Abstract
1. An experimental method for measuring ionic influxes during voltage clamp in the giant axon of Dosidicus is described; the technique combines intracellular perfusion with a method for controlling membrane potential.
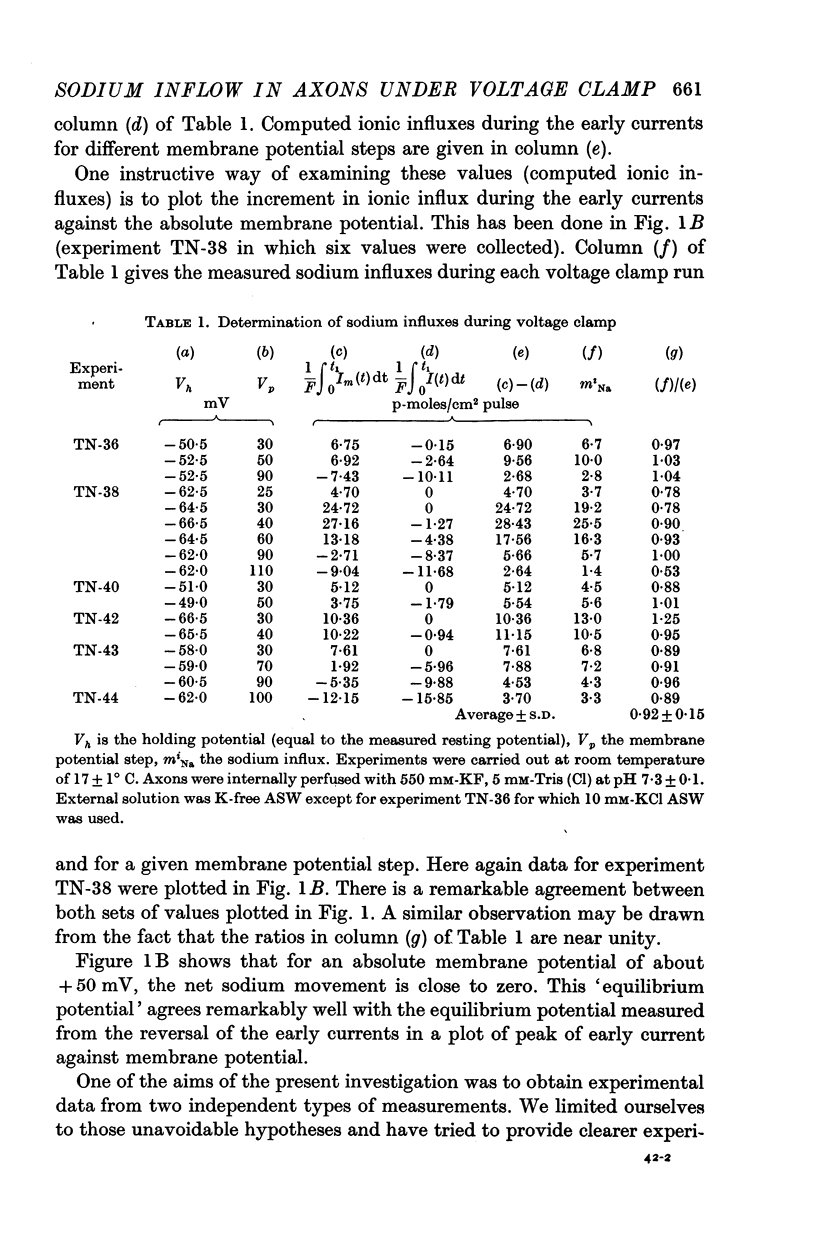
2. Sodium influx determinations were carried out while applying rectangular pulses of membrane depolarization. The ratio `measured sodium influx/computed ionic flux during the early current' is 0·92 ± 0·12.
3. Plots of measured sodium influx and computed ionic flux during the early current against membrane potential are very similar. There was evidence that the membrane potential at which the sodium influx vanishes is the potential at which the early current reverses.
Full text
PDF

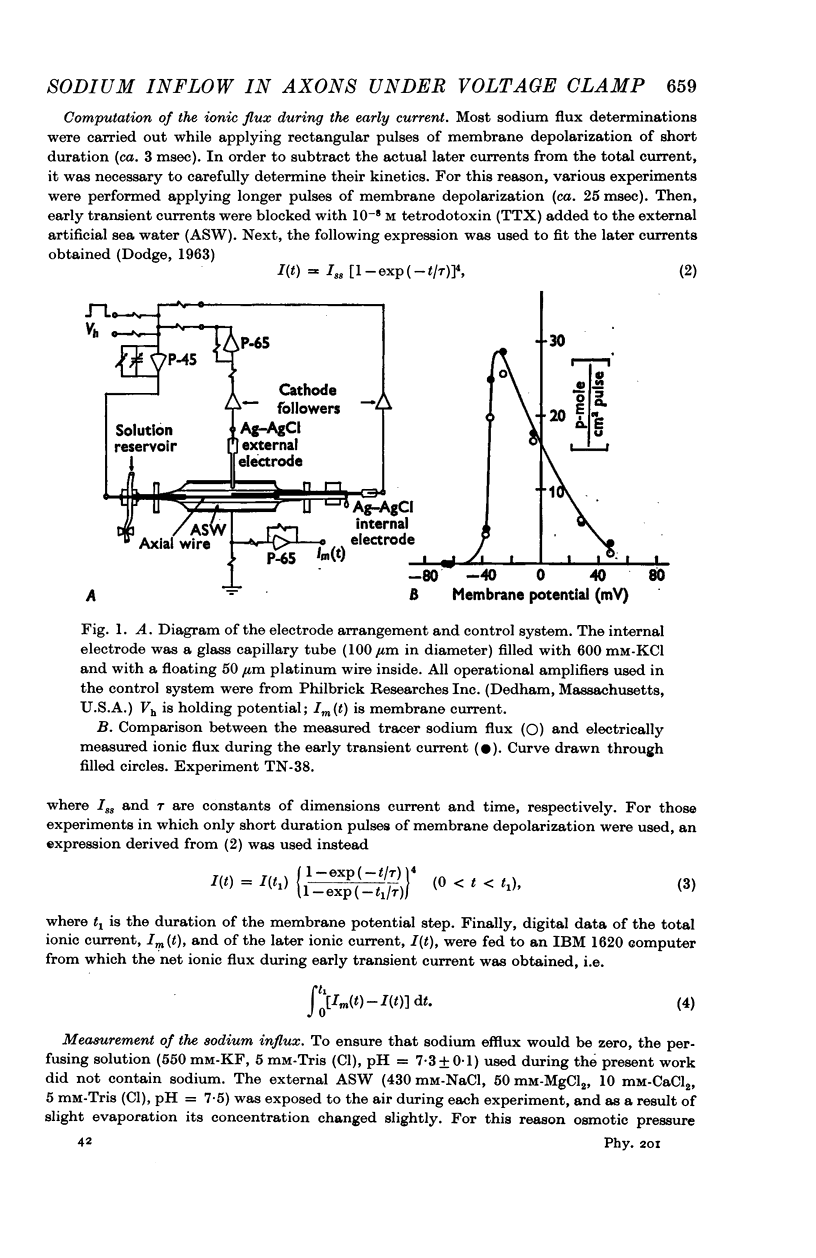




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINLEY F. J., Jr, MULLINS L. J. ION FLUXES AND TRANSFERENCE NUMBER IN SQUID AXONS. J Neurophysiol. 1965 May;28:526–544. doi: 10.1152/jn.1965.28.3.526. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Movement of radioactive potassium and membrane current in a giant axon. J Physiol. 1953 Aug;121(2):403–414. doi: 10.1113/jphysiol.1953.sp004954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic movements during nervous activity. J Physiol. 1951 Jun;114(1-2):119–150. doi: 10.1113/jphysiol.1951.sp004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLINS L. J., ADELMAN W. J., Jr, SJODIN R. A. Sodium and potassium ion effluxes from squid axons under voltage clamp conditions. Biophys J. 1962 May;2:257–274. doi: 10.1016/s0006-3495(62)86854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Atwater I. Effect of tetrodotoxin on the early outward currents in perfused giant axons. Proc Natl Acad Sci U S A. 1967 May;57(5):1350–1355. doi: 10.1073/pnas.57.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Canessa-Fischer M. Sodium movements in perfused squid giant axons. Passive fluxes. J Gen Physiol. 1968 Aug;52(2):240–257. doi: 10.1085/jgp.52.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANES A. M., BERMAN M. D. Kinetics of ion movement in the squid giant axon. J Gen Physiol. 1955 Nov 20;39(2):279–300. doi: 10.1085/jgp.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]


