Abstract
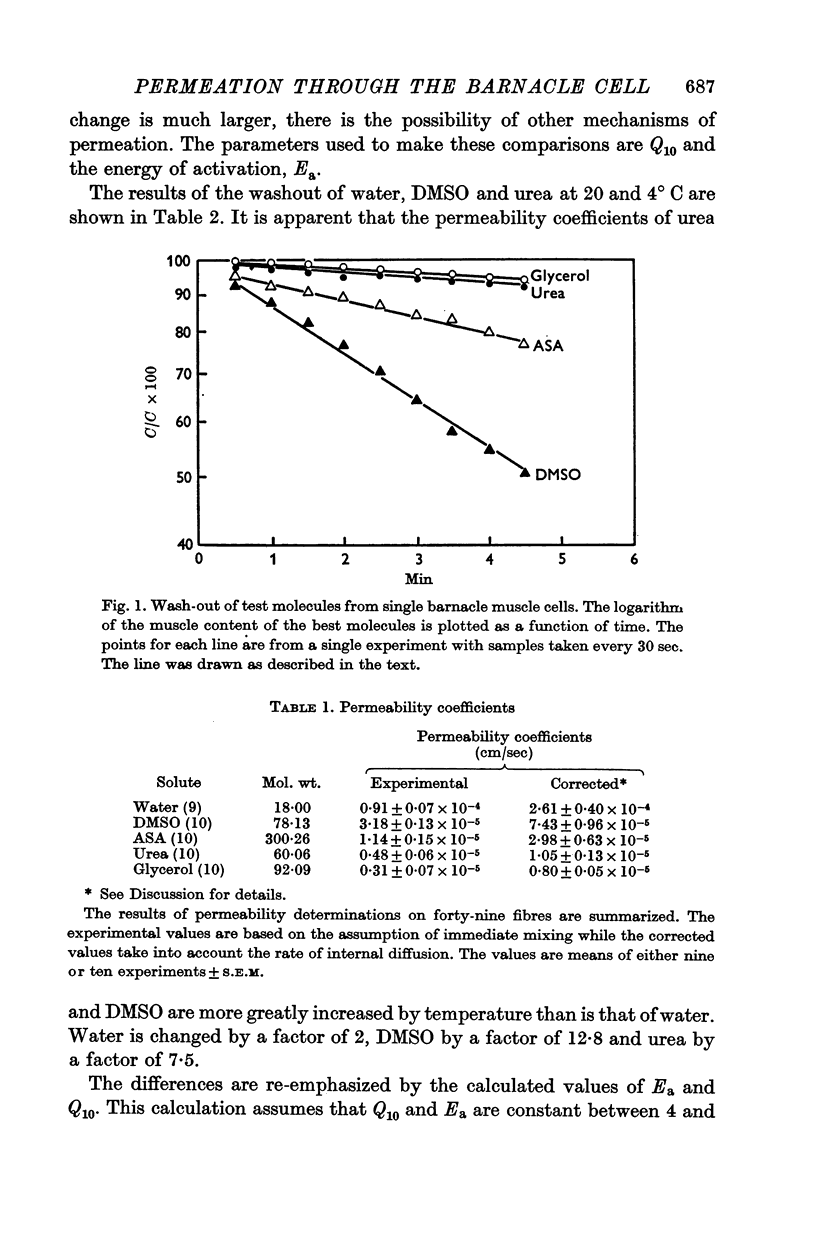
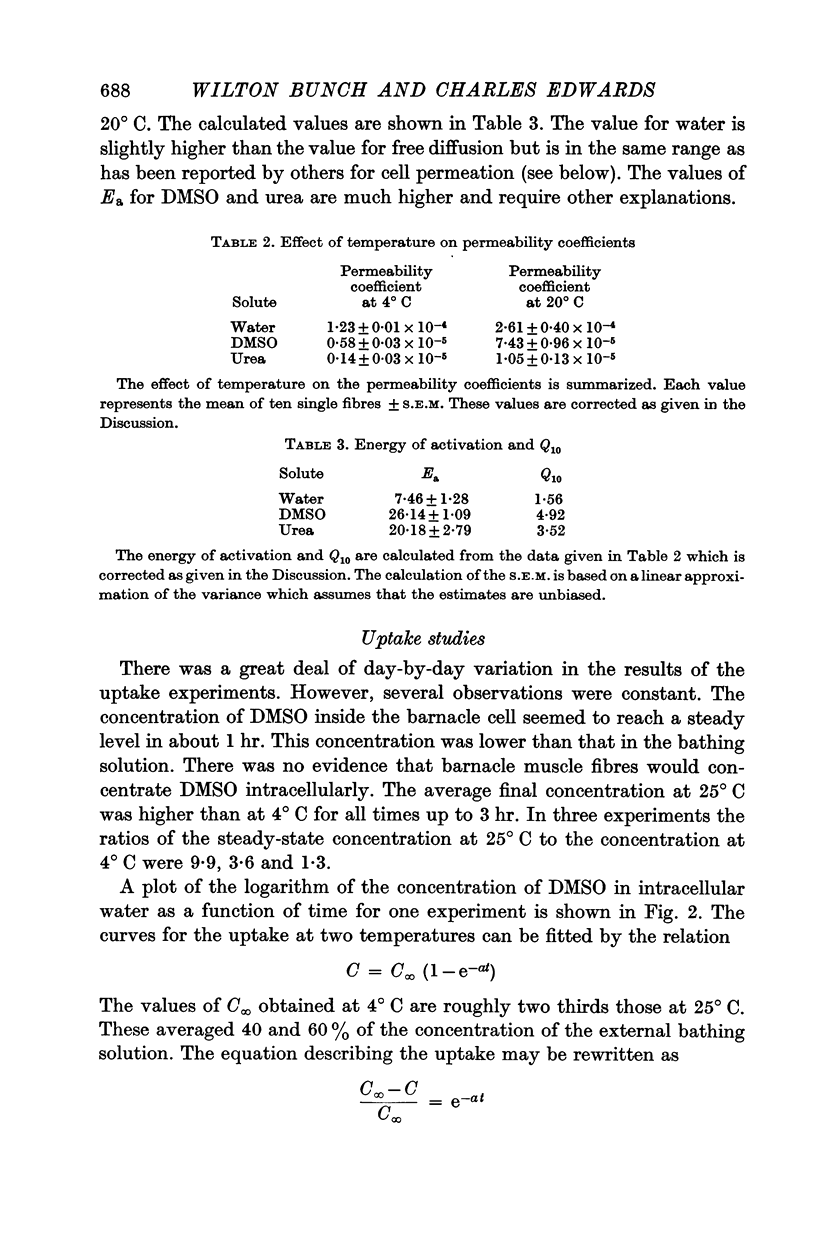
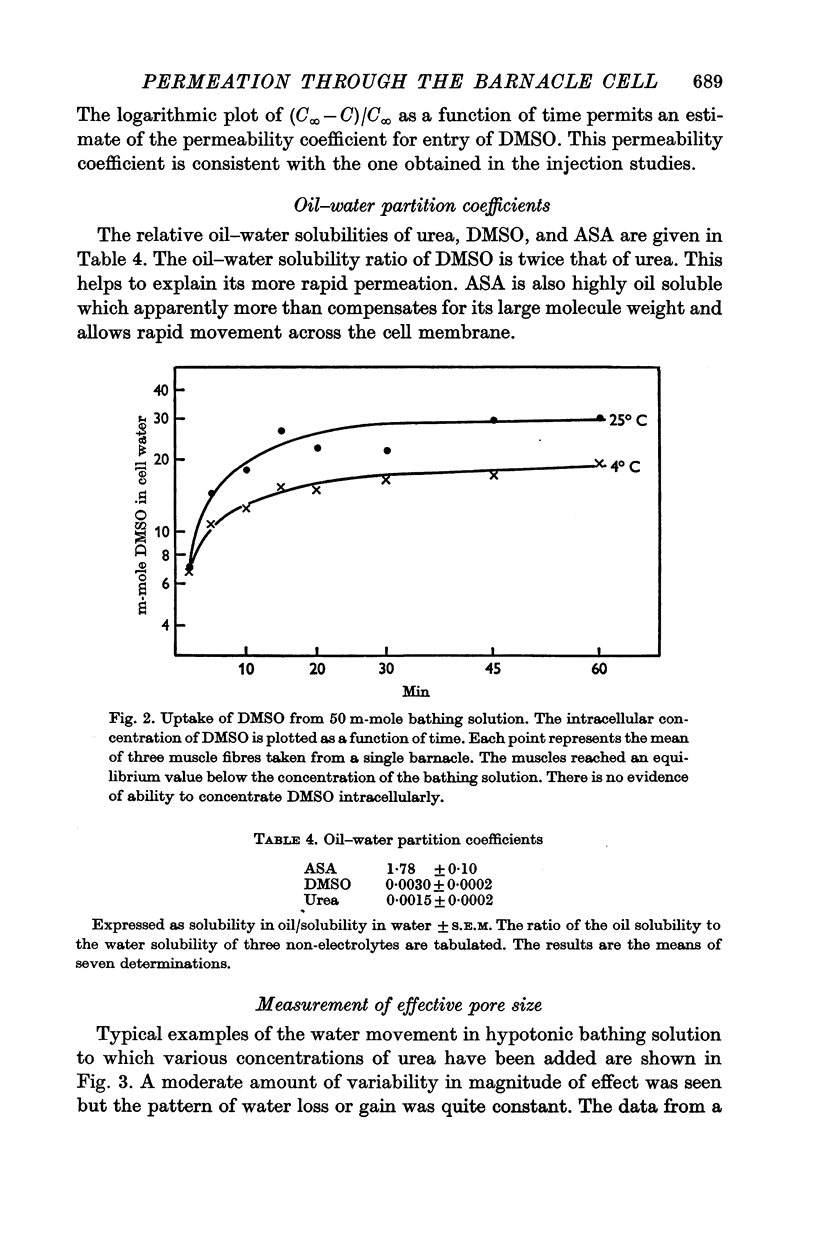
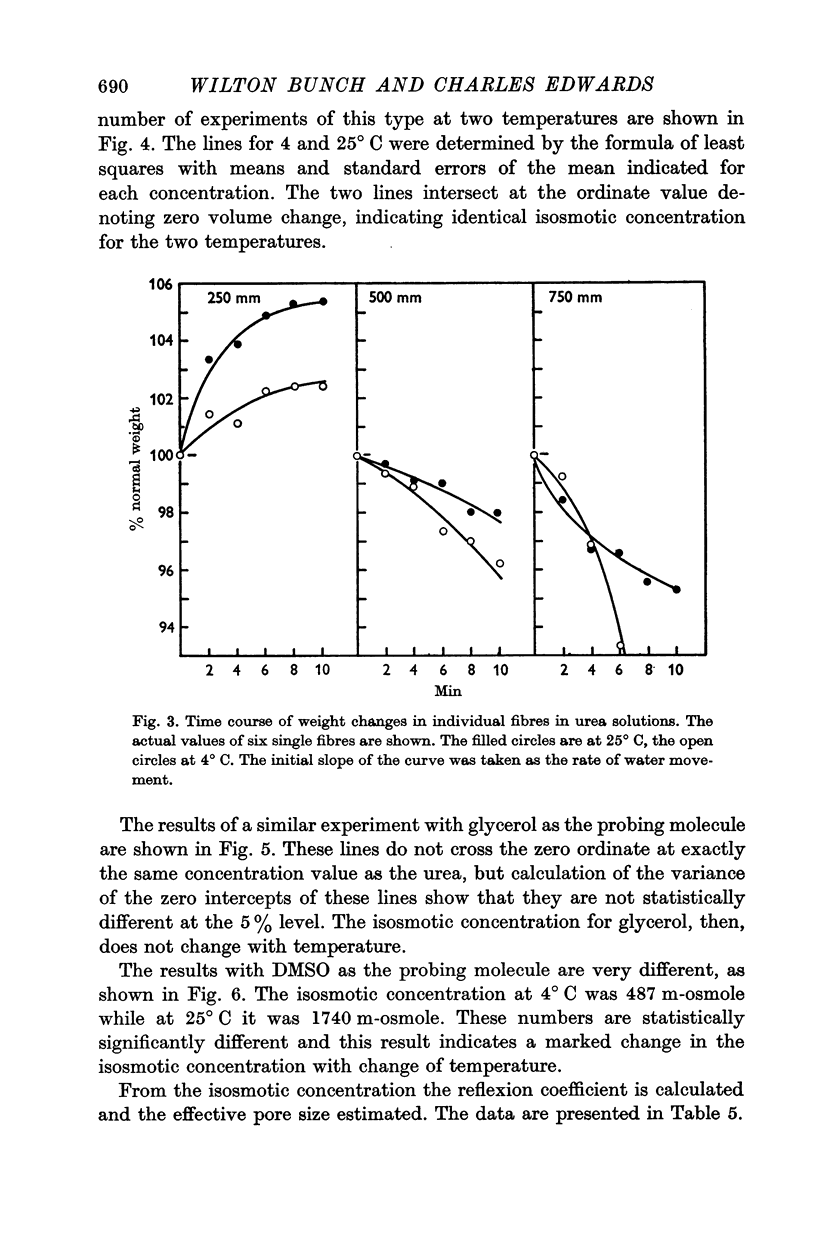
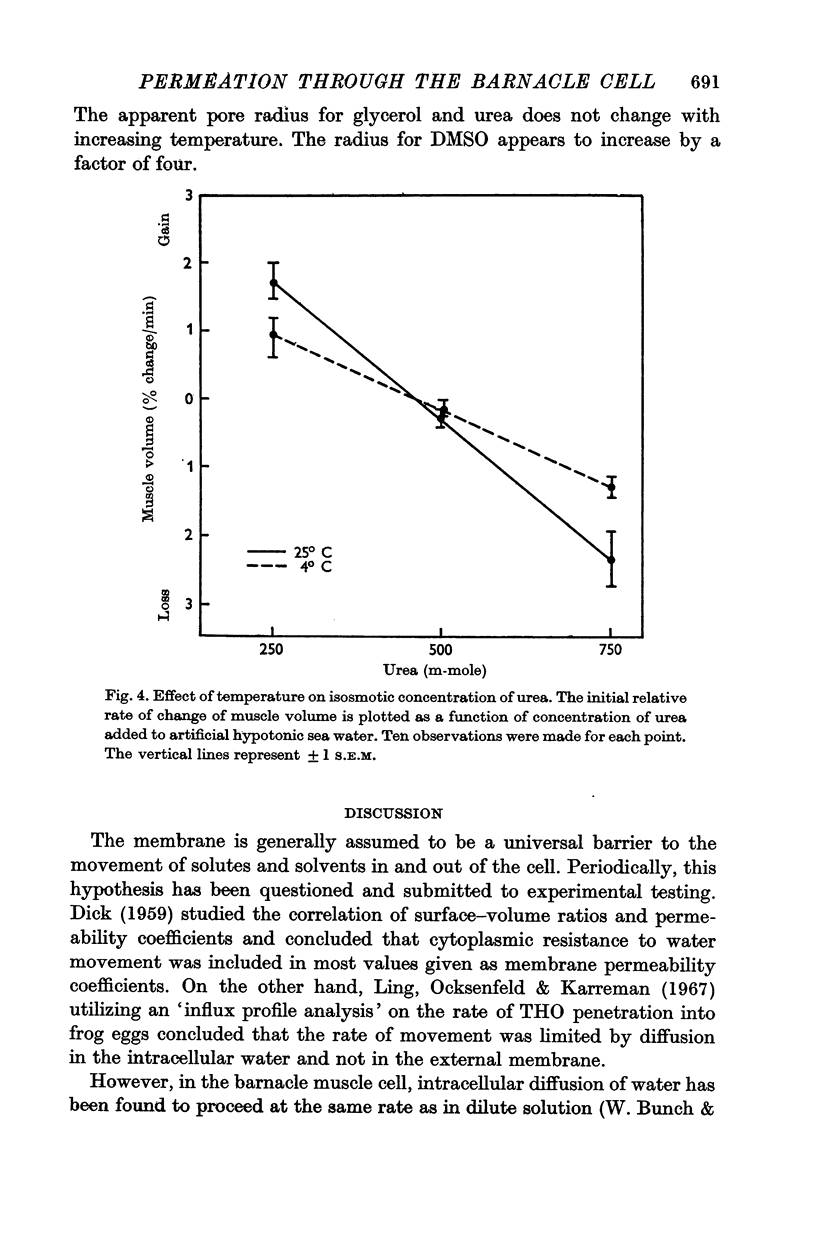
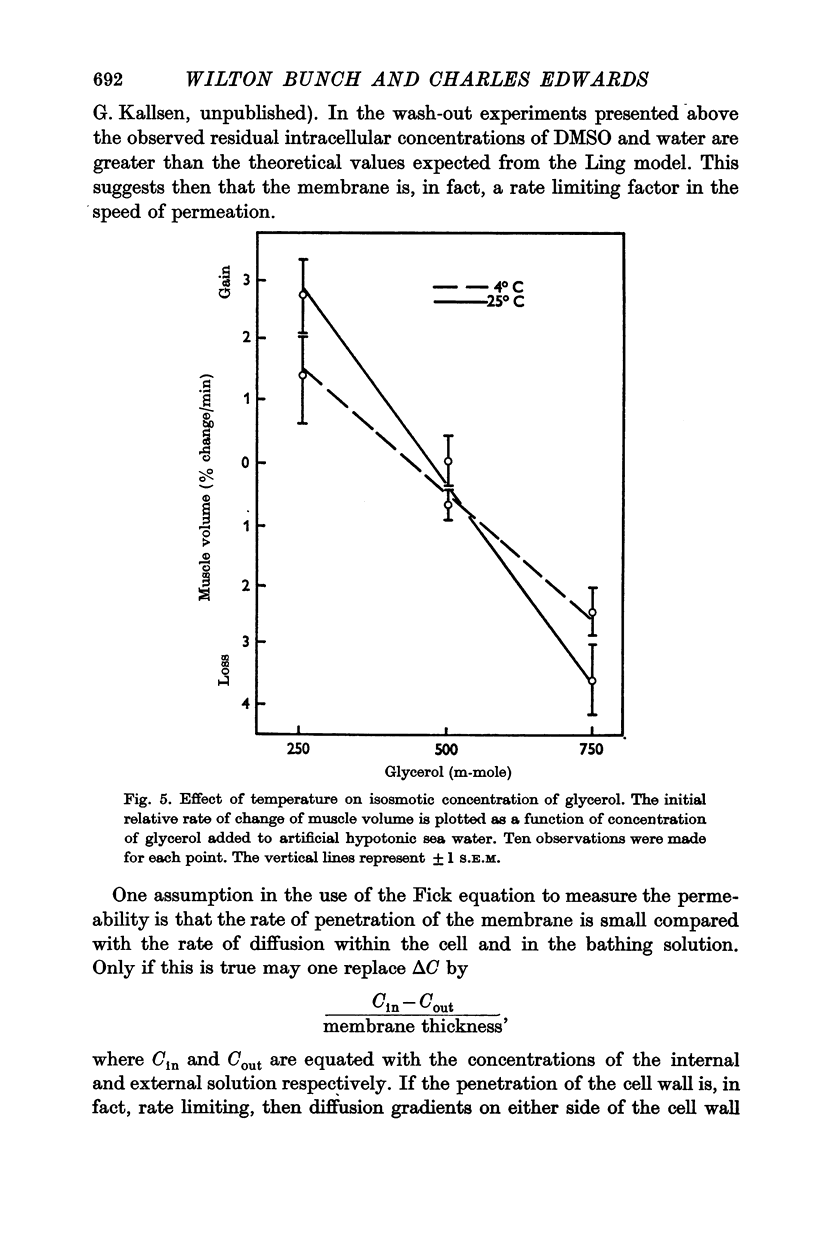
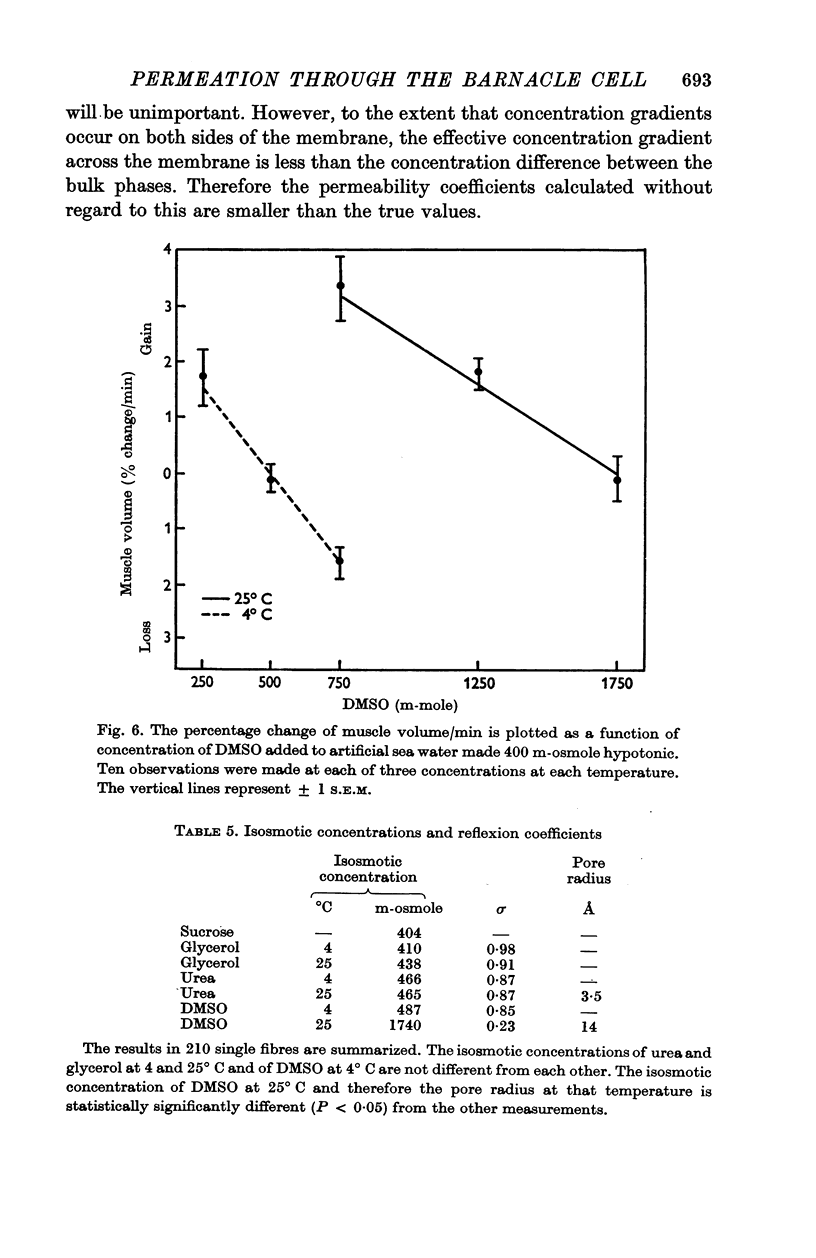
The rate of movement of non-electrolytes and tritiated water (THO) across the muscle cell membrane of the giant barnacle Balanus nubilus has been studied and permeability coefficient calculated. The rate of permeation is more closely related to the oil—water partition coefficient than to size of the molecule or degree of hydrogen bonding. Calculations based on efflux from an ideal cylinder suggest that the membrane acts as a significant barrier to movement of these molecules. The cell was unable to concentrate dimethyl sulphoxide (DMSO); the steady state was reached at about 60% of the extracellular concentration. The energies of activation for water, urea and DMSO are 7·5, 20·3 and 26·1 kcal/mol. At 4° C the apparent pore size measured with urea, glycerol and DMSO was 3·5 Å. At 25° C the apparent pore size for urea and glycerol is unchanged but that for DMSO is 14 Å.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DICK D. A. Osmotic properties of living cells. Int Rev Cytol. 1959;8:387–448. doi: 10.1016/s0074-7696(08)62736-9. [DOI] [PubMed] [Google Scholar]
- DOUGLASS D. C., FRISCH H. L., ANDERSON E. W. Self-diffusion of water in tobacco mosaic virus solutions. Biochim Biophys Acta. 1960 Nov 18;44:401–403. doi: 10.1016/0006-3002(60)91595-x. [DOI] [PubMed] [Google Scholar]
- Elford B. C. Deuterium oxide exchange in isolated guinea-pig taenia coli. J Physiol. 1967 Jan;188(2):29P–30P. [PubMed] [Google Scholar]
- FARRANT J. PERMEABILITY OF GUINEA-PIG SMOOTH MUSCLE TO NON-ELECTROLYTES. J Physiol. 1965 May;178:1–13. doi: 10.1113/jphysiol.1965.sp007610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN D. A., SOLOMON A. K. Determination of equivalent pore radius for human red cells by osmotic pressure measurement. J Gen Physiol. 1960 Sep;44:1–17. doi: 10.1085/jgp.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., CHICHIBU S., NAKA K. I. THE EFFECTS OF VARIOUS IONS ON RESTING AND SPIKE POTENTIALS OF BARNACLE MUSCLE FIBERS. J Gen Physiol. 1964 Sep;48:163–179. doi: 10.1085/jgp.48.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEMPLING H. G. Permeability of the Ehrlich ascites tumor cell to water. J Gen Physiol. 1960 Nov;44:365–379. doi: 10.1085/jgp.44.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling G. N., Ochsenfeld M. M., Karreman G. Is the cell membrane a universal rate-limiting barrier to the movement of water between the living cell and its surrounding medium? J Gen Physiol. 1967 Jul;50(6):1807–1820. doi: 10.1085/jgp.50.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. G., Hinke J. A. Sodium and water binding in single striated muscle fibers of the giant barnacle. Can J Physiol Pharmacol. 1966 Sep;44(5):837–848. doi: 10.1139/y66-102. [DOI] [PubMed] [Google Scholar]
- NEVIS A. H. Water transport in invertebrate peripheral nerve fibers. J Gen Physiol. 1958 May 20;41(5):927–958. doi: 10.1085/jgp.41.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]


