Abstract
1. The dry mass and nucleic acid content of both nerve cell bodies and their nucleoli were measured by interference microscopy and ultra-violet absorption microspectrography respectively: succinoxidase and acetylcholine hydrolase activities were also determined. Autoradiography was used to follow synthesis of deoxyribonucleic acid (DNA) by glial cells, and to follow nucleic acid and protein metabolism in muscle fibres.
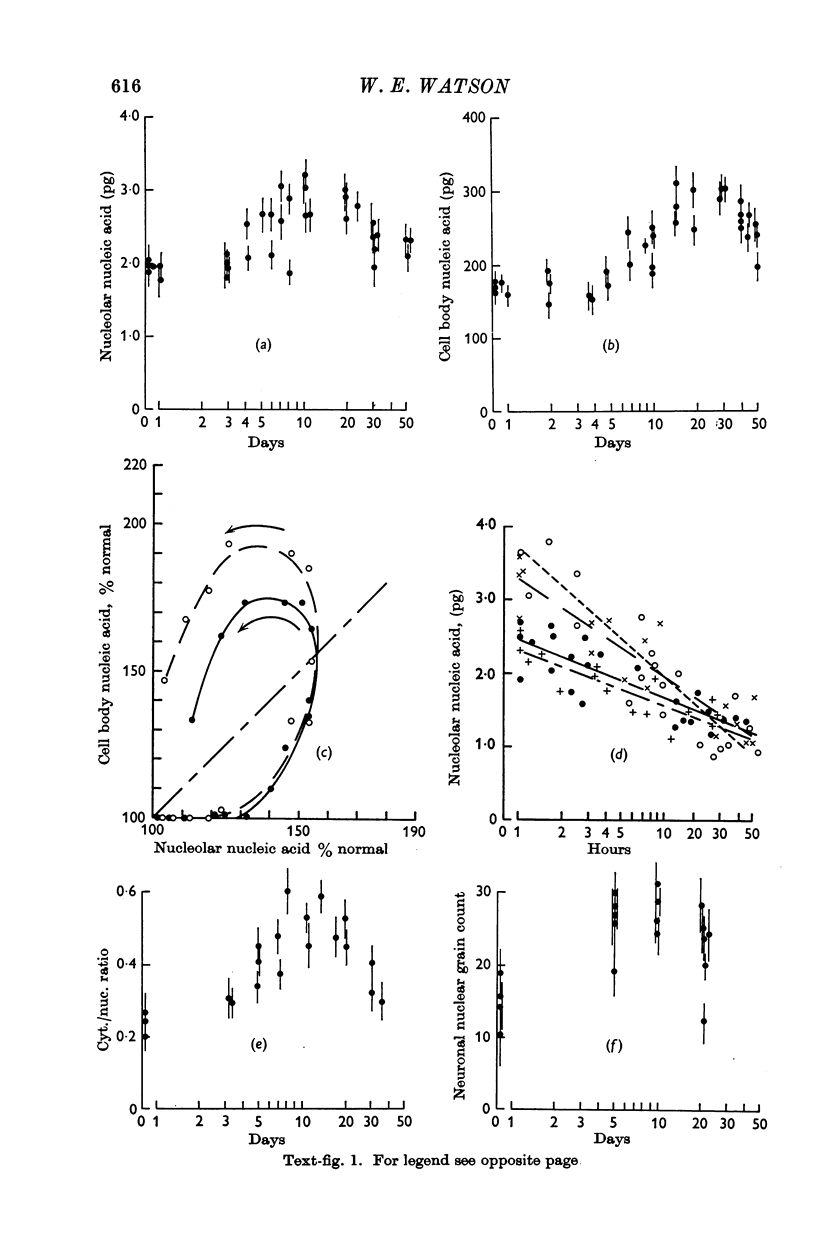
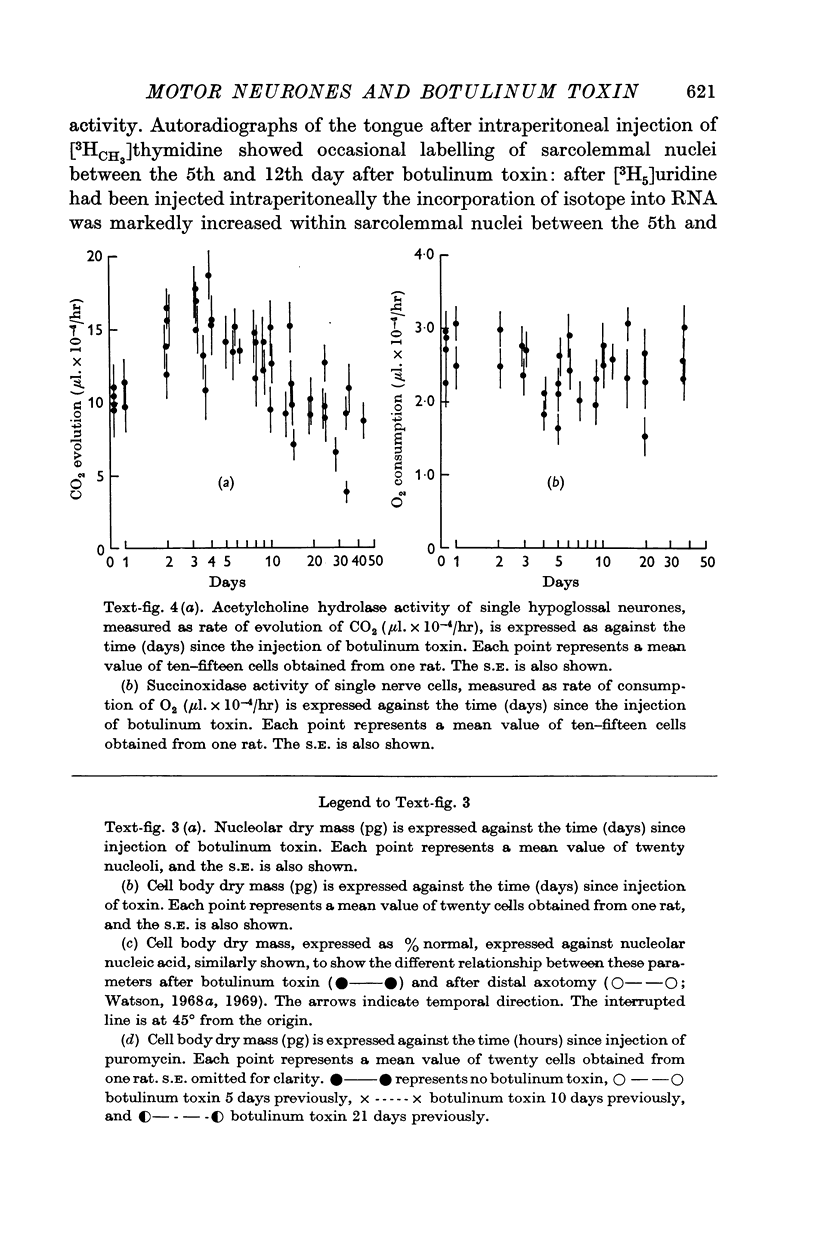
2. After injection of botulinum toxin the synthesis of ribosomal RNA by the neurone followed closely the pattern found after axotomy.
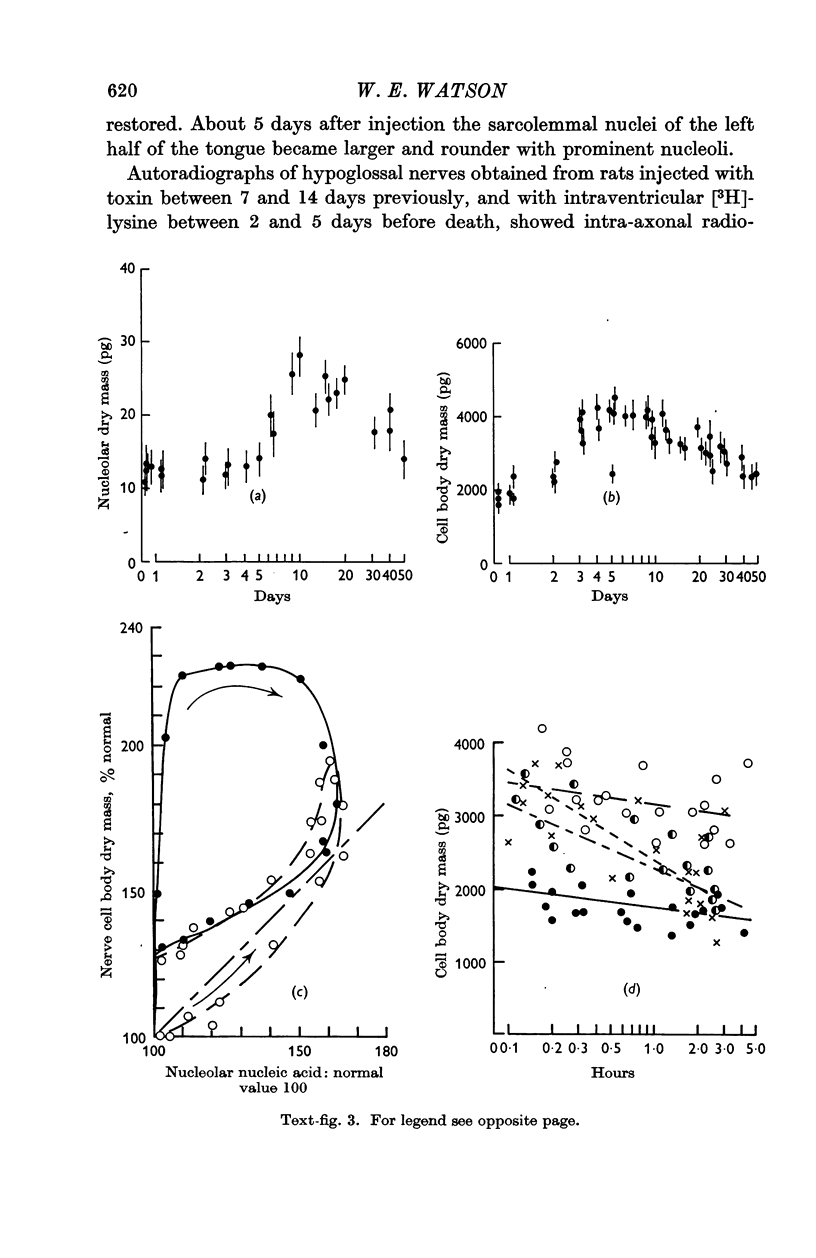
3. After injection of toxin neuronal dry mass increased before the rate of ribosomal RNA synthesis was raised. This early increase, which was not due to increased protein synthesis, probably represents a `damming back' of proteins within the nerve cell body.
4. After injection of toxin no local accumulation of microglial cells synthesizing DNA was found around the affected neurones: it is suggested that this reflects the intact system for intra-axonal transport under these conditions.
5. The affected muscles show increased nucleic acid and protein synthesis.
6. It is suggested that the results obtained indicate that membrane expansion or synthesis which occurs both in muscle and in neurone under these circumstances is the factor responsible for inducing directly or indirectly the changes found in nucleic acid metabolism after injection of botulinum toxin and after axotomy.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AITKEN J. T., BRIDGER J. E. Neuron size and neuron population density in the lumbosacral region of the cat's spinal cord. J Anat. 1961 Jan;95:38–53. [PMC free article] [PubMed] [Google Scholar]
- AMBACHE N. A further survey of the action of Clostridium botulinum toxin upon different types of autonomic nerve fibre. J Physiol. 1951 Mar;113(1):1–17. doi: 10.1113/jphysiol.1951.sp004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair G. S., Robinson M. E. The specific refraction increments of serum-albumin and serum-globulin. Biochem J. 1930;24(4):993–1011. doi: 10.1042/bj0240993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken J. T., Sharman M., Young J. Z. Maturation of regenerating nerve fibres with various peripheral connexions. J Anat. 1947 Jan;81(Pt 1):1–22.2. [PMC free article] [PubMed] [Google Scholar]
- Ambache N. The peripheral action of Cl. botulinum toxin. J Physiol. 1949 Mar 15;108(2):127–141. [PMC free article] [PubMed] [Google Scholar]
- BARER R. Interference microscopy and mass determination. Nature. 1952 Mar 1;169(4296):366–367. doi: 10.1038/169366b0. [DOI] [PubMed] [Google Scholar]
- BARER R., TKACZYK S. Refractive index of concentrated protein solutions. Nature. 1954 May 1;173(4409):821–822. doi: 10.1038/173821b0. [DOI] [PubMed] [Google Scholar]
- BRATTGARD S. O., EDSTROM J. E., HYDEN H. The chemical changes in regenerating neurons. J Neurochem. 1957;1(4):316–325. doi: 10.1111/j.1471-4159.1957.tb12088.x. [DOI] [PubMed] [Google Scholar]
- BROOKS V. B. An intracellular study of the action of repetitive nerve volleys and of botulinum toxin on miniature end-plate potentials. J Physiol. 1956 Nov 28;134(2):264–277. doi: 10.1113/jphysiol.1956.sp005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS V. B. The action of botulinum toxin on motor-nerve filaments. J Physiol. 1954 Mar 29;123(3):501–515. doi: 10.1113/jphysiol.1954.sp005067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN G. L., M'EWEN M. B., PRATT M. I. Macromolecular weight and size of deoxypentose nucleic acids. Nature. 1955 Jul 23;176(4473):161–162. doi: 10.1038/176161a0. [DOI] [PubMed] [Google Scholar]
- BURGEN A. S. V., DICKENS F., ZATMAN L. J. The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949 Aug;109(1-2):10–24. doi: 10.1113/jphysiol.1949.sp004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERF J. A., CHACKO L. W. Retrograde reaction in motoneuron dendrites following ventral root section in the frog. J Comp Neurol. 1958 Apr;109(2):205–219. doi: 10.1002/cne.901090205. [DOI] [PubMed] [Google Scholar]
- DAVIES H. G. Cell interferometry and the specific refraction increment of crystalline proteins. I. beta-Lactoglobulin. Biochim Biophys Acta. 1959 Mar;32(1):228–232. doi: 10.1016/0006-3002(59)90572-4. [DOI] [PubMed] [Google Scholar]
- DAVIES H. G., DEELEY E. M. An integrator for measuring the dry mass of cells and isolated components. Exp Cell Res. 1956 Aug;11(1):169–185. doi: 10.1016/0014-4827(56)90201-4. [DOI] [PubMed] [Google Scholar]
- DAVIES H. G., DEELEY E. M., DENBY E. F. Attempts at measurement of lipid, nucleic acid and protein content of cell nuclei by microscope-interferometry. Exp Cell Res. 1957;13(Suppl 4):136–149. [PubMed] [Google Scholar]
- DAVIES H. G. The determination of mass and concentration by microscope in interferometry. Gen Cytochem Methods. 1958;1:55–161. [PubMed] [Google Scholar]
- DE GROOT J. Neurosecretion in experimental conditions. Anat Rec. 1957 Feb;127(2):201–217. doi: 10.1002/ar.1091270207. [DOI] [PubMed] [Google Scholar]
- DROZ B. ACCUMULATION DE PROT'EINES NOUVELLEMENT SYNTH'ETIS'EES DANS L'APPAREL DE GOLGI DU NEURONE; 'ETUDE RADIOAUTOGRAPHIQUE EN MICROSCOPIE 'ELECTRONIQUE. C R Hebd Seances Acad Sci. 1965 Jan 4;260:320–322. [PubMed] [Google Scholar]
- DROZ B., LEBLOND C. P. AXONAL MIGRATION OF PROTEINS IN THE CENTRAL NERVOUS SYSTEM AND PERIPHERAL NERVES AS SHOWN BY RADIOAUTOGRAPHY. J Comp Neurol. 1963 Dec;121:325–346. doi: 10.1002/cne.901210304. [DOI] [PubMed] [Google Scholar]
- DROZ B., LEBLOND C. P. Migration of proteins along the axons of the sciatic nerve. Science. 1962 Sep 28;137(3535):1047–1048. doi: 10.1126/science.137.3535.1047. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Strich S. J. The effects of botulinum toxin on the pattern of innervation of skeletal muscle in the mouse. Q J Exp Physiol Cogn Med Sci. 1968 Jan;53(1):84–89. doi: 10.1113/expphysiol.1968.sp001948. [DOI] [PubMed] [Google Scholar]
- GIACOBINI E., HOLMSTEDT B. Cholinesterase content of certain regions of the spinal cord as judged by histochemical and cartesian diver technique. Acta Physiol Scand. 1958 Feb 10;42(1):12–27. doi: 10.1111/j.1748-1716.1958.tb01537.x. [DOI] [PubMed] [Google Scholar]
- GOLDBERG I. H., RABINOWITZ M. Actionmycin D inhibition of deoxyribonucleic acid-dependent synthesis of ribonucleic acid. Science. 1962 Apr 27;136(3513):315–316. doi: 10.1126/science.136.3513.315. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Woodland H. R. The cytoplasmic control of nuclear activity in animal development. Biol Rev Camb Philos Soc. 1968 May;43(2):233–267. doi: 10.1111/j.1469-185x.1968.tb00960.x. [DOI] [PubMed] [Google Scholar]
- JOSEFSSON J. O., THESLEFF S. Electromyographic findings in experimental botulinum intoxication. Acta Physiol Scand. 1961 Feb-Mar;51:163–168. doi: 10.1111/j.1748-1716.1961.tb02124.x. [DOI] [PubMed] [Google Scholar]
- KOENIG E. SYNTHETIC MECHANISMS IN THE AXON. I. LOCAL AXONAL SYNTHESIS OF ACETYLCHOLINESTERASE. J Neurochem. 1965 May;12:343–355. doi: 10.1111/j.1471-4159.1965.tb04235.x. [DOI] [PubMed] [Google Scholar]
- KOENIG E. SYNTHETIC MECHANISMS IN THE AXON. II. RNA IN MYELIN-FREE AXONS OF THE CAT. J Neurochem. 1965 May;12:357–361. doi: 10.1111/j.1471-4159.1965.tb04236.x. [DOI] [PubMed] [Google Scholar]
- Kerkut G. A., Shapira A., Walker R. J. The transport of 14C-labelled material from CNS to and from muscle along a nerve trunk. Comp Biochem Physiol. 1967 Dec;23(3):729–748. doi: 10.1016/0010-406x(67)90337-4. [DOI] [PubMed] [Google Scholar]
- LUBINSKA L., NIEMIERKO S., ODERFELD B., SZWARC L., ZELENA J. BIDIRECTIONAL MOVEMENTS OF AXOPLASM IN PERIPHERAL NERVE FIBRES. Acta Biol Exp (Warsz) 1963;23:239–247. [PubMed] [Google Scholar]
- MIANI N. ANALYSIS OF THE SOMATO-AXONAL MOVEMENT OF PHOSPHOLIPIDS IN THE VAGUS AND HYPOGLOSSAL NERVES. J Neurochem. 1963 Dec;10:859–874. doi: 10.1111/j.1471-4159.1963.tb11913.x. [DOI] [PubMed] [Google Scholar]
- MIANI N. Evidence of a proximo-distal movement along the axon of phospholipid synthesized in the nerve-cell body. Nature. 1962 Mar 3;193:887–888. doi: 10.1038/193887a0. [DOI] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAI J. Dissociated dorsal root ganglia in tissue culture. Am J Anat. 1956 Jul;99(1):81–129. doi: 10.1002/aja.1000990105. [DOI] [PubMed] [Google Scholar]
- NATHANS D. INHIBITION OF PROTEIN SYNTHESIS BY PUROMYCIN. Fed Proc. 1964 Sep-Oct;23:984–989. [PubMed] [Google Scholar]
- Ochs S., Johnson J., Ng M. H. Protein incorporation and axoplasmic flow in motoneuron fibres following intra-cord injection of labelled leucine. J Neurochem. 1967 Mar;14(3):317–331. doi: 10.1111/j.1471-4159.1967.tb09529.x. [DOI] [PubMed] [Google Scholar]
- REICH E., FRANKLIN R. M., SHATKIN A. J., TATUM E. L. Effect of actinomycin D on cellular nucleic acid synthesis and virus production. Science. 1961 Aug 25;134(3478):556–557. doi: 10.1126/science.134.3478.556. [DOI] [PubMed] [Google Scholar]
- ROUSER G., BAUMAN A. J., KRITCHEVSKY G. New methods for the separation and quantitative isolation of lipids. Initial applications to the study of beef brain and spleen lipids in Gaucher's disease. Am J Clin Nutr. 1961 Jan-Feb;9:112–123. doi: 10.1093/ajcn/9.1.112. [DOI] [PubMed] [Google Scholar]
- Roodyn D. B. Further study of factors affecting amino acid incorporation into protein by isolated mitochondria. Biochem J. 1965 Dec;97(3):782–793. doi: 10.1042/bj0970782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHADE J. P., VAN HARREVELD A. Volume distribution of moto- and interneurons in the peroneus-tibialis neuron pool of the cat. J Comp Neurol. 1961 Dec;117:387–398. doi: 10.1002/cne.901170310. [DOI] [PubMed] [Google Scholar]
- SIRLIN J. L., JACOB J., TANDLER C. J. TRANSFER OF THE METHYL GROUP OF METHIONINE TO NUCLEOLAR RIBONUCLEIC ACID. Biochem J. 1963 Dec;89:447–452. doi: 10.1042/bj0890447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstrand J. Proliferative changes in glial cells during nerve regeneration. Z Zellforsch Mikrosk Anat. 1965 Nov 15;68(4):481–493. doi: 10.1007/BF00347712. [DOI] [PubMed] [Google Scholar]
- TAYLOR E. W. THE MECHANISM OF COLCHICINE INHIBITION OF MITOSIS. I. KINETICS OF INHIBITION AND THE BINDING OF H3-COLCHICINE. J Cell Biol. 1965 Apr;25:SUPPL–SUPPL:160. doi: 10.1083/jcb.25.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THESLEFF S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960 Jun;151:598–607. doi: 10.1113/jphysiol.1960.sp006463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTAKOJI T., HSU T. C. NUCLEIC ACIDS AND PROTEIN SYNTHESIS OF ISOLATED CELLS FROM CHICK EMBRYONIC SPINAL GANGLIA IN CULTURE. J Exp Zool. 1965 Mar;158:181–201. doi: 10.1002/jez.1401580206. [DOI] [PubMed] [Google Scholar]
- Watson W. E. An autoradiographic study of the incorporation of nucleic-acid precursors by neurones and glia during nerve regeneration. J Physiol. 1965 Oct;180(4):741–753. doi: 10.1113/jphysiol.1965.sp007728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. Centripetal passage of labelled molecules among mammalian motor axons. J Physiol. 1968 May;196(2):122P–123P. [PubMed] [Google Scholar]
- Watson W. E. DNA synthesis in injured neurons of adult mice. J Neurochem. 1965 Sep-Oct;12(9):907–908. doi: 10.1111/j.1471-4159.1965.tb10277.x. [DOI] [PubMed] [Google Scholar]
- Watson W. E. Observations on the nucleolar and total cell body nucleic acid of injured nerve cells. J Physiol. 1968 Jun;196(3):655–676. doi: 10.1113/jphysiol.1968.sp008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. Quantitative observations upon acetylcholine hydrolase activity of nerve cells after axotomy. J Neurochem. 1966 Dec;13(12):1549–1550. doi: 10.1111/j.1471-4159.1966.tb04322.x. [DOI] [PubMed] [Google Scholar]
- Watson W. E. Some quantitative observations upon the oxidation of substrates of the tricarboxylic acid cycle in injured neurons. J Neurochem. 1966 Sep;13(9):849–856. doi: 10.1111/j.1471-4159.1966.tb05880.x. [DOI] [PubMed] [Google Scholar]
- Watson W. E. The change in dry mass of hypoglassal neurones induced by puromycin, and the effects of nerve injury. J Physiol. 1969 Apr;201(2):80P–81P. [PubMed] [Google Scholar]