Abstract
Objective: The purpose of this study was to examine the influence of prone and supine position in preterm infants during acute pain of blood collection.
Setting: Level III Neonatal Intensive Care Unit (NICU).
Study Design: Thirty-eight preterm infants (birthweight 1339 [590–2525] g, GA 29 [25–32] wks) were in 2 groups depending on their position in the isolette prior to and during heel lance at 32 weeks post-conceptional age. The study design was a comparison between groups (Prone, Supine) during 2 events (Baseline, Heel lance).
Outcome Measure: Pain measures were multidimensional, including behavioral (sleep–wake state and facial activity) and physiological (heart rate) responses measured continuously prior to (Baseline) and during blood collection (Lance).
Results: Both groups of infants displayed statistically significant shifts in sleep–wake state to greater arousal, and increased facial activity and heart rate, from Baseline to Lance. Prone position was associated with significantly more deep sleep during Baseline, compared with Supine position, but there were no differences in sleep-wake state during Lance. Minor increased facial activity was shown in some time segments of Baseline for infants in Supine compared with Prone, but did not differ overall between positions. Prone and Supine position did not affect heart rate significantly during Baseline or Lance events.
Conclusions: Prone position promotes deep sleep in preterm neonates at 32 weeks post-conceptional age when they are undisturbed. However, placement in prone position is not a sufficient environmental comfort intervention for painful invasive procedures such as heel lance for blood sampling in the NICU. Neonates require other environmental supports to promote coping with this stressful event.
Keywords: pain, position, premature neonates, facial activity, heart rate
The immaturity of the preterm infant central nervous system results in difficulties coping with pain and distress.1–3 Behavioral and physiologic instability associated with painful procedures can influence medical status and may affect subsequent clinical and developmental outcomes. Moreover, high levels of arousal such as crying are energy-consuming responses for the infants.4 Given the concerns that early repetitive pain may have long-term effects in these vulnerable infants,5,6 improving pain management in the neonatal intensive care unit (NICU) is a priority.
Pain is intrinsic to medical and nursing procedures in the NICU. For example, over 3,000 invasive procedures were recorded in 54 infants admitted to a NICU, 74% in infants below 31 weeks of gestation.7 Blood sampling by heel lance is the most common painful procedure.7 While severe pain such as chest tube insertion and surgery should be managed with pharmacological agents,8,9 minor pain or distress may be managed with environmental comfort measures.10–12 Effective environmental interventions enhance homeostasis and stability in preterm infants in the NICU and are essential elements of neonatal nursing care.10
Although there are a number of environmental pain intervention strategies, providing the infant with physical boundaries and maintaining a flexed position is thought to provide gentle stimulation simultaneously across the proprioceptive, thermal, and tactile sensory systems which may modify gate control mechanisms thereby altering pain transmission.10,11,13,14 In addition, the beneficial effects on arousal and respiratory function of prone positioning compared with supine have been reported in preterm infants. These include improved ventilation and oxygenation,15,16 more time in quiet sleep,17,18 fewer awakenings,19 less energy expenditure,18 and less crying.17 These findings have led to the assumption that infant position may alter pain response, and as such, infant position has been controlled in some pain studies in the NICU.20–22 However, only one study has examined prone position as a specific strategy for ameliorating pain.23 In this study, which was part of a larger investigation of methods of environmental support for procedural pain in preterm infants in the NICU, infants positioned in prone did not show altered pain responses compared with infants in a control group placed in either supine or side-lying. Stevens et al concluded that more work was needed to address prone position as a potential comfort intervention.23 To the best of our knowledge, there are no studies comparing prone with supine position during procedural pain in NICU. The answer to this question has important clinical implications, because it is possible that prone position per se might be a simple and inexpensive way to help ameliorate pain during procedures.
In undertaking research in pain in human preterm neonates, it is a challenge to balance the competing demands of experimental design, ethics, and clinical acceptability. Ideal experimental design would favor randomization of infant position. Lack of randomization would limit direct interpretation of the results. However, randomization to position would require changing the infant's position prior to the study session, and prior handling would likely affect the subsequent response.24 Furthermore, in studies of infants of low gestational age adapting to life outside the uterus, manipulation which is not warranted for clinical reasons appeared to be questionable ethically. Therefore, each infant was in the position judged by their bedside nurse to provide optimal comfort, and infant position was not altered for the purposes of the study. Instead we attempted to ensure that the 2 groups were clinically as comparable as possible.
The purpose of the present study was to examine the influence of prone and supine position on premature neonates' pain responses during the invasive routine procedure of heel lance for blood sampling in the NICU. Pain measures were multidimensional, including behavioral (sleep–wake state and facial activity) and physiological (heart rate [HR]) responses.
MATERIALS AND METHODS
Study Participants
Study participants were 38 neonates in the level III NICU at Children's & Women's Health Center of British Columbia who were part of a larger study of pain in the NICU. Criteria for inclusion were gestational age ≤32 weeks, absence of major congenital anomalies, and no known illicit drug use during pregnancy. The sample was divided into 2 groups based on the neonate positioning in the isolette prior to the heel lance procedure. Twenty-one neonates were in prone positioning (Prone Group) and 17 were in supine positioning (Supine Group). The sample size was based on Goto et al19 who included 16 preterm infants at 36 weeks PCA in each group and reported a significant difference in HR between prone and supine position.
Infant characteristics of the 38 neonates are provided in Table 1. Continuous variables were examined using independent samples t tests, and χ2 tests were used to examine the categorical variables. There were no significant differences (P ≤ 0.05) between the groups in infant characteristics.
TABLE 1.
Infant Characteristics Prone Group vs. Supine Group
| Infant Characteristics | Prone Group (N = 21) | Supine Group (N = 17) |
|---|---|---|
| Age at test day (days) mean (SD) | 18 (10) | 17 (12) |
| Gestational age at birth mean (SD) | 29.78 (1.56) | 29.85 (2.29) |
| Birthweight (g) mean (SD) | 1354 (386) | 1321 (539) |
| APGAR—5 minutes mean (SD) | 8.43 (0.98) | 8.41 (1.18) |
| Illness severity day 3 (SNAP-II) mean (SD) | 1.65 (3.1) | 2.82 (4.69) |
| Invasive procedures since birth mean (SD) | 62.38 (38.23) | 52.47 (34.44) |
| Invasive procedures prior 24 h mean (SD) | 1.67 (1.85) | 1.94 (2.70) |
| Total time of blood collection (sec) | 284.18 (107.45) | 277.92 (188.38) |
| Mechanical ventilation (days)—mean (SD) | 0.05 (0.22) | 0.18 (0.39) |
| Mother's age at delivery (years)—mean (SD) | 30 (7) | 32 (6) |
| Appropriate for gestational age (% AGA) | 81% | 76% |
| Gender (% boys) | 52% | 65% |
| Ethnicity (% white) | 67% | 77% |
Written informed consent was obtained from the mother or other legal guardian by the research nurse according to a protocol approved by the Clinical Research Ethics Board of the University of British Columbia, and the Research Review Committee of the Children's & Women's Health Center of B.C.
Measures
Facial Activity
The Neonatal Facial Coding System (NFCS) is a univariate behavioral pain scale25 which has been validated for use in preterm infants.26,27 The 7 NFCS face actions most frequently observed during procedural pain were coded from videotape: brow bulge, eye squeeze, nasolabial furrow, open mouth, vertical mouth stretch, horizontal mouth stretch, and taut tongue. Each face action was scored from videotape by a trained coder as 1/0 (occurred/did not occurred) for each of 10 2-second segments (ie, 20 sec) during 2 events, namely Baseline and Heel Lance for blood collection. Facial activity in each event (Baseline and Heel Lance), occurrences of the discrete facial actions, were examined both as a total score for each event (Baseline and Lance), and as scores summed separately during each time segment. To calculate the total score, all 7 facial actions during the total time (10-second segments for each facial action) of each event (Baseline and Heel Lance) were summed with a possible total score ranging from 0 to 70. Secondly, scores across time were calculated by summing the raw scores in each of the 10 2-second time segments during each event (Baseline and Heel Lance).
Sleep–wake State
Infant sleep–wake states were coded from videotape using Als28 system as follows: deep sleep = 1, active sleep = 2, drowsy = 3, quiet alert = 4, active alert = 5, and crying = 6. Sleep–wake state was coded by a trained coder during 10 minutes of Baseline, and during blood collection.
Heart Rate
Continuous electrocardiographic (ECG) activity was recorded from a single surface ECG lead and digitally sampled at 360Hz off-line using a specially adapted computer acquisition system and custom physiologic signal processing software.29
Procedure
The study was conducted in the level III Neonatal Intensive Care Unit of the Children's & Women's Health Center of British Columbia. Data collection was carried out during routine blood collection. The laboratory technician's standard protocol for sampling blood involved warming the foot, picking up the foot and rubbing it to disinfect the skin, applying a small disposable metal scalpel (4.9 mm long) for incision, and then squeezing the heel. The lance and incision were repeated as necessary to draw sufficient blood, and collection of the blood samples was either in micropipettes and/or on the absorbent card. Only the first heel lance incision was used for study data collection.
Each neonate in the Prone and Supine groups remained in the same position during both the Baseline and Heel Lance events, and video and physiologic recording were carried out continuously. Baseline was recorded prior to any contact by the laboratory technician. Two video cameras mounted on a mobile cart were used, one focused only on the face (face video) and another on the whole body (body video).
Behavioral video coding was carried out using the Observer (Noldus) software for video data analysis, which allows for slow motion and stop frame playback techniques.30 NFCS facial activity and infant sleep–wake states were coded independently by separate coders from the face video and body video respectively. All coders were blinded to the purpose of the study, the events, medical information, and subject characteristics.
Prospective medical chart review was carried out independently by a research nurse. Infant parameters including gestational age (GA) at birth, birth weight, 5 minute APGAR score, days of mechanical ventilation, number of invasive (skin breaking) procedures during the previous 24 hours, number of invasive (skin breaking) procedures since birth, and illness severity (measured using the Score for Neonatal Acute Physiology—SNAP-II)31 on day 3 since birth were obtained.
Data Analysis
The Statistical Package for Social Sciences (SPSS version 10.0) was used for data analysis. Sleep–wake states were analyzed using the Wilcoxen Signed Ranks test to examine change across events. The Mann-Whitney U test was used to compare the Prone versus Supine groups during each event. Continuous measures were examined using repeated measures analysis of variance to compare events (Baseline vs. Heel lance) and position (Prone vs. Supine), followed by t tests. The significance level for each test was set at P ≤ 0.05.
RESULTS
Sleep–wake States
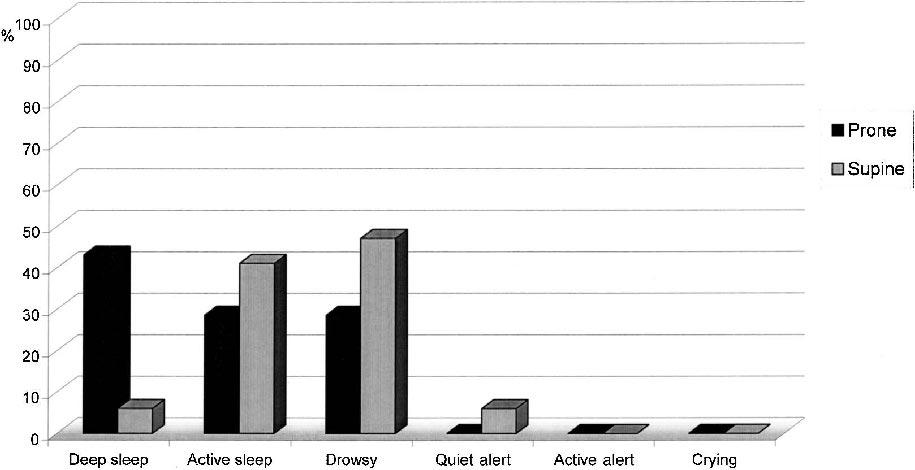
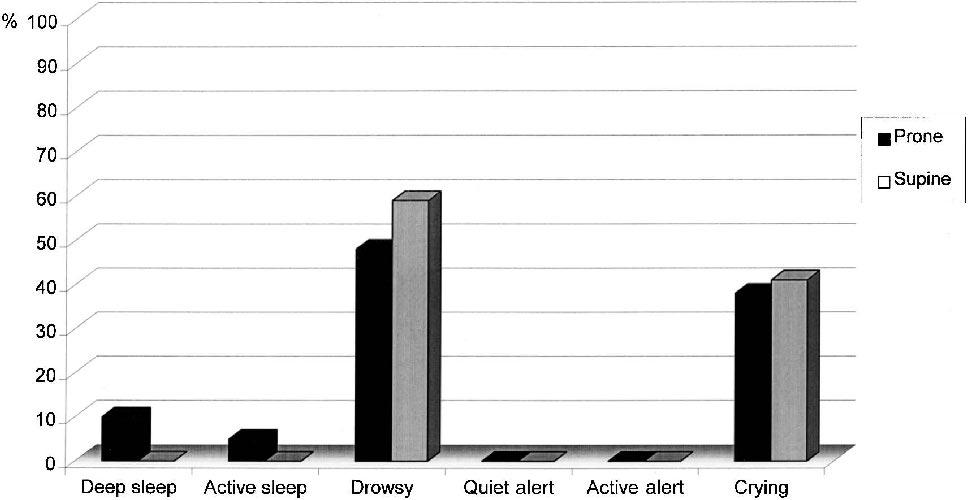
The frequency of each sleep–wake state by Position group and Event is presented in Table 2. The groups differed significantly in sleep–wake state during Baseline (Mann Whitney U = 103.5; P ≤ 0.02). During Baseline, more neonates in the Prone group were in deep sleep (44%) than in the Supine group (6%). During Heel Lance, the groups did not differ statistically significantly (Mann Whitney U = 158.0; P = 0.56). The infants in both groups shifted statistically significantly from more sleep or drowsy states during Baseline to more aroused states following Heel Lance (Z = –4.76, P = 0.0001). Following Heel Lance, in the Prone group, there were 3 neonates (14%) who stayed in deep or active sleep, whereas in the Supine group, there were none who remained asleep; this was not a statistically significant difference. These results are displayed graphically in Figures 1 and 2.
TABLE 2.
Sleep-wake States in the Prone and Supine Groups During Baseline and Heel Lance Events (Frequency; %)
|
Prone Group (N = 21) |
Supine Group (N = 17) |
|||
|---|---|---|---|---|
| Sleep-wake States | Baseline F (%) | Heel Lance F (%) | Baseline F (%) | Heel Lance F (%) |
| Deep sleep | 9 (44) | 2 (9) | 1 (6) | 0 (0) |
| Active sleep | 6 (28) | 1 (5) | 7 (41) | 0 (0) |
| Drowsy | 6 (28) | 10 (48) | 8 (47) | 10 (59) |
| Quiet alert | 0 (0) | 0 (0) | 1 (6) | 0 (0) |
| Crying | 0 (0) | 8 (38) | 0 (0) | 7 (41) |
FIGURE 1.
Sleep-wake state during baseline.
FIGURE 2.
Sleep-wake state during blood collection.
NFCS Facial Actions
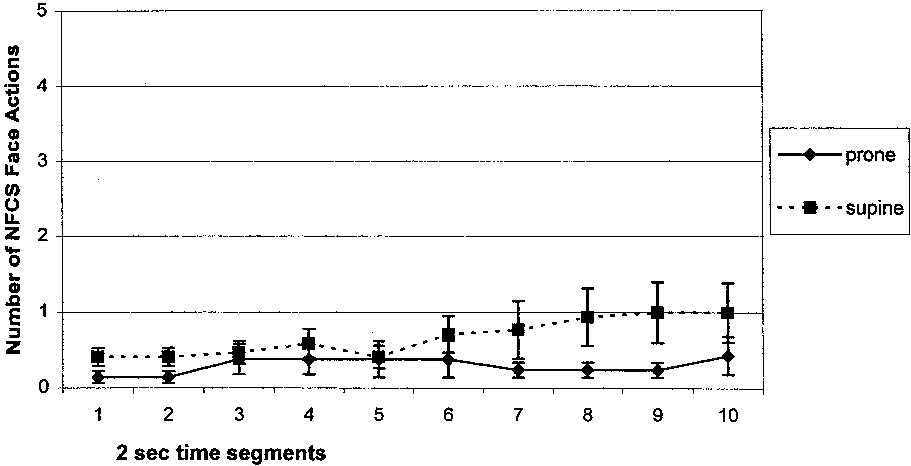
The means and standard deviations for total facial activity by Position group and Event are presented in Table 3. The total NFCS facial activity increased significantly from Baseline to Heel Lance in both groups, a main effect of Event (F [1,36] = 68.32, P = 0.0001). There was no statistically significant main effect of Position between the Prone and Supine groups in the total NFCS score during the Baseline or Heel Lance events (F [1,36] = 0.88, P = 0.35), and no significant interaction between Event and Position (F [1,36] = 0.03, p = 0.88). The NFCS face actions were compared for the Prone and Supine groups in 2 second time segments for Baseline (segment 1–10) and for Heel Lance (segments 11–20). The only statistically significant difference was in segment 9 in Baseline (t [36] = 2.03, P = 0.05); trends were seen in Baseline time segment 1 (t [36] = 1.91, P = 0.06), segment 2 (t [36] = 1.91, P = 0.06) and segment 8 (t [36] = 1.98, P = 0.056), with the Supine group displaying higher means compared with the Prone group (displayed in Fig. 3). There were no significant differences between the variances of the Prone and Supine groups for each of the Heel Lance segments using Levene's test for equality of variances. None of the Heel Lance segments approached statistical significance. Heel Lance segments 12–14 were re-analyzed using nonparametric statistics (Mann-Whitney U test), however the results remained non-significant. Therefore, we concluded that there were no differences between the Prone and Supine groups during the invasive event of blood collection.
TABLE 3.
NFCS Total Facial Activity and Heart Rate in Prone and Supine Groups During Baseline and Heel Lance Events (Mean; SD)
|
Prone Group (N = 21) |
Supine Group (N = 17) |
|||
|---|---|---|---|---|
| Baseline | Heel Lance | Baseline | Heel Lance | |
| NFCS total score | 2.95 (5.68) | 32.52 (21.29) | 6.70 (7.25) | 35.18 (18.77) |
| Heart rate | 160.07 (13.77) | 179.04 (17.35) | 154.89 (12.14) | 173.64 (9.23) |
FIGURE 3.
Mean NFCS facial activity during baseline.
Heart Rate
The means and standard deviations for mean HR by Position group and Event are presented in Table 3. Mean HR increased significantly in both groups from Baseline to Heel Lance, a main effect of Event (F [1,36] = 68.96, P = 0.0001). There was no statistically significant main effect of Position between the Prone and Supine groups in mean HR during the Baseline and the Heel Lance events (F [1,36] = 1.89, P = 0.18), and no significant interaction between Event and Position (F [1,36] = 0.002, p = 0.96).
DISCUSSION
The purpose of this study was to examine whether placement in prone position may ameliorate acute procedural pain responses in preterm infants in the NICU, compared with placement in supine. There are major challenges to studying pain in the NICU in this vulnerable population. We know that unnecessary handling disturbs the regulatory systems of potentially unstable premature infants. While some studies attempt to control for infant position, this would necessitate additional handling for experimental purposes only, which in itself might alter the immediate pain response.24 Furthermore, this avoidable handling appeared to be unwarranted interference with an otherwise stable infant. Thus we decided not to randomize infants to position. Instead, to reduce bias, we tried to ensure that the two groups of infants were comparable in perinatal characteristics (gestational age at birth, post-conceptional age, birth weight, APGAR score, early illness severity, previous pain procedures since birth, and duration of mechanical ventilation). In addition, to ensure that other influencing variables related to caregiving and infant condition did not differ between the groups in the hours preceding the observation period, the number of invasive (skin breaking) procedures in the 24 hour period immediately prior to the target pain response were compared and did not differ. Moreover, the total time of blood collection was similar in the two groups.
Previous studies which compared prone with supine positioning in preterm infants in the NICU have focused on the benefits of prone position for promoting sleep18,19 (although some investigators have reported no differences),32 for reducing energy expenditure, and for improving respiratory function.33 Our findings confirm that prone position facilitates deep sleep when premature neonates at 32 weeks post-conceptional age are undisturbed. In addition, less facial activity was observed in prone compared with supine position during part of baseline, but did not differ overall. Therefore we concluded that the decreased facial activity observed in prone position was likely mediated by prone position promoting sleep while the infants were undisturbed. Consistent with previous studies of pain in preterm neonates, increased arousal from baseline to the invasive event was evident in shifts in sleep–wake states, increased facial activity, and HR.23,27,34,35 However, despite the difference in sleep–wake state during baseline, prone position did not diminish pain reactivity to lance. This finding was consistent with a previous study of environmental interventions during pain in preterm infants of comparable gestational age.36 In that study, simulated rocking promoted quiet sleep, but was not effective in blunting pain response. While prior sleep–wake state alters infant pain response in term born infants,25 it is not clear whether this relationship holds to the same degree in preterm infants. Infant sleep–wake state becomes established at around 36 weeks gestation,37 and prior to this state is less organized and more diffuse.38 More work is needed to understand immature sleep–wake state in preterm infants in relation to pain and stress in the NICU.
Inherently, there are a legion of uncontrolled variables in relation to human infant pain research in a NICU setting. This study suggests that prone or supine infant position does not appear to differentially modulate pain response to blood collection by heel lance. A limitation of this study is that position can be seen on the videotape, which may have affected behavioral, but not physiological parameters. However, the video coders were unaware of the purpose of the study, and the physiological findings during the skin breaking phase of the study were consistent with the behavioral results.
Although the potential benefits of prone position for preterm infants remains controversial,39 in term born infants, the recommendation to place infants in non-prone positions for sleep has been associated with a significant decrease in sudden infant death syndrome.40
Our results show that signs of discomfort and reactivity to the painful medical procedure could be observed in neonates in either the prone or supine position. The findings of our study confirmed those of Stevens et al23 that prone positioning did not decrease pain responses when compared with infants either supine or side-lying. The main contribution of our study was to clarify that the findings of the previous study23 were not due to having combined supine with side-lying in the same group. Together, based on these 2 studies, we conclude that prone or supine positioning should not be considered an environmental comfort intervention for preterm infants for ameliorating discomfort associated with the heel lance procedure for blood sampling in the NICU. The management of position per se is not sufficient for assisting preterm neonates to cope with this painful and potentially stressful event.
ACKNOWLEDGMENTS
The authors thank the staff and families of the Special Care Nursery at B.C. Children's Hospital for their participation in this study, and the staff of the Biobehavioral Research Unit.
Footnotes
Supported by the National Institutes of Health grant HD39783, Canadian Institutes of Health Research grant MOP42469, and a Senior Scholar Award from the Michael Smith Foundation for Health Research to R.E.G., a Visiting Scholar Award (held at U.B.C.) funded by the Foundation for Research Support of the State of Sao Paulo, Brazil to M.B.M.L., and a Canadian Institutes of Health Research/Canadian Occupational Therapy Foundation Post Doctoral Fellowship to L.H.
REFERENCES
- 1.Als H. Toward a synactive theory of development: promise for the assessment and support of infant individuality. Infant Ment Health J. 1982;3:229–243. [Google Scholar]
- 2.Als H, Lawhon G, Duffy FH, et al. Individualized developmental care for the very low-birth-weight preterm infant: medical and neurofunctional effects. JAMA. 1994;272:853–858. [PubMed] [Google Scholar]
- 3.Als H. Reading the premature infant. In: Goldson EE, editor. Nurturing the Premature Infant: Developmental Interventions in the Neonatal Intensive Care Nursery. Oxford University Press; New York, NY: 1999. pp. 18–85. [Google Scholar]
- 4.Fuller BF. Fluctuations in established pain behaviors. Clin Nurs Res. 2000;9:298–316. doi: 10.1177/10547730022158609. [DOI] [PubMed] [Google Scholar]
- 5.Anand KJS. Effects of perinatal pain and stress. In: Mayer EA, Saper CB, editors. Progress in Brain Research. Vol. 122. Elsevier Science; Amsterdam: 2000. pp. 117–129. [DOI] [PubMed] [Google Scholar]
- 6.Grunau RE. Long-term consequences of pain in human neonates. In: Anand KJS, Stevens BJ, McGrath PJ, editors. Pain in Neonates. 2nd Vol. 10. Elsevier Science; Amsterdam: 2000. pp. 55–76. [Google Scholar]
- 7.Barker DP, Rutter N. Exposure to invasive procedures in neonatal intensive care unit admission. Arch Dis Child. 1995;72:F47–F48. doi: 10.1136/fn.72.1.f47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott CS, Riggs KW, Ling EW, et al. Morphine pharmacokinetics and pain assessment in premature babies. J Pediatr. 1999;135:423–429. doi: 10.1016/s0022-3476(99)70163-0. [DOI] [PubMed] [Google Scholar]
- 9.Stevens B, Gibbins S, Franck LS. Treatment of pain in the neonatal intensive care unit. Pediatr Clin North Am. 2000;47:633–650. doi: 10.1016/s0031-3955(05)70230-3. [DOI] [PubMed] [Google Scholar]
- 10.Corff KLE, Seidman R, Venkataraman MBBS, et al. Facilitated tucking: a nonpharmacological comfort measure for pain in preterm neonates. JOGNN. 1995;24:143–145. doi: 10.1111/j.1552-6909.1995.tb02456.x. [DOI] [PubMed] [Google Scholar]
- 11.Franck LS, Lawhon G. Environmental and behavioral strategies to prevent and manage neonatal pain. Semin Perinato. 1998;22:434–443. doi: 10.1016/s0146-0005(98)80059-1. [DOI] [PubMed] [Google Scholar]
- 12.Stevens B, Gibbins S, Franck LS. Treatment of pain in the neonatal intensive care unit. Pediatr Clin North Am. 2000;47:633–650. doi: 10.1016/s0031-3955(05)70230-3. [DOI] [PubMed] [Google Scholar]
- 13.Fearon I, Kisilevsky BS, Hains SMJ, et al. Swaddling after heel lance: age-specific effects on behavioral recovery in preterm infants. J Dev Behav Pediatr. 1997;18:222–232. [PubMed] [Google Scholar]
- 14.Gardner S, Lubchenco LO. The neonatal and the environment: impact on development. In: Merestein GB, Gardner SL, editors. Handbook of Neonatal Intensive Care. 4th Mosby; Toronto: 19851998. [Google Scholar]
- 15.Martin RJE, Herrell N, Rubin D, et al. Effects of supine and prone positions on arterial oxygen tension in the preterm infant. Pediatrics. 1979;63:528–531. [PubMed] [Google Scholar]
- 16.Hutchinson AA, Ross KR, Russel G. The effect of posture on ventilation and lung mechanisms in preterm and light-for-date infants. Pediatrics. 1979;64:429–432. [PubMed] [Google Scholar]
- 17.Brackbill Y, Douthitt TC, West H. Psychophysiologic effects in the neonate of prone versus supine placement. J Pediatr. 1973;82:82–84. doi: 10.1016/s0022-3476(73)80017-4. [DOI] [PubMed] [Google Scholar]
- 18.Masterson J, Zucker C, Schulze K. Prone and supine positioning effects on energy expenditure and behavior of low birth weight neonates. Pediatrics. 1987;80:689–692. [PubMed] [Google Scholar]
- 19.Goto KG, Mirmiran M, Adams M, et al. More awakenings and heart variability during supine sleep in preterm infants. Pediatrics. 1999;10:603–609. doi: 10.1542/peds.103.3.603. [DOI] [PubMed] [Google Scholar]
- 20.McIntosh N, Van Veen L, Barmeyer H. The pain of heel prick and its measurement in preterm infants. Pain. 1993;52:71–74. doi: 10.1016/0304-3959(93)90116-7. [DOI] [PubMed] [Google Scholar]
- 21.Johnston CC, Stevens BJ, Franck LS, et al. Factors explaining lack of response to heel lance in preterm newborns. J Obstetr Gynecol Neonat Nurs. 1999;28:587–594. doi: 10.1111/j.1552-6909.1999.tb02167.x. [DOI] [PubMed] [Google Scholar]
- 22.Johnston CC, Sherrard A, Stevens B, et al. Do cry features reflect pain intensity in preterm neonates. Biol Neonate. 1999;76:120–140. doi: 10.1159/000014150. [DOI] [PubMed] [Google Scholar]
- 23.Stevens B, Johnston C, Franck L, et al. The efficacy of developmentally sensitive interventions and sucrose for relieving procedural pain in very low birth weight neonates. Nurs Res. 1999;48:35–43. doi: 10.1097/00006199-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Porter FL, Wolf CM, Miller JP. The effect of handling and immobilization on the response to acute pain in newborn infants. Pediatrics. 1998;102:1383–1389. doi: 10.1542/peds.102.6.1383. [DOI] [PubMed] [Google Scholar]
- 25.Grunau RVE, Craig KD. Pain expression in neonates:facial action and cry. Pain. 1987;28:395–410. doi: 10.1016/0304-3959(87)90073-X. [DOI] [PubMed] [Google Scholar]
- 26.Craig KD, Whitfield MF, Grunau RVE, et al. Pain in the preterm neonate: behavioral and physiological indices. Pain. 1993;52:287–299. doi: 10.1016/0304-3959(93)90162-I. [DOI] [PubMed] [Google Scholar]
- 27.Grunau RE, Oberlander T, Holsti L, et al. Bedside application of the Neonatal Facial Coding System in pain assessment of premature neonates. Pain. 1998;76:277–286. doi: 10.1016/S0304-3959(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 28.Als H. Manual for the Naturalistic Observation of Newborn Behavior (Preterm and Fullterm Infants) The Children's Hospital; Boston: 1984. [Google Scholar]
- 29.HR View Software [computer program]. Version 2.0. Boston Medical Technologies; Brighton, MA: 1996. [Google Scholar]
- 30.Noldus Information Technology . The Observer, Base Package for Windows. Reference Manual. Version 3.0 ed. The Netherlands; Wageningen: 1995. [Google Scholar]
- 31.Lee SK, Ohlsson A, Synnes AR, et al. Mortality variations and illness severity (SNAP-II) in Canadian NICUs. Pediatr Res. 1999;45:248A. [Google Scholar]
- 32.Bozynski ME, Naglie RA, Nicks JJ, et al. Lateral positioning of the stable ventilated very-low-birth weight infant: effect on transcutaneous oxygen and carbon dioxide. Am J Dis Child. 1988;142:200–202. doi: 10.1001/archpedi.1988.02150020102039. [DOI] [PubMed] [Google Scholar]
- 33.Mizuno K, Itabashi K, Okayama K. Effect of body position on the blood gases and ventilation volume of infants with chronic lung disease before and after feeding. Am J Perintol. 1995;12:275–277. doi: 10.1055/s-2007-994473. [DOI] [PubMed] [Google Scholar]
- 34.Grunau RE, Holsti L, Whitfield MF, et al. Are twitches, startles, and body movements pain indicators in extremely low birth weight infants? Clin J Pain. 2000;16:37–45. doi: 10.1097/00002508-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Johnston CC, Stevens BJ, Yang F, et al. Differential response to pain by very premature neonates. Pain. 1995;61:471–479. doi: 10.1016/0304-3959(94)00213-X. [DOI] [PubMed] [Google Scholar]
- 36.Johnston CC, Stremler RL, Stevens BJ, et al. Effectiveness of oral sucrose and simulated rocking on pain response in preterm neonates. Pain. 1997;72:193–199. doi: 10.1016/s0304-3959(97)00033-x. [DOI] [PubMed] [Google Scholar]
- 37.Nijuis J, van de Pas M. Behavioral states and their ontogeny: human studies. Semin Perinatol. 1992;16:206–210. [PubMed] [Google Scholar]
- 38.Holditch-Davis D. The development of sleeping and waking sates in highrisk preterm infants. Inf Beh Dev. 1990;13:513–531. [Google Scholar]
- 39.Kneene DJ, Wimmer JE, Mathew OP. Does supine positioning increase apnea, bradycardia, and desaturation in preterm infants? J Perinatol. 2000;1:17–20. doi: 10.1038/sj.jp.7200301. [DOI] [PubMed] [Google Scholar]
- 40.American Academy of Pediatrics Task Force on Infant Positioning and SIDS Positioning and sudden infant death syndrome (SIDS). Update. Pediatrics. 1996;98:1216–1218. [PubMed] [Google Scholar]


