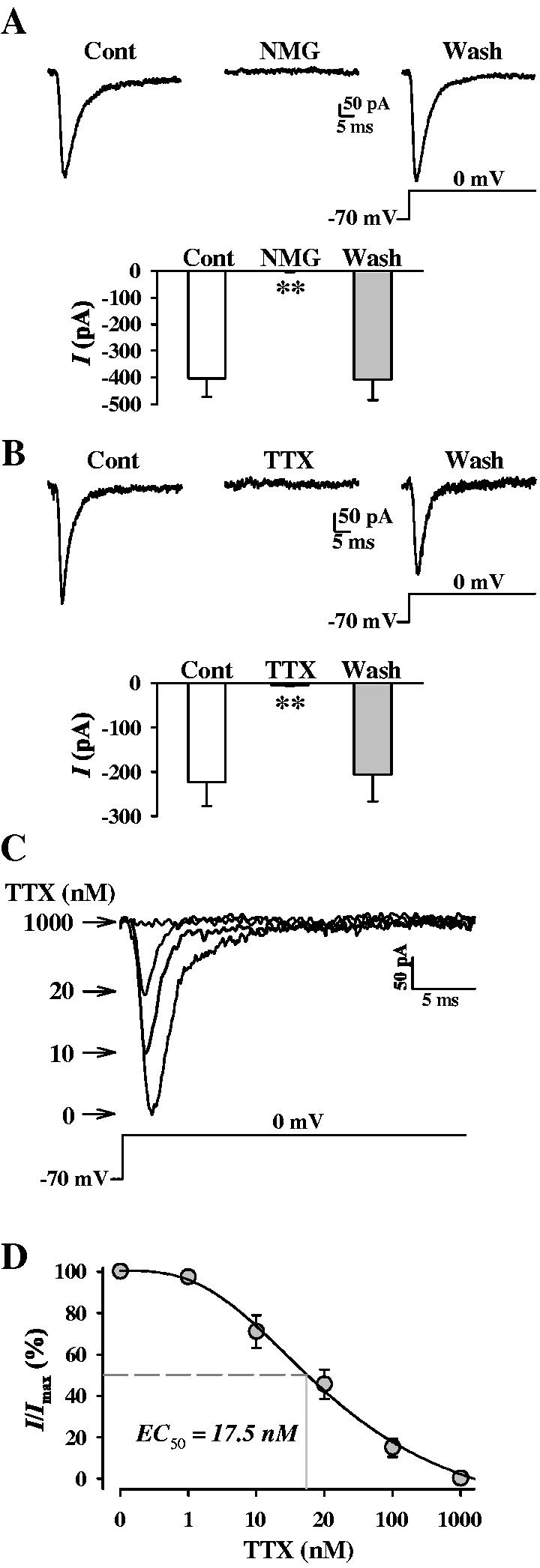
Pharmacological characterization of INa. A. Representative current traces (top), elicited by depolarizing a cell from a holding potential o f-70 mV to 0 mV, in PASMC before (Control), during (NMG), and after (Wash) replacement of extracellular Na+ with equimolar N-methyl-d-glucamine (NMG). Summarized (n = 4 experiments) current amplitude measured at 0 mV is presented in the histogram below. B. Representative currents (top), elicited by depolarizing a cell from a holding potential of -70 mV to 0 mV, in PASMC before (Control), during (TTX), and after (Wash) extracellular application of 1 μM TTX. The cells were superfused with Ca2+-free bath solution and dialyzed with K+-free and Cs+-containing pipette solution. Summarized (n = 4 experiments) current amplitude measured at 0 mV is presented in the histogram below. C. Representatives currents recorded at 0 mV in the absence (0) or presence of incremental (10, 20, 1000 nM) TTX. D. Concentration-response curve for TTX. Currents as represented in C were normalized and plotted as a function of [TTX]. I/Imax points were fit to determine EC50 (17.5 nM) (n = 3-5 cells for each concentration).
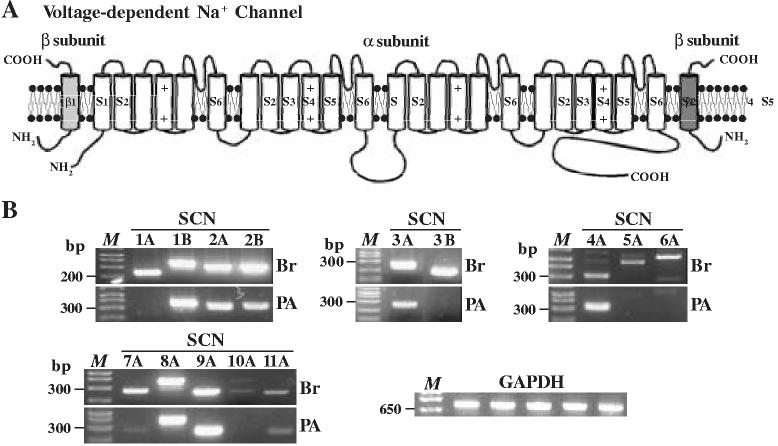
Voltage-gated Na+ channel mRNA expressed in human PASMC. A complex of three glycoprotein subunits forms functional Na+ channels: a pore-forming σ subunit and two β subunits that modulate channel gating and membrane expression (Fig. 4A) [11]. Using RT-PCR and specifically designed primers, we detected the transcripts of seven pore-forming α subunits: SCN2A, SCN3A, SCN4A, SCN7A, SCN8A, SCN9A, and SCN11A in human PASMC (Fig. 4B); all of which form TTX-sensitive Na+ channels [15]. The mRNA expression level of SCN2A, SCN3A, SCN4A, SCN8A, and SCN9A in human PASMC is comparable to that in brain tissues; suggesting that they are dominant α subunits for forming functional Na+ channels in PASMC. In addition, two β subunit transcripts were detected in human PASMC: SCN1B and SCN2B (Fig. 4B). The transcripts of SCN1A, SCN5A, SCN6A, and SCN10A were not detectable in human PASMC, but were all expressed in brain tissues (Fig. 4B).
Figure 4.
. The mRNA expression of voltage-gated Na+ channels in human PASMC. A. Schematic diagram showing structural arrangement of Na+ channel α and β (β1 and β2) subunits. B. RT-PCR amplified products from human brain tissues (Br) and cultured human PASMC (PA) are displayed on agarose gel for SCN1A (227 bp), 1B (361 bp), 2A (309 bp), 2B (331 bp), 3A (241 bp), 3B (193 bp), 4A (312 bp), 5A (466 bp), 6A (507 bp), 7A (259 bp), 8A (380 bp), 9A (247 bp), 10A (260 bp), and 11A (239 bp), as well as for an internal GAPDH (719 bp) as control. “M”, 100 bp DNA ladder. Data are representative of 3-5 experiments.
DISCUSSION
Putative roles of voltage-gated Na+ channels in regulating [Ca2+]cyt and resting Em. The inward INa we observed in human PASMC possessed biophysical and pharmacological characteristics similar to those previously identified in other human VSMC [4, 5, 7, 16, 28]: sensitivity to TTX (EC50 17.5 nM), -60 to -50 mV activation threshold, -15 to 0 mV peak amplitude potential, -70 to -65 mV half-inactivation voltage, -25 to -15 mV half-activation voltage, and activation (τact ∼1 ms) and inactivation time (τinact ≤ 4 ms) constants. Similarities in the window currents, -60 to -10 mV in 3human PASMC and other VSMC [4, 7], also suggest that Na+ channels are active over a wide range of physiological Em, and that they may contribute to the regulation of resting Em in human PASMC. However, in unpublished observations, we found that TTX did not significantly alter human PASMC Em (-41.2±2.6 mV, -46.2±3.1 mVin TTX, n=4, data not shown) indicating that Na+ channel activity does not modulate Em in cultured normal human PASMC.
Na+ influx also may regulate [Ca2+]cyt by different mechanisms. i) Voltage-gated Na+ channels are essential in the generation of action potentials in many excitable cells, thereby subsequently regulating [Ca2+]cytt. ii) Enhanced TTX-sensitive INa causes a localized transient increase in [Na+]cyt, activating reverse-mode Na+/Ca2+ exchange (NCX) which increases [Ca2+]cyt in the subsarcolemmal space [19] and triggers either further Ca2+ release from the sarcoplasmic reticulum (SR) (causing contraction) or replenishes SR Ca2+ pools by SERCA-mediated Ca2+ re-uptake [1, 29]. iii) TTX-sensitive Na+ channels can conduct Ca2+ ions in the absence of extracellular Na+ or in the presence of trace doses of steroids such as ouabain and digoxin [6, 29]; this can contribute to local and global Ca2+ signaling, especially in heart failure patients treated with digoxin [29]. The permeability of Na+ channels to Ca2+ may also play a role in the contractile-to-proliferative cellular transition. iv) Transient receptor potential (TRP) channels, non-selective cation channels which allow permeation of both Ca2+ and Na+, are believed to underlie store-operated Ca2+ (SOC) channels [22]. Na+ influx via (SOC) [3] can also alter Ca2+ signalling by shifting Em, resulting in reversed NCX activity (i.e., enhanced Ca2+ influx) due to increased opening of voltage-gated Na+ channels and enhanced Na+ influx. Conversely, an enhanced local rise in [Na+]cyt due to enhanced INa activity, could have a similar effect on both NCX activity and [Ca2+]cyt. In a recent study, we demonstrated that human PASMC express high levels of NCX-encoding proteins, NCX1 and NCKX3 [37]. Enhanced reverse-mode NCX activity produced not only an increase in [Ca2+]cyt, but also promoted PASMC proliferation [37]. Preliminary experiments conducted within our lab suggest that TTX-sensitive Na+ channel activation does not influence the histamine-induced release of Ca2+ from the SR in cultured human PASMC (Mauban and Yuan, unpublished observations), or serum-induced PASMC proliferation (Remillard and Yuan, unpublished observations). Furthermore, in isolated rat intrapulmonary arteries, we also observed a lack of effect of TTX on 25 mM K+ or phenylephrine-induced contractions (Wallner and Yuan, unpublished observations). Coupled with our observations regarding Em, these findings suggest that, while the regulation of [Ca2+]cyt and resting Em are intimately related, normal activity of TTX-sensitive Na+ channels may not play a significant role in modulating Em or [Ca2+]cyt in quiescent or agonist-stimulated PASMC.
Na+ channel α subunit mRNA expression in human PASMC. Multiple voltage-gated Na+ channel α and β subunits were expressed in human PASMC. However, it was apparent that some showed higher expression level than others. SCN2A, 3A, 4A, 8A and 9A subunits, which encode for TTX-sensitive Na+ channels [15], are highly expressed in human PASMC, whereas the subunits encoding for TTX-resistant Na+ channels (SCN5A, 6A, 7A, 10A and 11A) [15] are minimally expressed. The RT-PCR data are consistent with our electrophysiological experiments in which TTX-sensitive INa were recorded in human PASMC. Although mRNA expression does not directly reflect expression of functional channel proteins, the results from this study provide important insights into the existence and identification of functional voltage-gated Na+ channels in human PASMC. Further study using combined approaches of molecular biology and electrophysiology would be necessary to define the nature of the Na+ channel α and β subunits that underlie native INa in human PASMC.
Implication of Na+ channel activity in pulmonary vascular pathophysiology. Em and Ca2+ are important modulators of pulmonary vascular tone and PASMC growth. Ion channels, which can both control and be controlled by Em, therefore are important mediators of the pulmonary vascular response. Indeed, dysfunctional K+ channels and upregulated transient receptor potential (TRP) channels have been implicated in the development of idiopathic pulmonary arterial hypertension [9, 32, 35, 36]. Although there is no direct evidence to suggest alterations of Na + channel function in sustained pulmonary vasoconstriction and excessive pulmonary vascular medial hypertrophy in patients with pulmonary arterial hypertension, a recent cDNA microarray study showed that the mRNA expression of the voltage-gated Na+ channel β1 (SCN1B) subunit was increased in lungs tissues from patients with idiopathic and familial pulmonary arterial hypertension [14]. Recent findings from our own laboratory have substantiated this finding (Fantozzi, Huang, Zhang, Platoshyn, Remillard, Thistlethwaite, and Yuan, unpublished observations). Furthermore, other studies have indicated that expression of atypical (SCN6A/7A) Na+ channel genes occurs during pregnancy [10, 17]. Based on these findings, It will be interesting to evaluate whether Na+ channel expression and function are altered in PASMC from patients with pulmonary arterial hypertension, and whether Na+ current-mediated regulation of [Ca2+]cyt is important in the modulation of pulmonary vasoconstriction and PASMC proliferation and de-differentiation in this pathological model.
In summary, the observations from this study indicate that a) cultured human PASMC express multiple voltage-gated Na+ channel α (SCN2A-4A and SCN8A-9A) and β (SCN1B and 2B) subunits, the expression level of putative TTX-sensitive Na+ subunits is greater than that of TTX-insensitive Na+ channel subunits; and b) the rapidly inactivating voltage-gated Na+ current present in cultured human PASMC is TTX-sensitive and the window currents are in the voltage range of -60 to -20 mV. The presence of functional voltage-gated Na+ channels in cultured human PASMC suggests that these channels may participate in the regulation of membrane potential, excitation-contraction coupling, cell volume, and cytoplasmic Ca2+ homeostasis. Our current findings, however, suggest that voltage-gated Na+ channel activity does not regulate key physiological processes (i.e., Em, Ca2+ release) in normally proliferating human PASMC. However, based on observations showing that SCN1B is upregulated in pulmonary vascular tissues in patients with idiopathic pulmonary arterial hypertension, we speculate that changes in voltage-gated Na+ channel activity may relate to the development of the sustained pulmonary vasoconstriction and excessive pulmonary vascular remodeling in these patients.
Acknowledgment
This work was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL 64945, HL 66012, and HL 54043). We thank Ann Nicholson, M. S. for technical assistance.
REFERENCES
- 1.Aggarwal R, Shorofsky SR, Goldman L, Balke CW. Tetrodotoxin-blockable calcium currents in rat ventricular myocytes: a third type of cardiac cell sodium current. J Physiol. 1997;505:353–369. doi: 10.1111/j.1469-7793.1997.353bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amédée T, Renaud JF, Jmari K, Lombet A, Mironneau J, Mironneau M. The presence of Na+ channels in myometrial smooth muscle cells is revealed by specific neurotoxins. Biochem Biophys Res Comm. 1986;137:675–681. doi: 10.1016/0006-291x(86)91131-9. [DOI] [PubMed] [Google Scholar]
- 3.Arnon A, Hamlyn JM, Blaustein MP. Na+ entry via store-operated channels modulates Ca2+ signaling in arterial myocytes. Am J Physiol Cell Physiol. 2000;278:C163–C173. doi: 10.1152/ajpcell.2000.278.1.C163. [DOI] [PubMed] [Google Scholar]
- 4.Boccara G, Choby C, Frapier JM, Quignard JF, Nargeot J, Dayanithi G, Richard S. Regulation of Ca2+ homeostasis by atypical Na+ currents in cultured human coronary myocytes. Circ Res. 1999;85:606–613. doi: 10.1161/01.res.85.7.606. [DOI] [PubMed] [Google Scholar]
- 5.Choby C, Mangoni ME, Boccara G, Nargeot J, Richard S. Evidence for tetrodotoxin-sensitive sodium currents in primary cultured myocytes from human, pig and rabbit arteries. Pflügers Arch. 2000;440:149–152. doi: 10.1007/s004240000268. [DOI] [PubMed] [Google Scholar]
- 6.Cole WC, Chartier D, Martin M, Leblanc N. Ca2+ permeation through Na+ channels in guinea pig ventricular myocytes. Am J Physiol. 1997;273:H128–H137. doi: 10.1152/ajpheart.1997.273.1.H128. [DOI] [PubMed] [Google Scholar]
- 7.Cox RH, Zhou Z, Tulenko TN. Voltage-gated sodium channels in human aortic smooth muscle cells. J Vasc Res. 1998;35:310–317. doi: 10.1159/000025600. [DOI] [PubMed] [Google Scholar]
- 8.Deshpande MA, Wang J, Preiksaitis HG, Laurier LG, Sims SM. Characterization of a voltage-dependent Na+ current in human esophageal smooth muscle. Am J Physiol Cell Physiol. 2002;283:C1045–C1055. doi: 10.1152/ajpcell.00359.2001. [DOI] [PubMed] [Google Scholar]
- 9.Fantozzi I, Zhang S, Platoshyn O, Remillard CV, Cowling RT, Yuan JX-J. Hypoxia increases AP-1 binding activity by enhancing capacitative Ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1233–L1245. doi: 10.1152/ajplung.00445.2002. [DOI] [PubMed] [Google Scholar]
- 10.Felipe A, Knittle TJ, Doyle KL, Tamkun MM. Primary structure and differential expression during development and pregnancy of a novel voltage-dependent sodium channel in the mouse. J Biol Chem. 1994;269:30125–30131. [PubMed] [Google Scholar]
- 11.Felix R. Channelopathies: ion channel defects linked to heritable clinical disorders. J Med Genet. 2000;37:729–740. doi: 10.1136/jmg.37.10.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fozzard HA, January CT, Makielski JC. New studies of the excitatory sodium currents in heart muscle. Circ Res. 1985;56:475–485. doi: 10.1161/01.res.56.4.475. [DOI] [PubMed] [Google Scholar]
- 13.George ALJ, Knittle TJ, Tamkun MM. Molecular cloning of an atypical voltage-gated sodium channel expressed in human heart and uterus; evidence for a distinct gene family. Proc Natl Acad Sci U S A. 1992;89:4893–4897. doi: 10.1073/pnas.89.11.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geraci M, Moore M, Gesell T, Yeager M, Alger L, Golpon H, Gao B, Loyd JE, Tuder RM, Voelkel NF. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res. 2001;88:555–562. doi: 10.1161/01.res.88.6.555. [DOI] [PubMed] [Google Scholar]
- 15.Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- 16.James AF, Okada T, Horie M. A fast transient outward current in cultured cells from human pulmonary artery smooth muscle. Am J Physiol. 1995;268:H2358–H2365. doi: 10.1152/ajpheart.1995.268.6.H2358. [DOI] [PubMed] [Google Scholar]
- 17.Knittle TJ, Doyle KL, Tamkun MM. Immunolocalization of the mNaV2.3 Na+ channel in mouse heart: upregulation in myometrium during pregnancy. Am J Physiol. 1996;270:C688–C696. doi: 10.1152/ajpcell.1996.270.2.C688. [DOI] [PubMed] [Google Scholar]
- 18.Kuriyama H, Kitamura K, Nabata H. Pharmacological and physiological significance of ion channels and factors that modulate them in vascular tissues. Pharmacol Rev. 1995;47:387–573. [PubMed] [Google Scholar]
- 19.Leblanc N, Hume JR. Sodium current induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990;248:372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Sims S, Jiao Y, Chow LH, Pickering JG. Evidence from a novel human cell clone that adult vascular smooth muscle cells can convert reversibly between noncontractile and contractile phenotypes. Circ Res. 1999;85:338–348. doi: 10.1161/01.res.85.4.338. [DOI] [PubMed] [Google Scholar]
- 21.Mandegar M, Remillard CV, Yuan JX-J. Ion channels in pulmonary arterial hypertension. Prog Cardiovasc Dis. 2002;45:81–114. doi: 10.1053/pcad.2002.127491. [DOI] [PubMed] [Google Scholar]
- 22.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 23.Mironneau J, Yamamoto T, Sayet I, Arnaudeau S, Rakotoarisoa L, Mironneau C. Effect of dihydropyridines on calcium channels in isolated smooth muscle cells from rat vena cava. Br J Pharmacol. 1992;105:321–328. doi: 10.1111/j.1476-5381.1992.tb14253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muraki K, Imaizumi Y, Watanabe M. Sodium currents in smooth muscle cells freshly isolated from stomach fundus of the rat and ureter of the guinea-pig. J Physiol. 1991;442:351–375. doi: 10.1113/jphysiol.1991.sp018797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohya Y, Sperelakis N. Fast Na+ and slow Ca2+ channels in single uterine muscle cells from pregnant rats. Am J Physiol. 1989;257:C408–C412. doi: 10.1152/ajpcell.1989.257.2.C408. [DOI] [PubMed] [Google Scholar]
- 26.Okabe K, Kajioka S, Nakao K, Kitamura K, Kuriyama H, Weston AH. Actions of cromakalim on ionic currents recorded from single smooth muscle cells of the rat portal vein. J Pharmacol Exp Ther. 1990;252:832–839. [PubMed] [Google Scholar]
- 27.Okabe K, Kitamura K, Kuriyama H. The existence of a highly tetrodotoxin sensitive Na channel in freshly dispersed smooth muscle cells of the rabbit main pulmonary artery. Pflügers Arch. 1988;411:423–428. doi: 10.1007/BF00587722. [DOI] [PubMed] [Google Scholar]
- 28.Quignard J-F, Ryckwaert F, Albat B, Nargeot J, Richard S. A novel tetrodotoxin-sensitive Na+ current in cultured human coronary myocytes. Circ Res. 1997;80:377–382. [PubMed] [Google Scholar]
- 29.Santana LF, Gómez AM, Lederer WJ. Ca2+ flux through promiscuous cardiac Na+ channels: slip-mode conductance. Science. 1998;279:1027–1033. doi: 10.1126/science.279.5353.1027. [DOI] [PubMed] [Google Scholar]
- 30.Sashihara S, Tshuji S, Matsui T. Oncogenes and signal transduction pathways involved in the regulation of Na+ channel expression. Crit Rev Oncol. 1998;9:19–34. doi: 10.1615/critrevoncog.v9.i1.20. [DOI] [PubMed] [Google Scholar]
- 31.Sturek M, Hermsmeyer K. Calcium and sodium channels in spontaneously contracting vascular muscle cells. Science. 1986;233:475–478. doi: 10.1126/science.2425434. [DOI] [PubMed] [Google Scholar]
- 32.Sweeney M, McDaniel SS, Platoshyn O, Zhang S, Yu Y, Lapp BR, Zhao Y, Thistlethwaite PA, Yuan JX-J. Role of capacitative Ca2+ entry in bronchial contraction and remodeling. J Appl Physiol. 2002;92:1594–1602. doi: 10.1152/japplphysiol.00722.2001. [DOI] [PubMed] [Google Scholar]
- 33.Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Fast Na+ current in circular smooth muscle cells of the large intestine. Pflügers Arch. 1993;423:485–491. doi: 10.1007/BF00374945. [DOI] [PubMed] [Google Scholar]
- 34.Yoshino M, Wang SY, Kao CY. Sodium and calcium inward currents in freshly dissociated smooth myocytes of rat uterus. J Gen Physiol. 1997;110:565–577. doi: 10.1085/jgp.110.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan JX-J, Aldinger AM, Juhaszova M, Wang J, Conte JVJ, Gaine SP, Orens JB, Rubin LJ. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- 36.Yuan X-J, Wang J, Juhaszova M, Gaine SP, Rubin LJ. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet. 1998;351:726–727. doi: 10.1016/S0140-6736(05)78495-6. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Yuan JX-J, Barrett KE, Dong H. Role of Na+/Ca2+ exchange in regulating cytosolic Ca2+ in cultured human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol. 2005;288:C245–C252. doi: 10.1152/ajpcell.00411.2004. [DOI] [PubMed] [Google Scholar]