Abstract
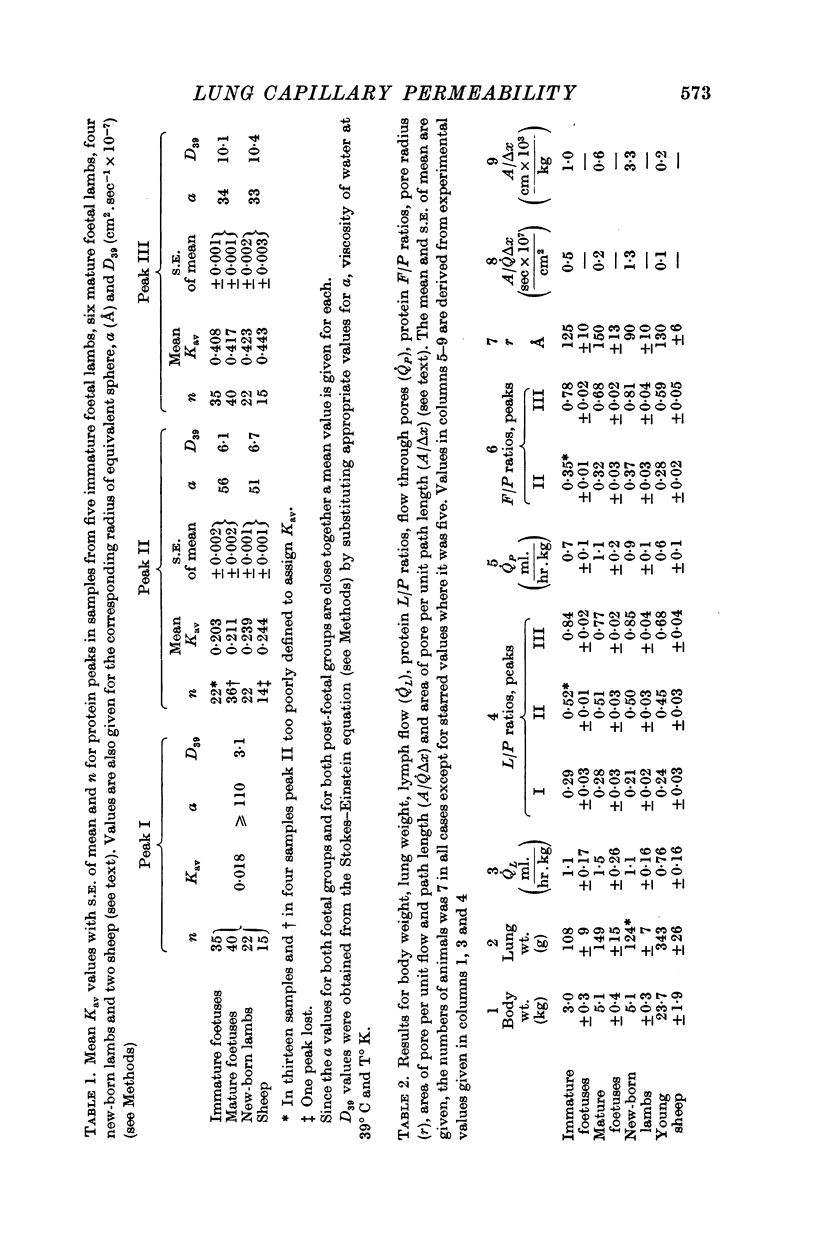
1. The permeability of lung capillaries to macromolecules was investigated in immature and mature foetal lambs, new-born lambs and young sheep. The placental circulation of the foetal animals was maintained intact after delivery by Caesarian section. New-born lambs and sheep were mechanically ventilated. Samples of plasma and lymph that had drained from the lung via the thoracic duct were collected over a period of 1-5 hr.
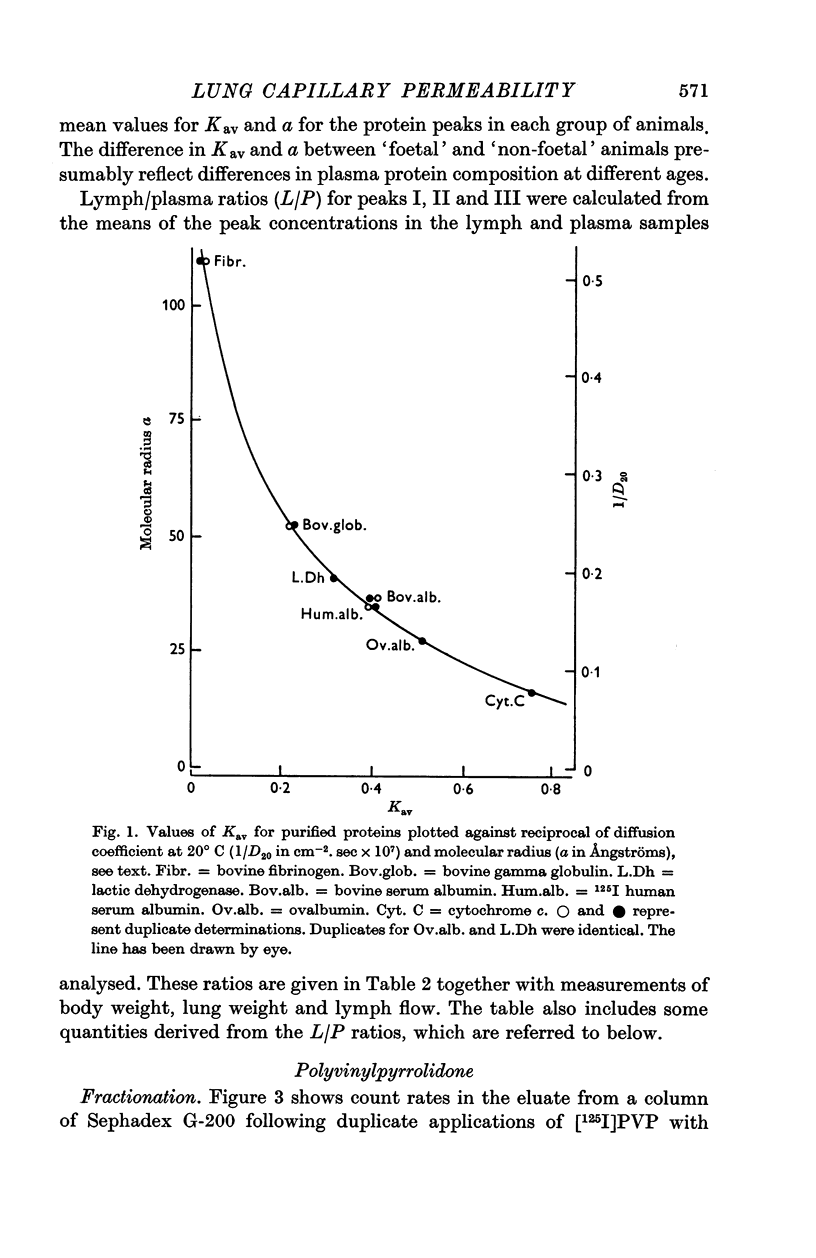
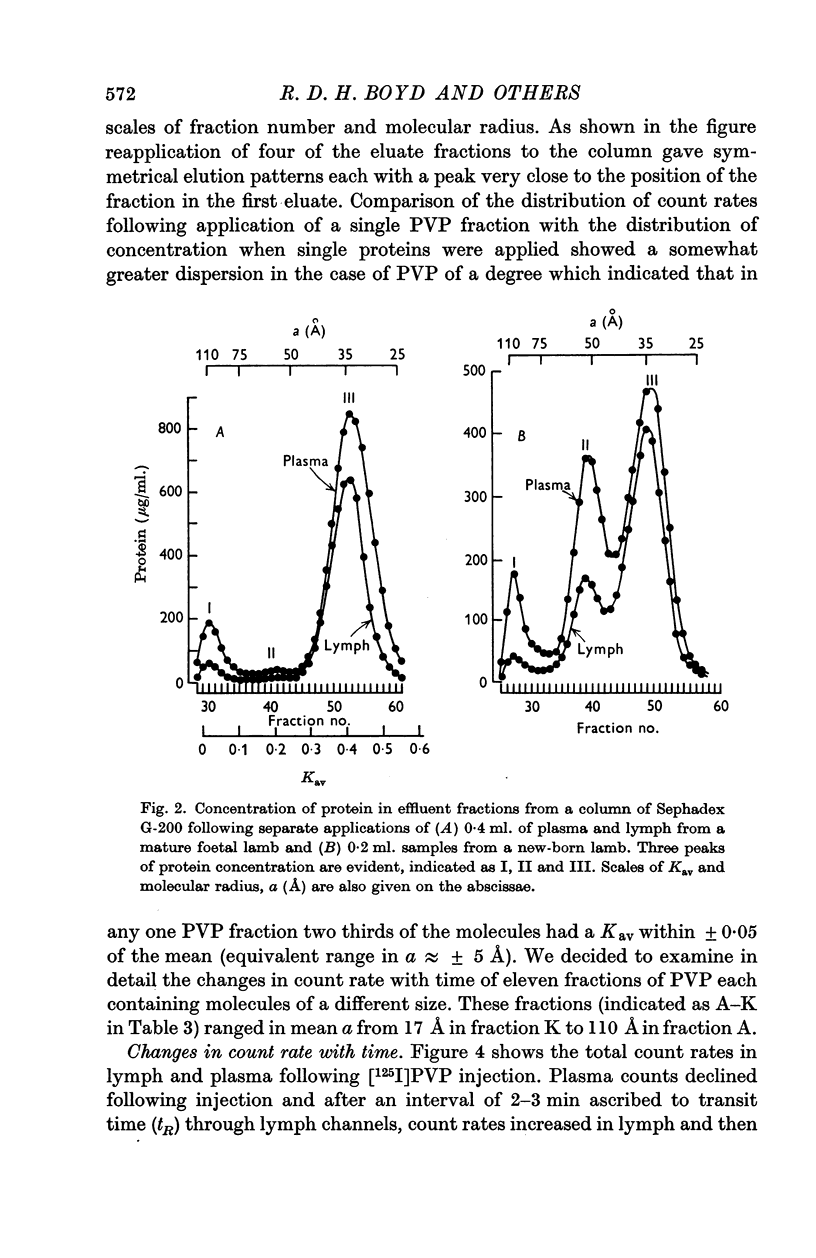
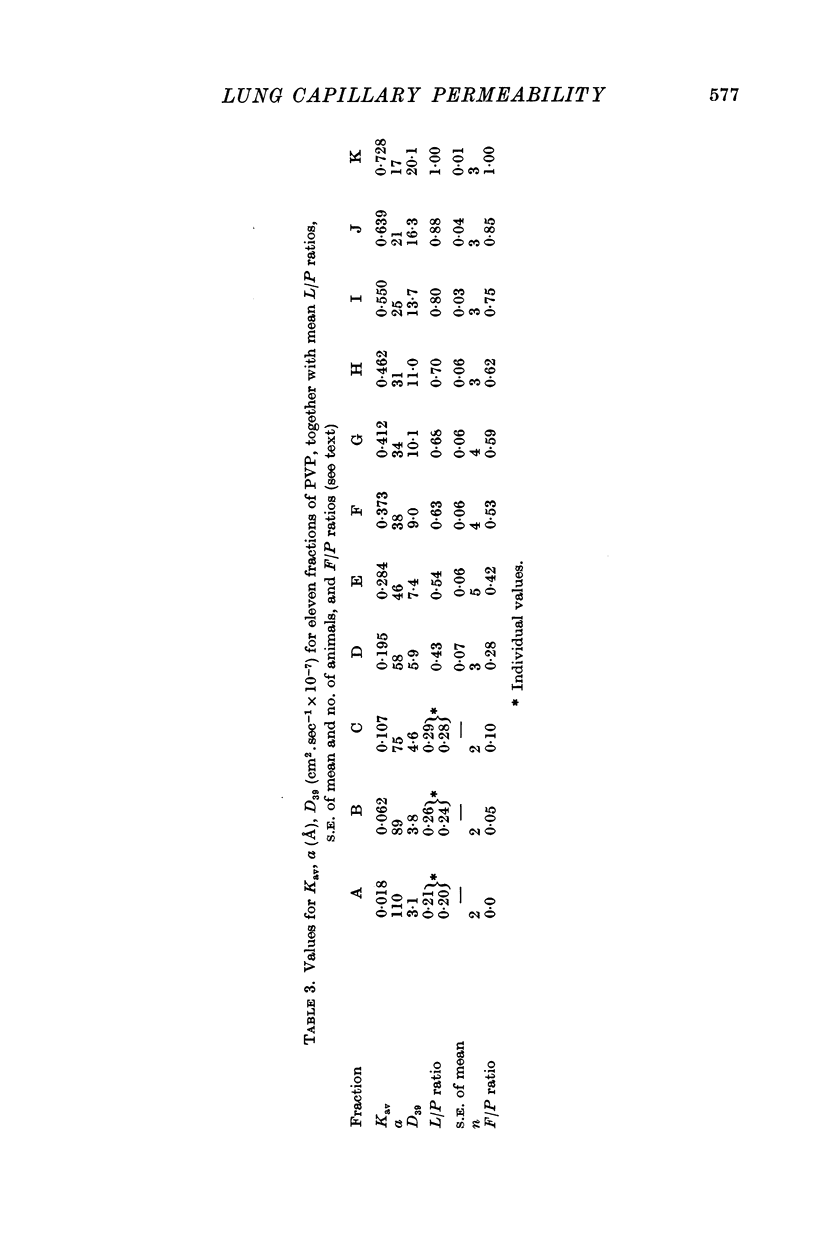
2. The proteins in plasma and lymph samples were separated by fractionation on columns of Sephadex G-200. Plasma yielded three peaks of protein concentration. The Kav value of each peak was determined, and, by calibrating the columns with known proteins, the mean radius of equivalent sphere (a) of the proteins in peak I was shown to be similar to that of fibrinogen ≥ 110 Å, peak II to γ-globulin ≈ 54 Å and peak III to albumin ≈ 34 Å. Lung lymph contained the same three constituent peaks as plasma but in lower concentration. In all four groups mean lymph/plasma concentration (L/P) ratio was significantly different for each of the three peaks, being lowest for the largest molecules (peak I) and highest for the smallest (peak III).
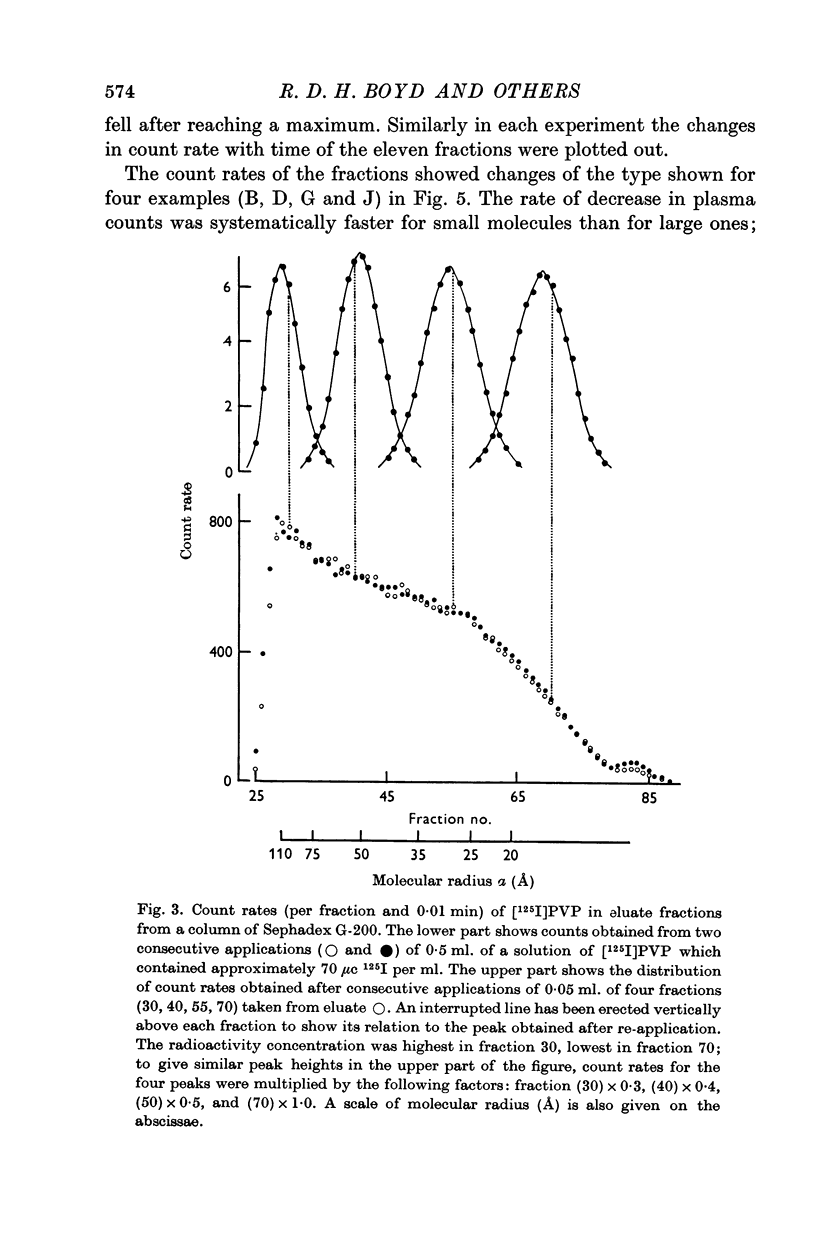
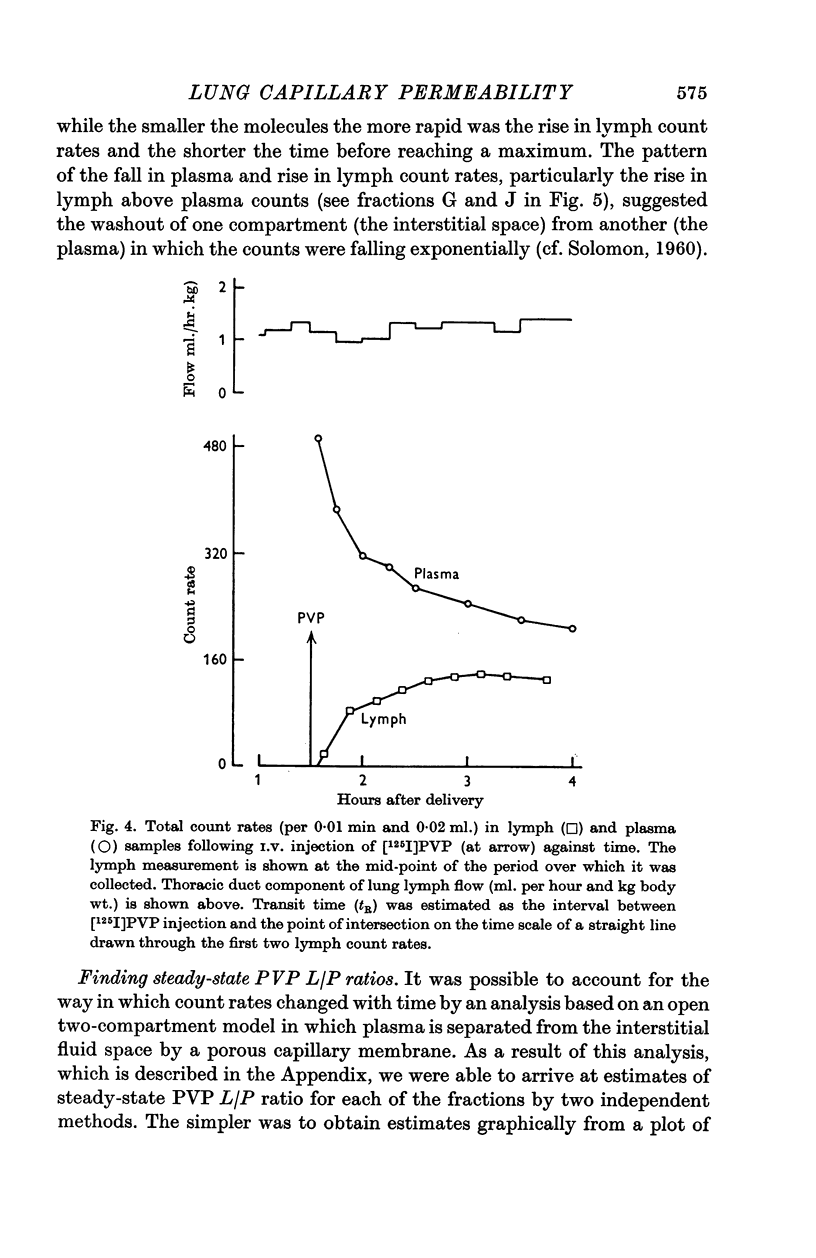
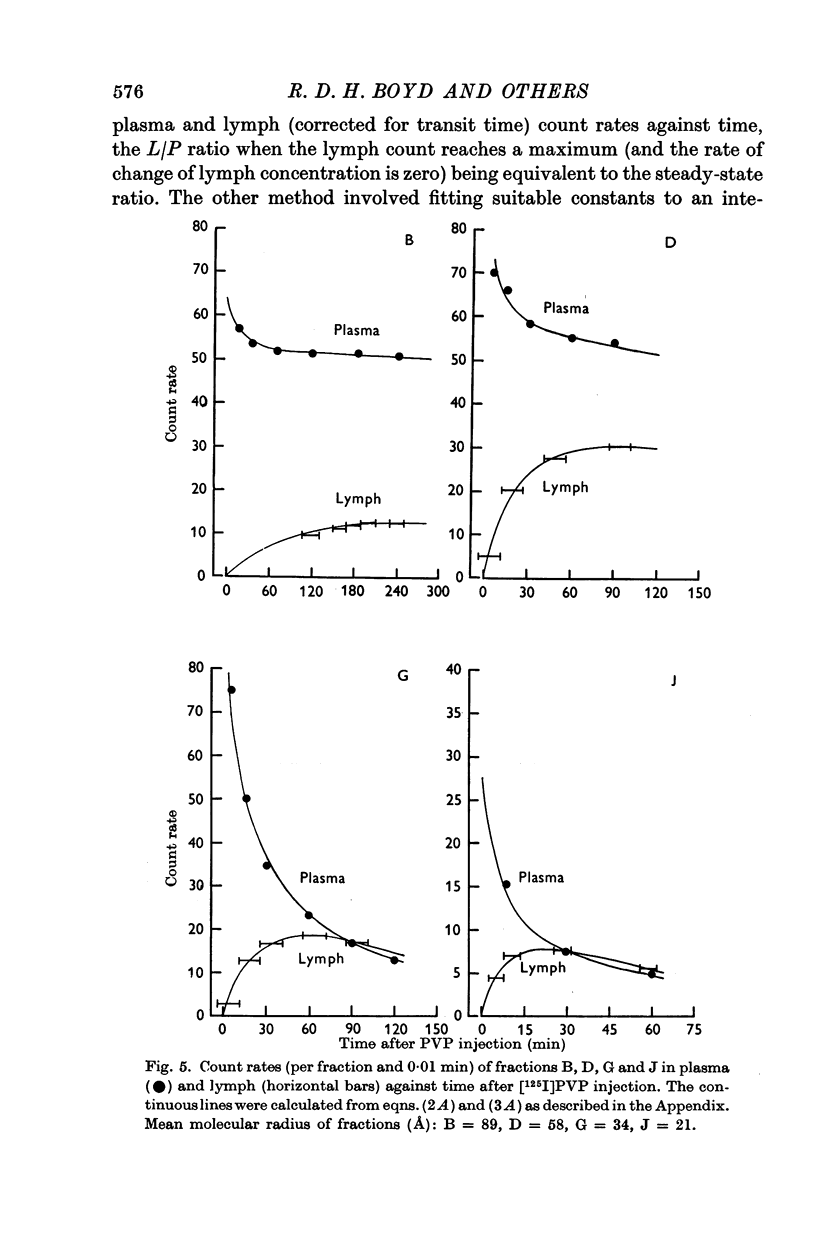
3. In five mature foetal lambs polydisperse polyvinylpyrrolidone labelled with 125I ([125I]PVP) was injected I.V. early in the experiment: count rates in fractionated samples showed for plasma a continuous decline with time after injection, and for lung lymph an increase to a maximum then a decline. Steady-state L/P ratios for eleven fractions of PVP of differing molecular size ranging from 110 to 17 Å were derived by compartmental analysis. For a given molecular size PVP L/P ratios were similar to protein L/P values.
4. The regression of PVP L/P ratio on Kav was linear (correlation coefficient r = 0·99), and the slope of the regression of protein L/P ratio on Kav was significantly steeper for new-born lambs than for mature foetuses (P < 0·025) and sheep (P < 0·005), and steeper for immature foetuses than sheep (P < 0·01).
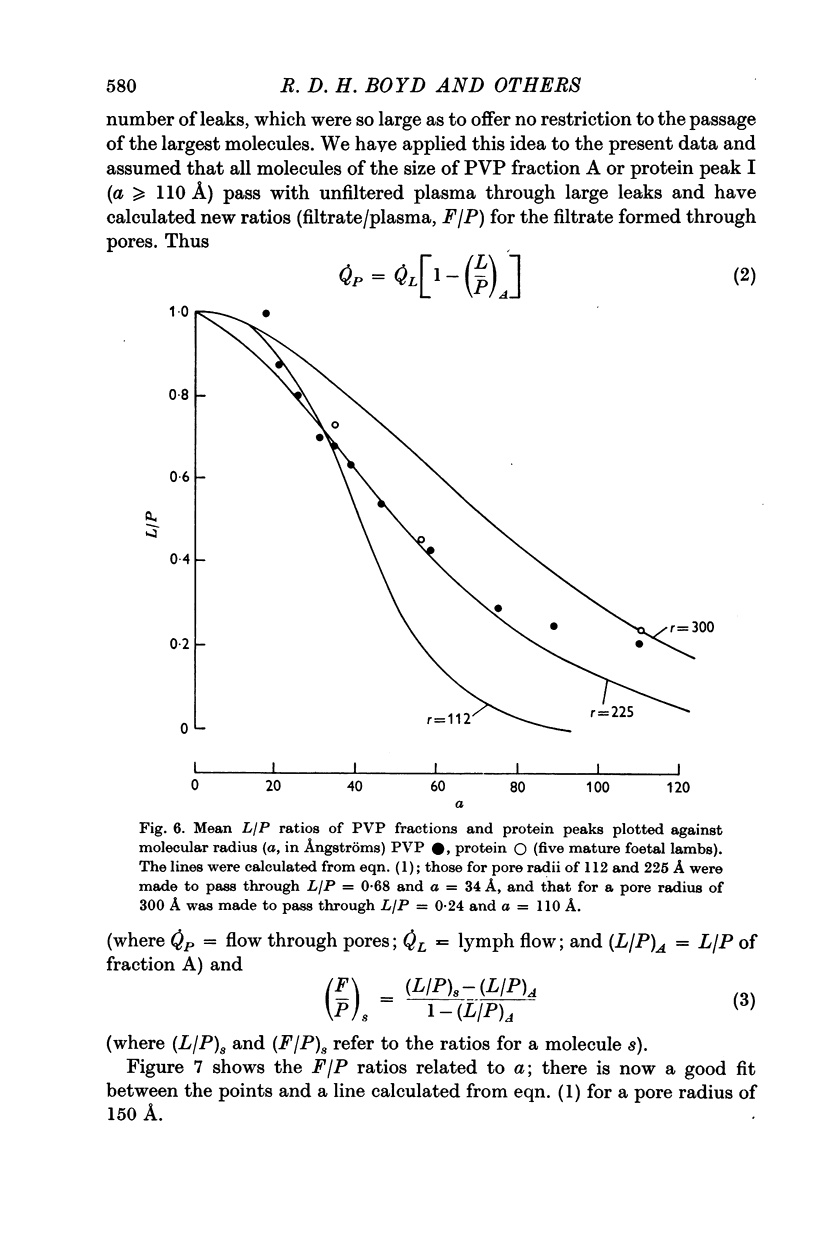
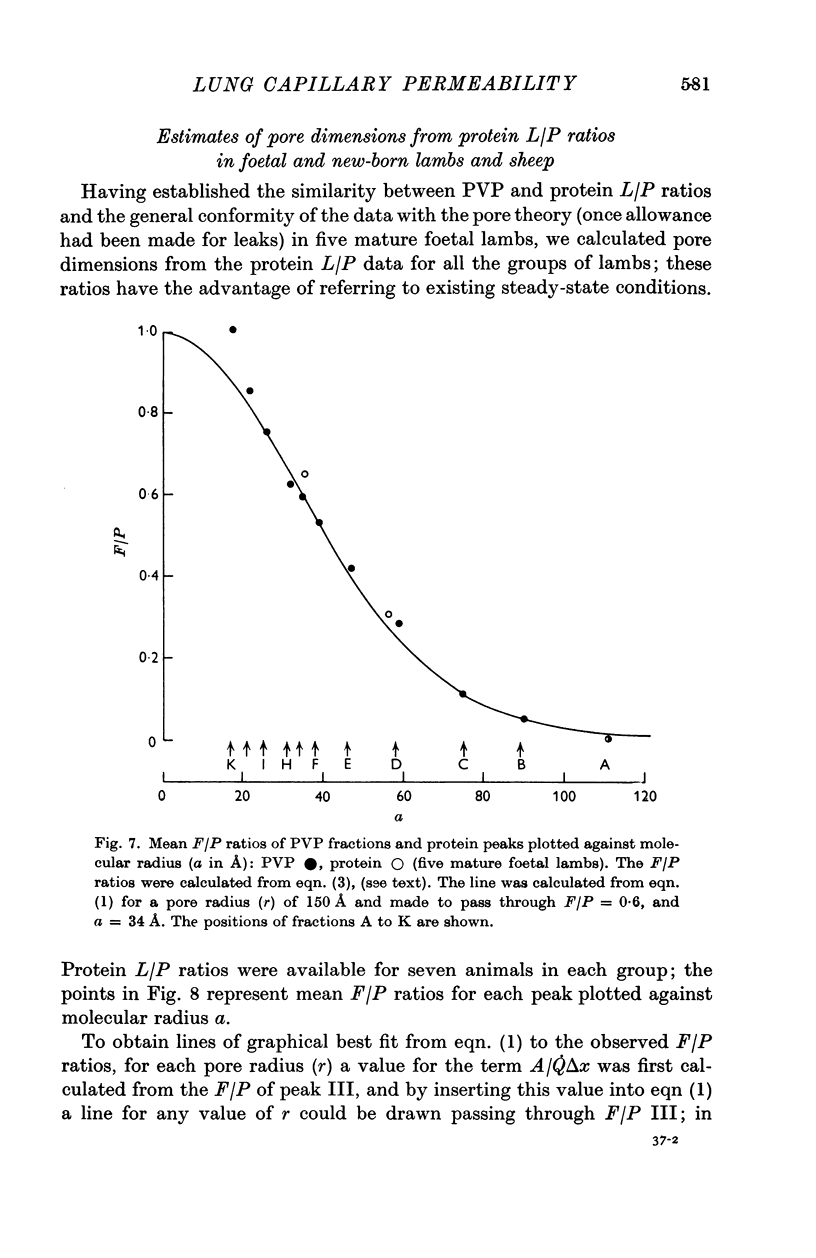
5. PVP and protein L/P ratios (mature foetuses) plotted against a showed a sigmoid relation with agreement between the two sets of L/P ratios. The goodness of fit between our experimental results and Landis & Pappenheimer's (1963) capillary pore theory (eqn. (1)) was examined: L/P ratios for the larger molecules (≥ 75 Å) appeared to be too high. By recalculating ratios on the assumption that the largest molecules (110 Å) escape unrestricted from the capillary via leaks, the discrepancy disappears.
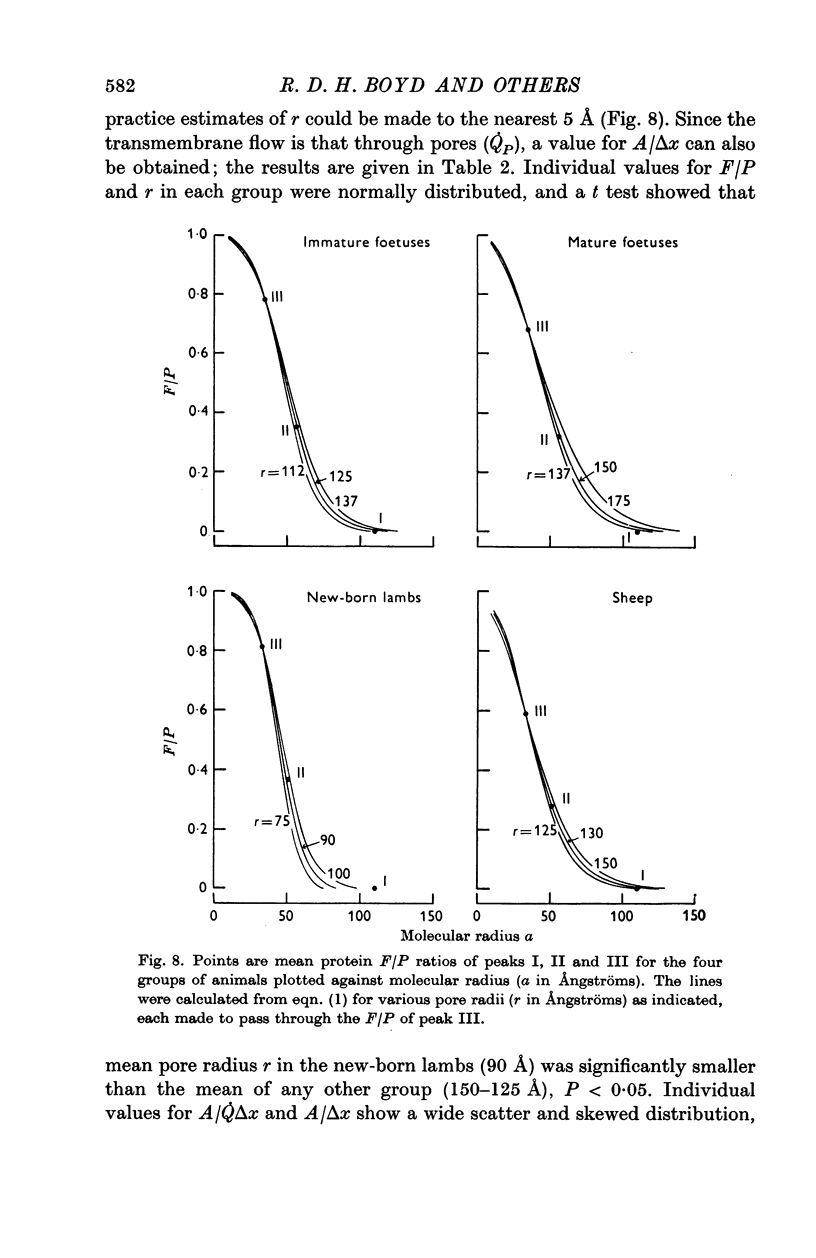
6. Values for pore radius (r), and pore area per unit path length (A/Δx) have been calculated for each of the four groups; r ranged from 90 to 150 Å, A/Δx from 3·3 to 0·2 cm × 103.kg-1. In new-born lambs the value of r was significantly smaller, and A/Δx larger than that of any other group. The inferences to be drawn from these results are discussed.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREESE R., D'SILVA J. L., SHAW D. M. Interfibre fluid from guinea-pig muscle. J Physiol. 1962 Jun;162:44–53. doi: 10.1113/jphysiol.1962.sp006913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES G. S., MOTT J. C., WIDDICOMBE J. G., WYATT D. G. Changes in the lungs of the new-born lamb. J Physiol. 1953 Jul;121(1):141–162. doi: 10.1113/jphysiol.1953.sp004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAJL-PECZALSKA K. PLASMA PROTEIN COMPOSITION OF HYALINE MEMBRANE IN THE NEWBORN AS STUDIES BY IMMUNOFLUORESCENCE. Arch Dis Child. 1964 Jun;39:226–231. doi: 10.1136/adc.39.205.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GITLIN D., CRAIG J. M. The nature of the hyaline membrane in asphyxia of the newborn. Pediatrics. 1956 Jan;17(1):64–71. [PubMed] [Google Scholar]
- Gibbs D. F., Bright C. M. Improved automated methods of protein determination. J Clin Pathol. 1968 Nov;21(6):777–780. doi: 10.1136/jcp.21.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys P. W., Normand I. C., Reynolds E. O., Strang L. B. Pulmonary lymph flow and the uptake of liquid from the lungs of the lamb at the start of breathing. J Physiol. 1967 Nov;193(1):1–29. doi: 10.1113/jphysiol.1967.sp008340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDIS E. M. HETEROPOROSITY OF THE CAPILLARY WALL AS INDICATED BY CINEMATOGRAPHIC ANALYSIS OF THE PASSAGE OF DYES. Ann N Y Acad Sci. 1964 Aug 27;116:765–773. doi: 10.1111/j.1749-6632.1964.tb52544.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E., SCHOEFL G. I. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol. 1961 Dec;11:607–626. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYANT N. B. Observation on the metabolism of human gamma globulin labelled by radioactive iodine. Clin Sci. 1952 May;11(2):191–201. [PubMed] [Google Scholar]
- PAPPENHEIMER J. R. Passage of molecules through capillary wals. Physiol Rev. 1953 Jul;33(3):387–423. doi: 10.1152/physrev.1953.33.3.387. [DOI] [PubMed] [Google Scholar]
- RENKIN E. M. TRANSPORT OF LARGE MOLECULES ACROSS CAPILLARY WALLS. Physiologist. 1964 Feb;60:13–28. [PubMed] [Google Scholar]
- SHIRLEY H. H., Jr, WOLFRAM C. G., WASSERMAN K., MAYERSON H. S. Capillary permeability to macromolecules: stretched pore phenomenon. Am J Physiol. 1957 Aug;190(2):189–193. doi: 10.1152/ajplegacy.1957.190.2.189. [DOI] [PubMed] [Google Scholar]
- STERLING K. The turnover rate of serum albumin in man as measured by I131-tagged albumin. J Clin Invest. 1951 Nov;30(11):1228–1237. doi: 10.1172/JCI102542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger-Keeley E. E., Karnovsky M. J. The ultrastructural basis of alveolar-capillary membrane permeability to peroxidase used as a tracer. J Cell Biol. 1968 Jun;37(3):781–793. doi: 10.1083/jcb.37.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A. K. Characterization of biological membranes by equivalent pores. J Gen Physiol. 1968 May;51(5 Suppl):335S+–335S+. [PubMed] [Google Scholar]
- WINNE D. DIE CAPILLARPERMEABILITAET HOCHMOLEKULARER SUBSTANZEN. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 Mar 18;283:119–136. [PubMed] [Google Scholar]


