Abstract
1. The nervous activity in single afferent gastric vagal units was recorded electrophysiologically from halothane-anaesthetized sheep with spontaneous reticulo-ruminal movements present.
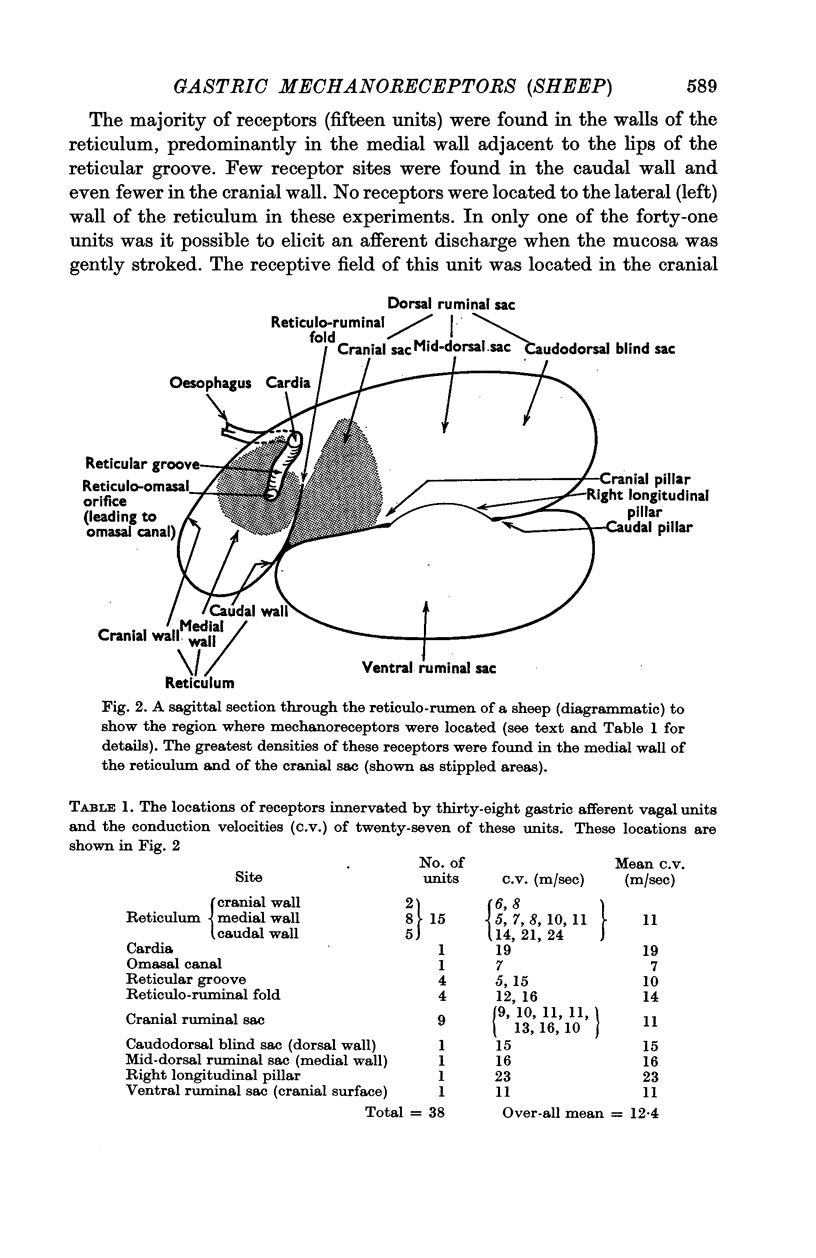
2. Sixty-six afferent units innervating gastric mechanoreceptors were isolated from fifteen sheep. The receptors were located mainly in the medial walls of the reticulum and the cranial sac of the dorsal rumen, and also in the reticular groove, the reticulo-ruminal fold, the dorsal and ventral sacs of the rumen and the omasal canal.
3. The mean conduction velocity (C.V.) for twenty-seven units was 12·4 ± 1·0 m/sec (S.E.). For units with a pathway in the dorsal vagal trunk, the mean C.V. was 14·5 ± 1·0 m/sec (S.E.) and for units with a pathway in the ventral vagal trunk the mean C.V. was 6·6 ± 0·5 m/sec (S.E.).
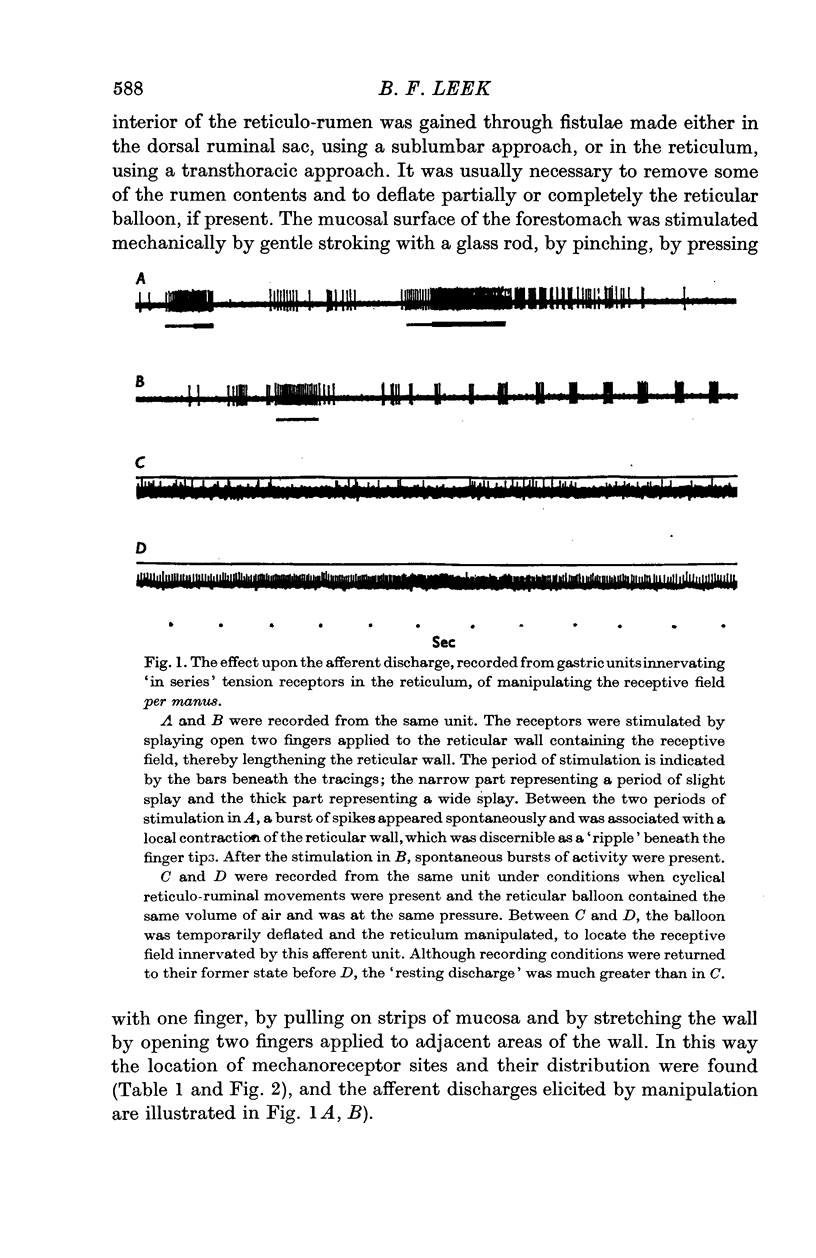
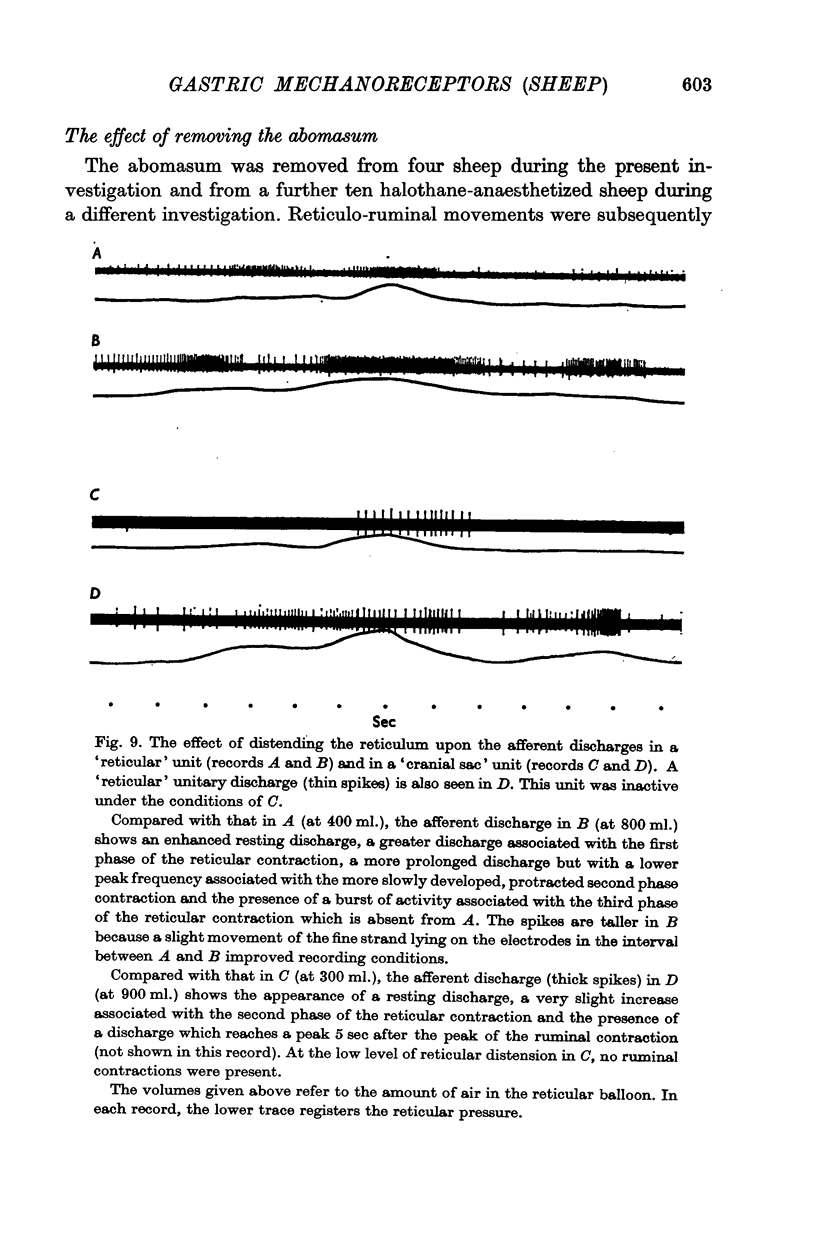
4. From the receptors a slowly adapting response was elicited by tangential lengthening. These were tension receptors in series with contractile elements, as they were excited by increased tensions developed both passively by inflation of the viscus and actively by muscular contractions.
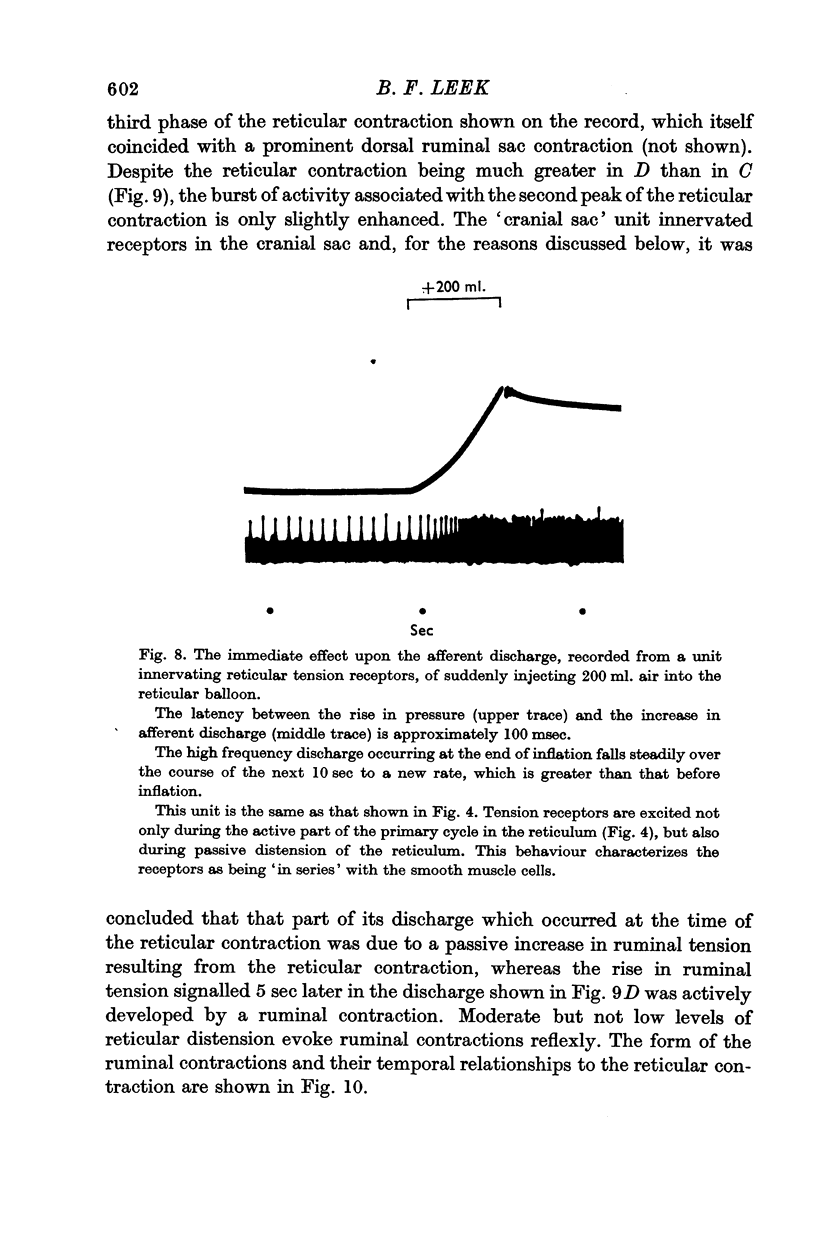
5. Receptors in the reticulum and the rumen appeared to be situated deep in the muscle layers, whereas those in the reticular groove structures seemed to be more superficial and gave the in series tension receptor response as well as a response to light pressure.
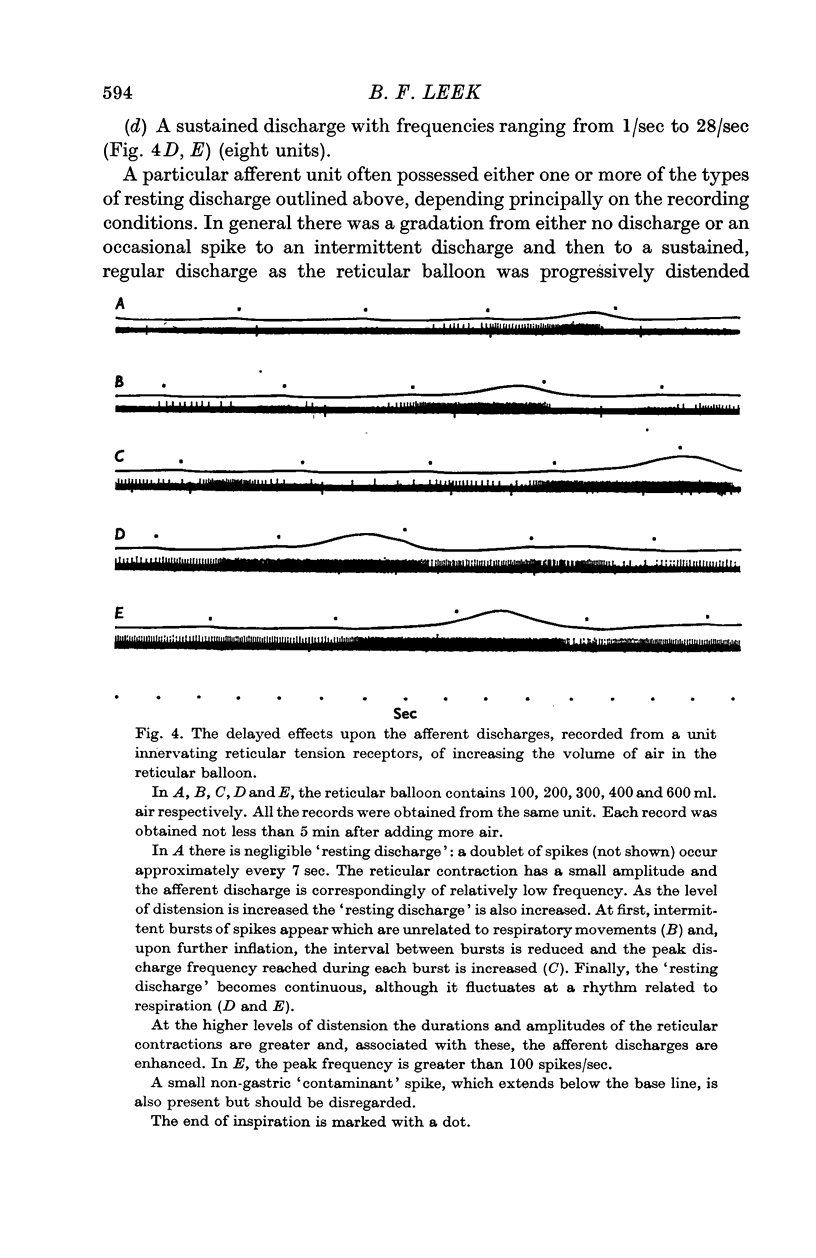
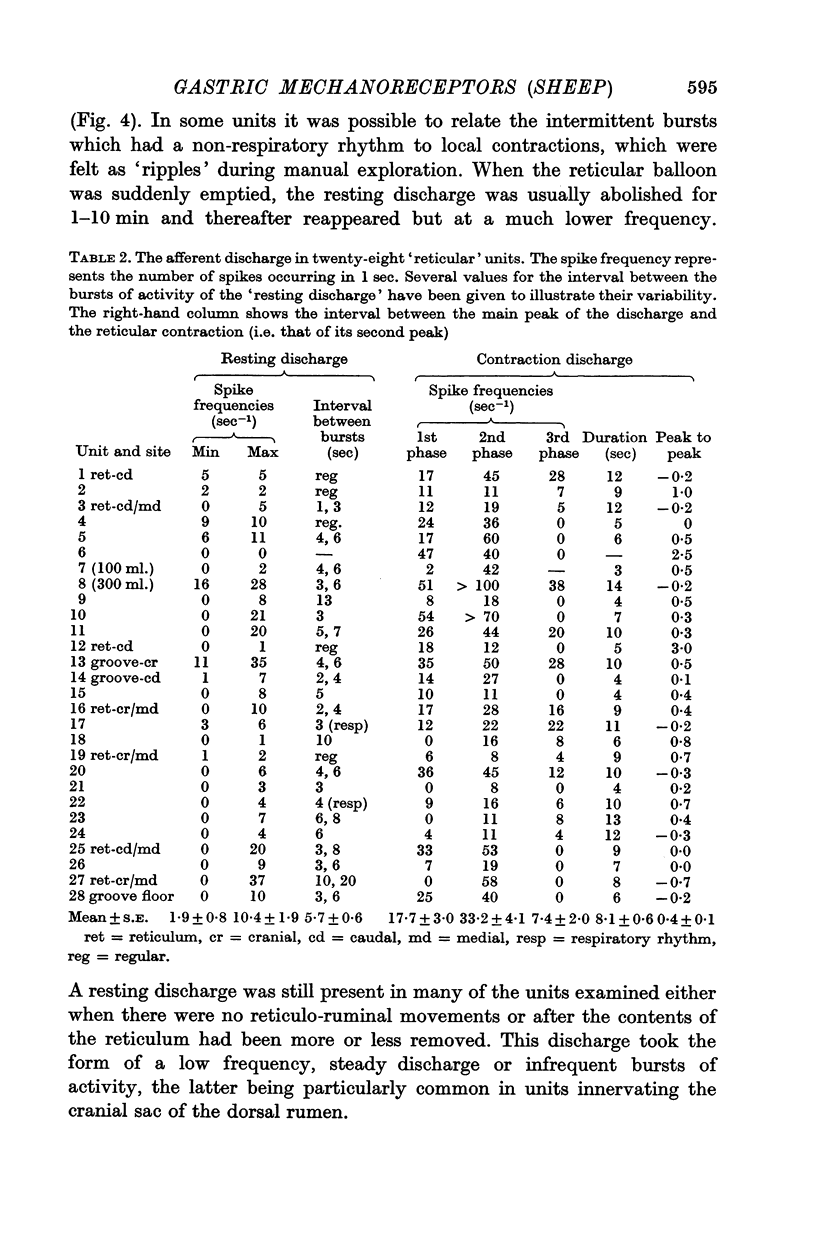
6. A resting discharge in tension receptor units was usually absent at low levels of distension but appeared and increased as the level of distension was raised. Intermittency and fluctuations in the resting discharge were related to intrinsic local movement involving the receptive fields. Increasing distension enhanced the intrinsic movements.
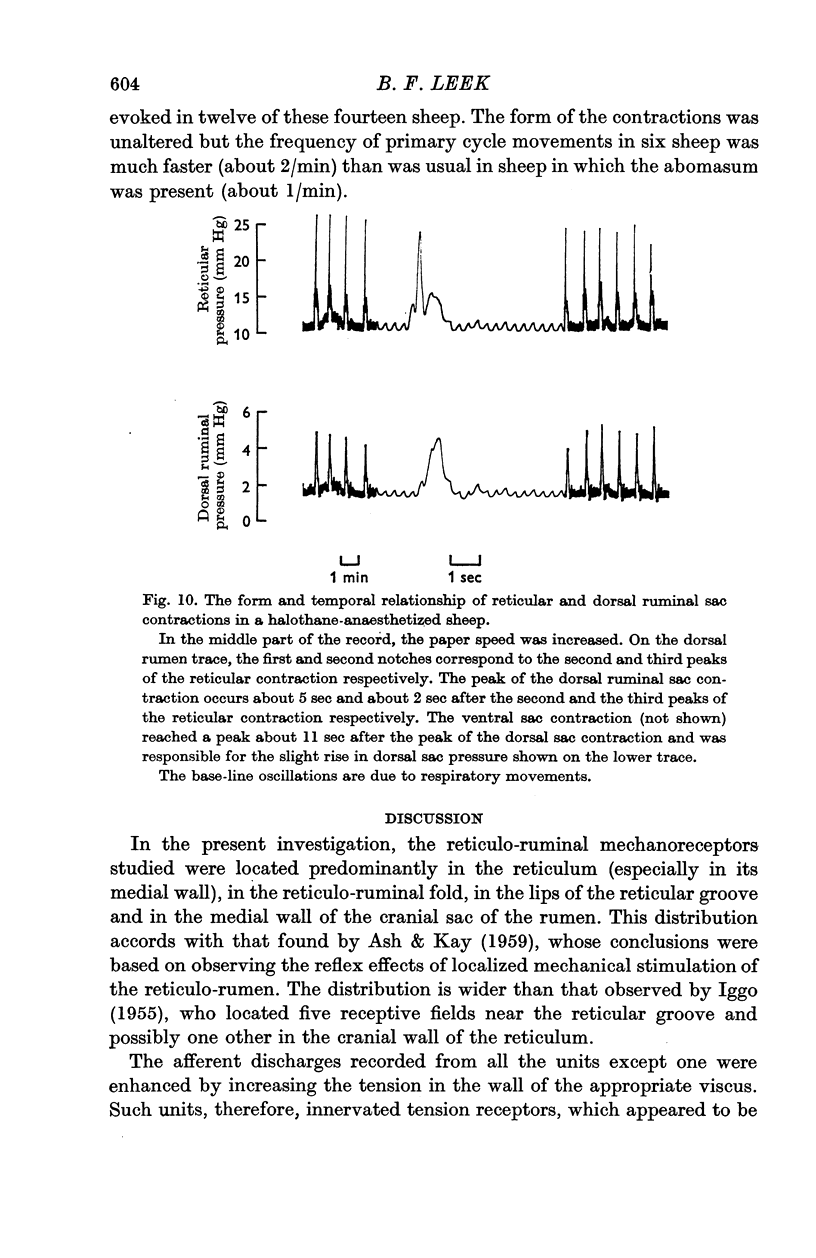
7. Even after the removal of the abomasum, reticular and ruminal (primary cycle) movements were evoked by distending the reticulum. It is possible that this manoeuvre enhanced intrinsic movements, which, in turn, caused an increased excitatory afferent input to the `gastric centres' from in series reticular tension receptors.
8. The enhanced afferent discharge from reticular tension receptors elicited by an isometrically recorded reticular contraction reflexly inhibited the subsequent (primary cycle) contraction of the rumen.
9. Very few receptors were located in the caudal regions of the rumen whereas the cranial sac is richly supplied with tension receptors. The idea that the cranial sac may serve as the reflexogenic zone for secondary cycle movements of the rumen is discussed.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASH R. W., KAY R. N. Stimulation and inhibition of reticulum contractions, rumination and parotid secretion from the forestomach of conscious sheep. J Physiol. 1959 Dec;149:43–57. doi: 10.1113/jphysiol.1959.sp006324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGHERTY R. W., HABEL R. E., BOND H. E. Esophageal innervation and the eructation reflex in sheep. Am J Vet Res. 1958 Jan;19(70):115–128. [PubMed] [Google Scholar]
- HABEL R. E. A study of the innervation of the ruminant stomach. Cornell Vet. 1956 Oct;46(4):555–633. [PubMed] [Google Scholar]
- IGGO A. Central nervous control of gastric movements in sheep and goats. J Physiol. 1956 Jan 27;131(1):248–256. doi: 10.1113/jphysiol.1956.sp005460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A. Receptors in the stomach and the bladder. J Physiol. 1954 Nov 29;126(2):29–30P. [PubMed] [Google Scholar]
- IGGO A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955 Jun 28;128(3):593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A. The electrophysiological identification of single nerve fibres, with particular reference to the slowest-conducting vagal afferent fibres in the cat. J Physiol. 1958 Jun 18;142(1):110–126. doi: 10.1113/jphysiol.1958.sp006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Leek B. F. An electrophysiological study of single vagal efferent units associated with gastric movements in sheep. J Physiol. 1967 Jul;191(1):177–204. doi: 10.1113/jphysiol.1967.sp008244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Leek B. F. An electrophysiological study of some reticulo-ruminal and abomasal reflexes in sheep. J Physiol. 1967 Nov;193(1):95–119. doi: 10.1113/jphysiol.1967.sp008345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. H. Nerve endings in mammalian muscle. J Physiol. 1933 Apr 13;78(1):1–53. doi: 10.1113/jphysiol.1933.sp002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. The conduction velocities of respiratory and cardiovascular afferent fibres in the vagus nerve. J Physiol. 1953 Aug;121(2):341–359. doi: 10.1113/jphysiol.1953.sp004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. VAGAL AFFERENT FIBRES. Ergeb Physiol. 1963;52:74–156. [PubMed] [Google Scholar]
- TITCHEN D. A. Reflex stimulation and inhibition of reticulum contractions in the ruminant stomach. J Physiol. 1958 Apr 3;141(1):1–21. doi: 10.1113/jphysiol.1958.sp005950. [DOI] [PMC free article] [PubMed] [Google Scholar]


