Abstract
1. A method is described by which a selective lesion can be made in vitro in the transverse tubules of frog skeletal muscle.
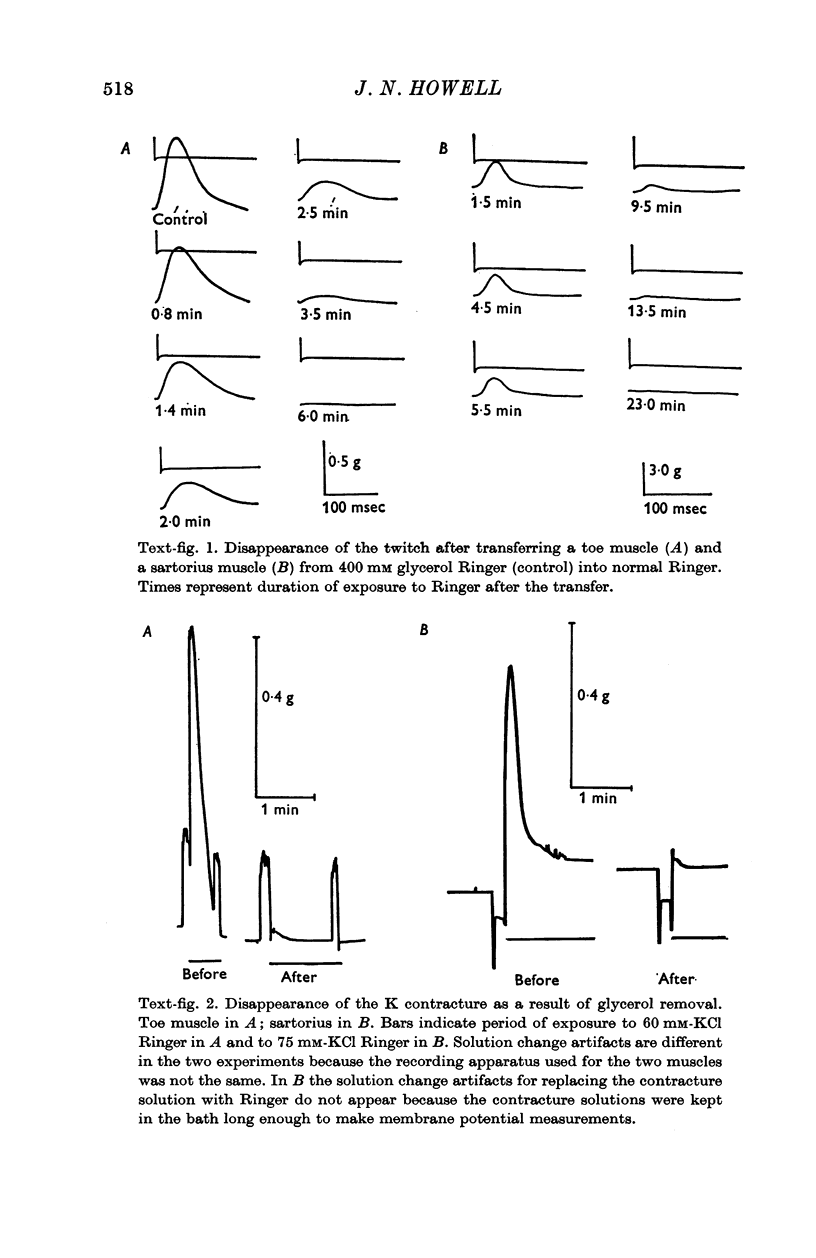
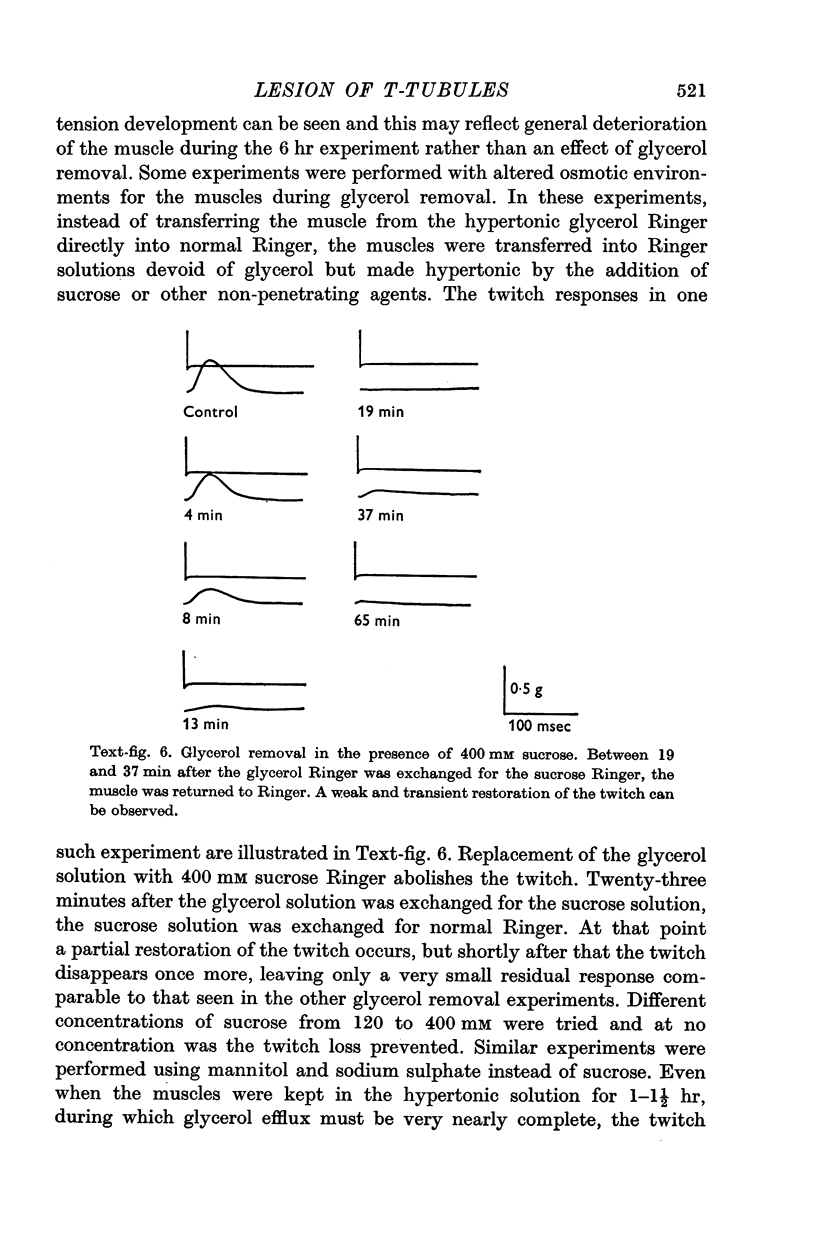
2. The method consists of exposing the muscle for 1 hr or more to a buffered salt solution made hypertonic by the inclusion of 400 mM glycerol and then returning the muscle to an isotonic salt solution. The lesion is induced during the washout of the glycerol.
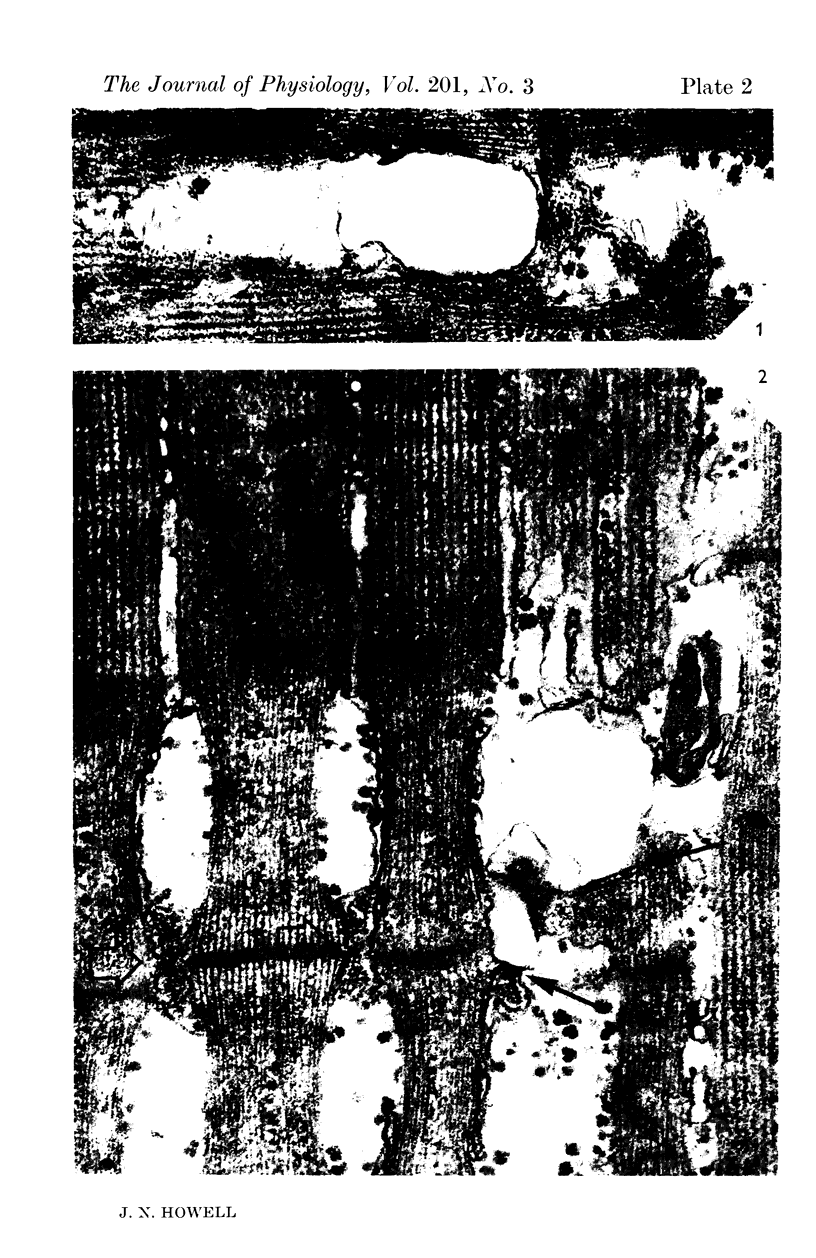
3. Electron micrographs reveal that the lesion consists of a rearrangement of the T-system membranes in such a way that the continuity of the tubules is lost. The membranes appear to coalesce into large vesicles scattered irregularly throughout the sarcoplasm.
4. The glycerol treatment results in a depression of the resting potential of up to 30 mV. The treated fibres are depolarized by high concentrations of K as are normal muscle fibres.
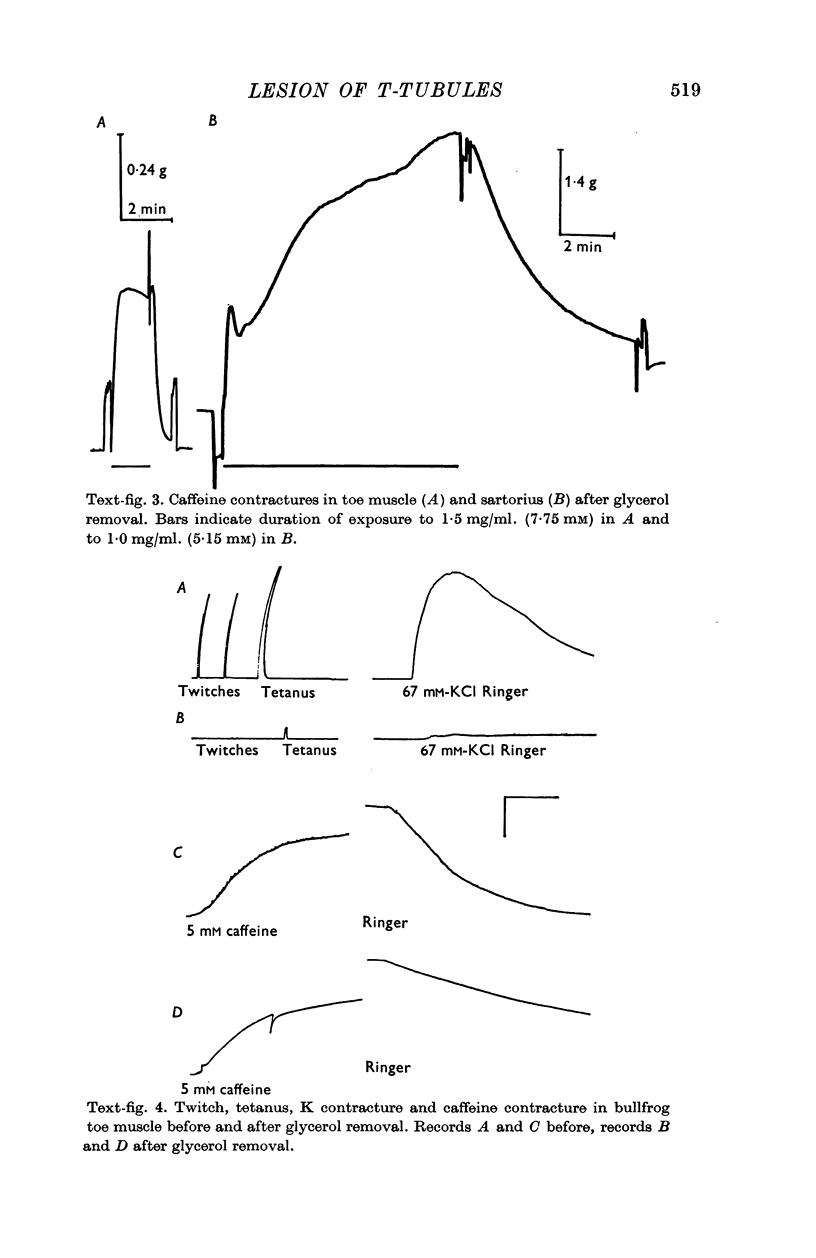
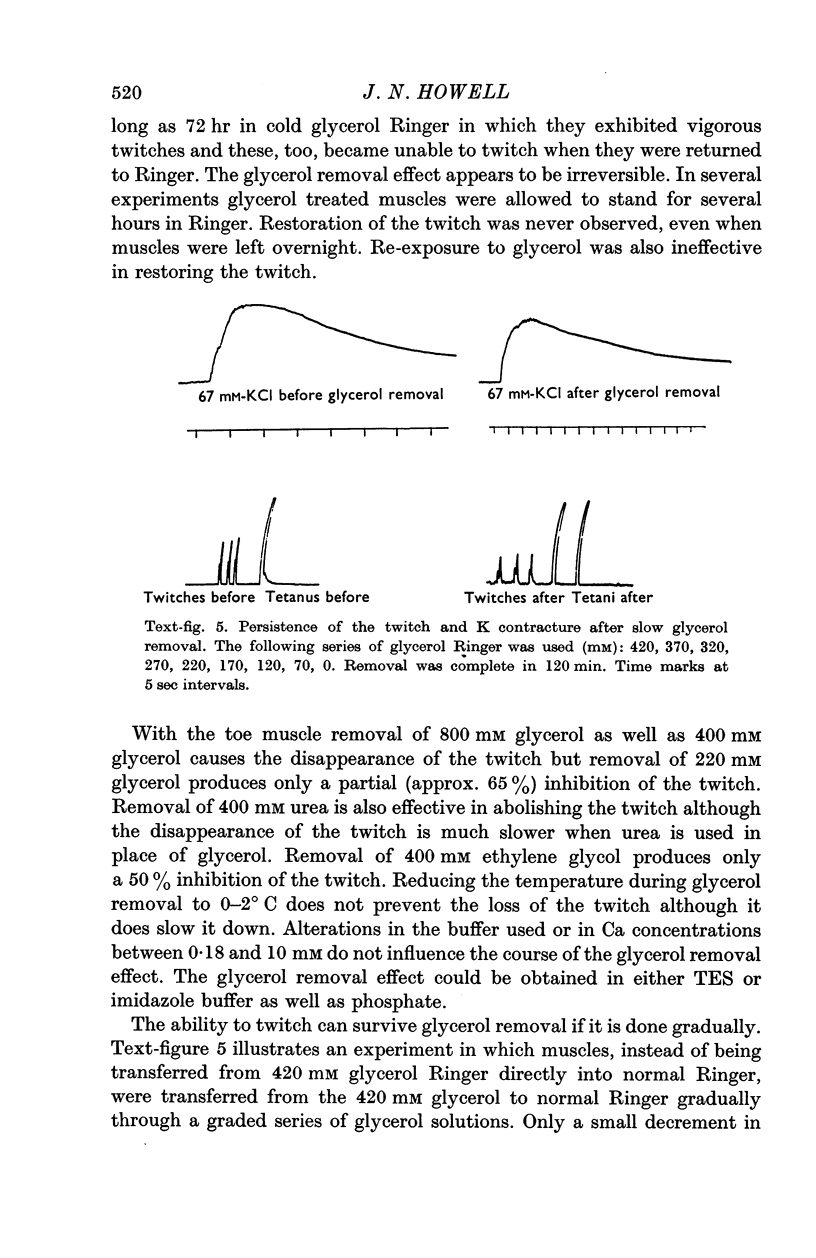
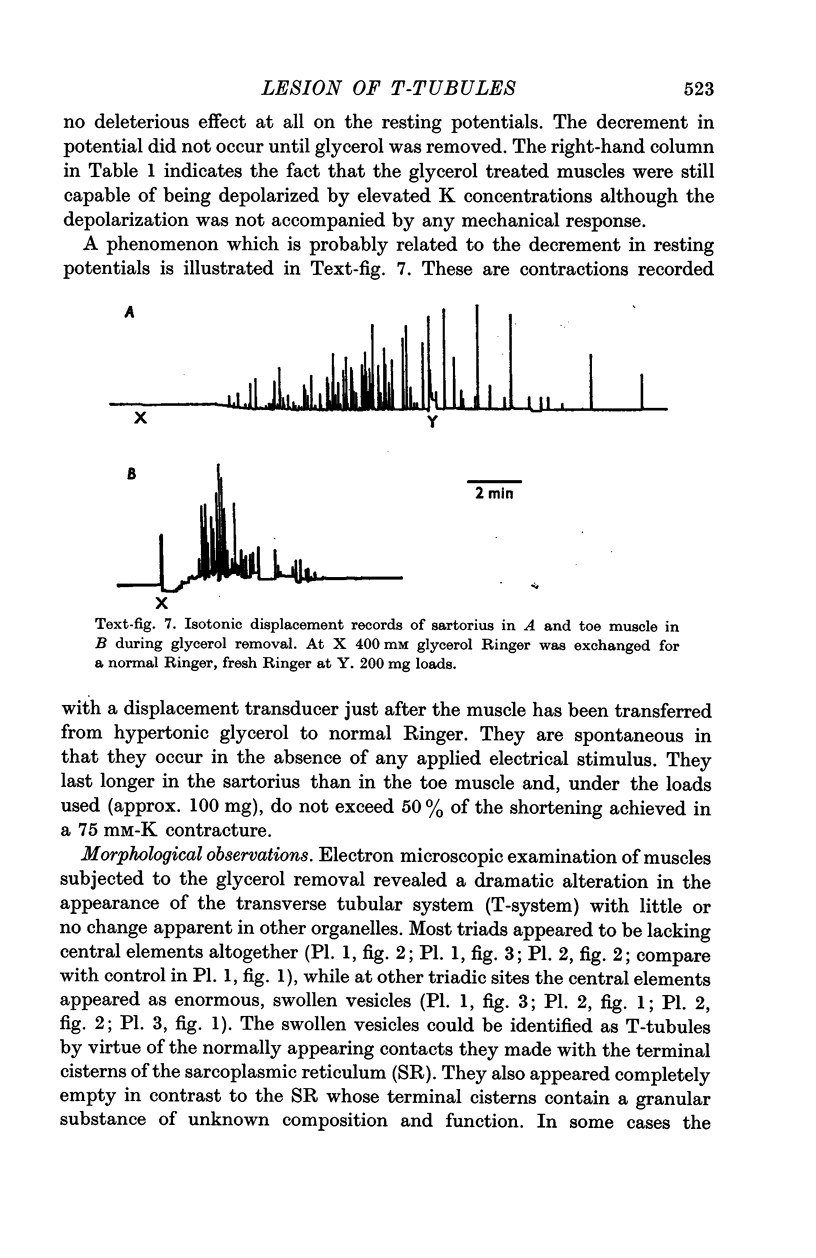
5. T-tubule lesioned fibres are unable to respond mechanically either to electrical stimulation or to elevated K but they do contract in the presence of caffeine and relax when the caffeine is removed.
6. Problems concerning the variability of the procedure are presented and certain considerations concerning the mechanism of the effect are discussed.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIANCHI C. P., SHANES A. M. Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J Gen Physiol. 1959 Mar 20;42(4):803–815. doi: 10.1085/jgp.42.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., WALSTER G. STUDIES ON THE MICRO-INJECTION OF VARIOUS SUBSTANCES INTO CRAB MUSCLE FIBRES. J Physiol. 1963 Nov;169:353–372. doi: 10.1113/jphysiol.1963.sp007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSAPO A., SUZUKI T. The effectiveness of the longitudinal field, coupled with depolarization in activating frog twitch muscles. J Gen Physiol. 1958 May 20;41(5):1083–1098. doi: 10.1085/jgp.41.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A. P., Leo B. Effects of ATP on the interaction of Ca++, Mg++, and K+ with fragmented sarcoplasmic reticulum isolated from rabbit skeletal muscle. J Gen Physiol. 1967 May;50(5):1327–1352. doi: 10.1085/jgp.50.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B., Eisenberg R. S. Selective disruption of the sarcotubular system in frog sartorius muscle. A quantitative study with exogenous peroxidase as a marker. J Cell Biol. 1968 Nov;39(2):451–467. doi: 10.1083/jcb.39.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B., Eisenberg R. S. Transverse tubular system in glycerol-treated skeletal muscle. Science. 1968 Jun 14;160(3833):1243–1244. doi: 10.1126/science.160.3833.1243. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., Gage P. W. Frog skeletal muscle fibers: changes in electrical properties after disruption of transverse tubular system. Science. 1967 Dec 29;158(3809):1700–1701. doi: 10.1126/science.158.3809.1700. [DOI] [PubMed] [Google Scholar]
- Endo M. Entry of fluorescent dyes into the sarcotubular system of the frog muscle. J Physiol. 1966 Jul;185(1):224–238. doi: 10.1113/jphysiol.1966.sp007983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, GOLDSTEIN D. A., HELLAM D. C. THE AFTER-POTENTIAL THAT FOLLOWS TRAINS OF IMPULSES IN FROG MUSCLE FIBERS. J Gen Physiol. 1964 May;47:929–952. doi: 10.1085/jgp.47.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJINO M., FUJINO S. DIE BEZIEHUNG ZWISCHEN COFFEIN-KONTRAKTUR UND CALCIUM AM FROSCHSKELETMUSKEL. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964;278:478–486. [PubMed] [Google Scholar]
- FUJINO M., YAMAGUCHI T., SUZUKI K. 'Glycerol effect' and the mechanism linking excitation of the plasma membrane with contraction. Nature. 1961 Dec 23;192:1159–1161. doi: 10.1038/1921159a0. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Action potentials without contraction in frog skeletal muscle fibers with disrupted transverse tubules. Science. 1967 Dec 29;158(3809):1702–1703. doi: 10.1126/science.158.3809.1702. [DOI] [PubMed] [Google Scholar]
- Gruener R. Caffeine contractures in sarcolemma-free muscle fibres. J Physiol. 1967 Jul;191(2):106P–108P. [PubMed] [Google Scholar]
- HILL A. V. The abrupt transition from rest to activity in muscle. Proc R Soc Lond B Biol Sci. 1949 Oct;136(884):399–420. doi: 10.1098/rspb.1949.0033. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The effect of sudden changes in ionic concentrations on the membrane potential of single muscle fibres. J Physiol. 1960 Sep;153:370–385. doi: 10.1113/jphysiol.1960.sp006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARTH J. V. The effect of hypertonic solutions on the velocity of shortening of the frog's sartorius. J Physiol. 1957 Jun 18;137(1):23–4P. [PubMed] [Google Scholar]
- HUXLEY A. F., TAYLOR R. E. Local activation of striated muscle fibres. J Physiol. 1958 Dec 30;144(3):426–441. doi: 10.1113/jphysiol.1958.sp006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUTSCHA W., PAUSCHINGER P., BRECHT K. [On the problem of inhibition of the interaction of urea and thiourea in living tonic and phasic skeletal muscle]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963;277:194–206. [PubMed] [Google Scholar]
- Krolenko S. A., Adamian S. Ia. Pronitsaemost' myshechnykh volokon dlia neélektrolitov. Tsitologiia. 1967 Feb;9(2):185–192. [PubMed] [Google Scholar]
- Krolenko S. A., Adamian S. Ia, Shvinka N. E. Vakuolizatsiia skeletnykh myshechnykh volokon. I. Vakuolizatsiia volokon posle vykhoda iz nikh riada nizkomolekuliarnykh neèlektrolitov. Tsitologiia. 1967 Nov;9(11):1346–1353. [PubMed] [Google Scholar]
- Krolenko S. A. Vakuolizatsiia skeletnykh myshechnykh volokon. II. Lokalizatsiia ferritina i ul'trastruktura volokon. Tsitologiia. 1968 Jul;10(7):803–811. [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- SANDOW A. Contracture responses of skeletal muscle. Am J Phys Med. 1955 Feb;34(1):145–160. [PubMed] [Google Scholar]
- STEN-KNUDSEN O. Is muscle contraction initiated by internal current flow? J Physiol. 1960 May;151:363–384. doi: 10.1113/jphysiol.1960.sp006444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E., Steinbach A. Persistence of excitation contraction coupling in "slow" muscle fibres after a treatment that destroys transverse tubules in "twitch" fibres. Nature. 1968 May 18;218(5142):681–682. doi: 10.1038/218681a0. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. G. The effect of disruption of the T-tubules on calcium efflux from frog skeletal muscle. Comp Biochem Physiol. 1968 Jul;26(1):377–379. doi: 10.1016/0010-406x(68)90345-9. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI T., MATSUSHIMA T., FUJINO M., NAGAI T. The excitation-contraction coupling of the skeletal muscle and the 'glycerol effect'. Jpn J Physiol. 1962 Apr 15;12:129–142. doi: 10.2170/jjphysiol.12.129. [DOI] [PubMed] [Google Scholar]










