Abstract
1. The relationship between motor terminal polarization and miniature end-plate potential (m.e.p.p.) frequency was examined in the presence of various Ca, Mg and K concentrations ([Ca], [Mg] and [K]) and also at modified bathing osmolarity levels. The polarization changes were obtained with `electrotonic' and `focal' polarizing currents and with rapid changes in bathing [K].
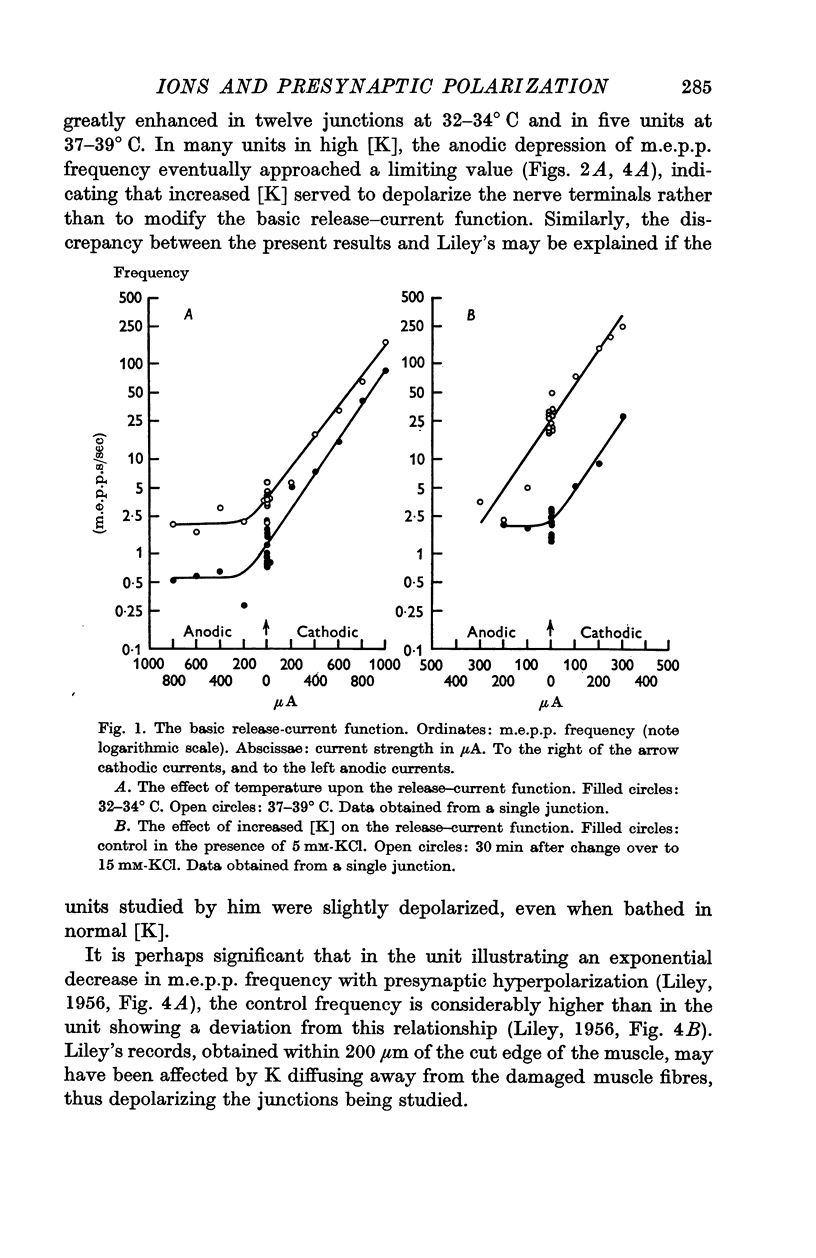
2. M.e.p.p. frequency increased exponentially with electrotonic depolarizing currents, but failed to decrease similarly with hyperpolarizing currents. An increase in bathing [K] to 15 mM increased the sensitivity of the terminals to presynaptic hyperpolarization.
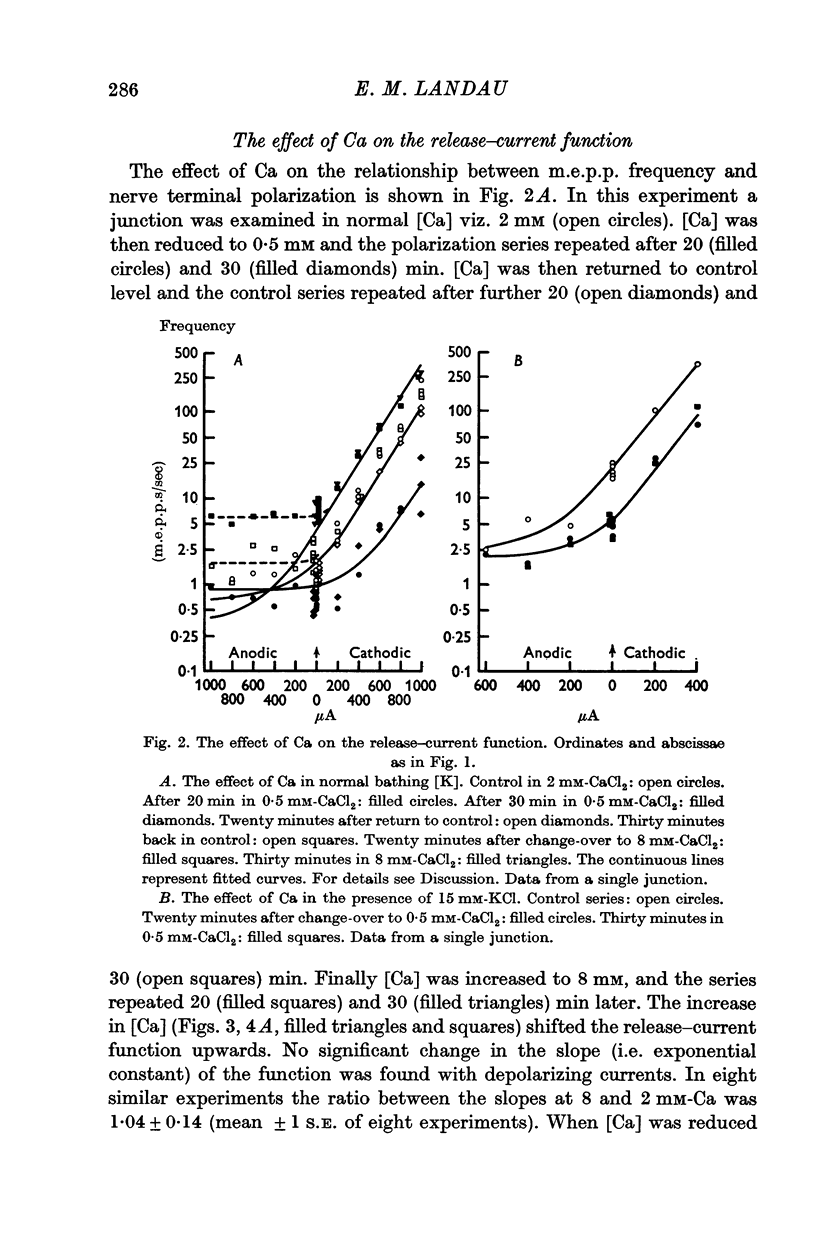
3. The slope, on semilogarithmic coordinates, of the function relating m.e.p.p. frequency to electrotonic polarizing currents (the release-current function) was unchanged when bathing [Ca] was raised from 2 to 8 mM. When [Ca] was reduced to 0·5 mM the slope of this function was reduced initially but eventually approached the same slope as in control [Ca]. A similar effect was also found in the presence of 15 mM-KCl.
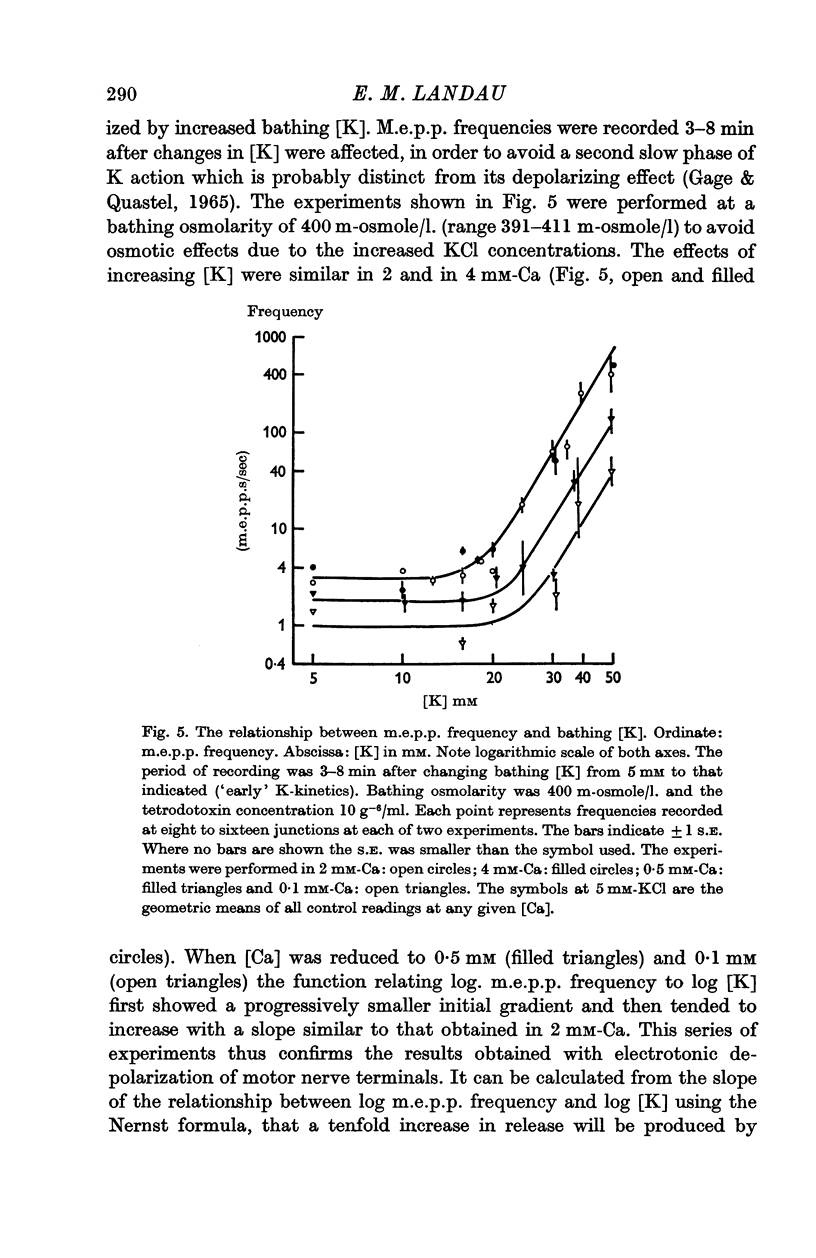
4. The relationship between m.e.p.p. frequency and log [K], at various [Ca], resembled the relationships between m.e.p.p. frequency and presynaptic polarizing currents.
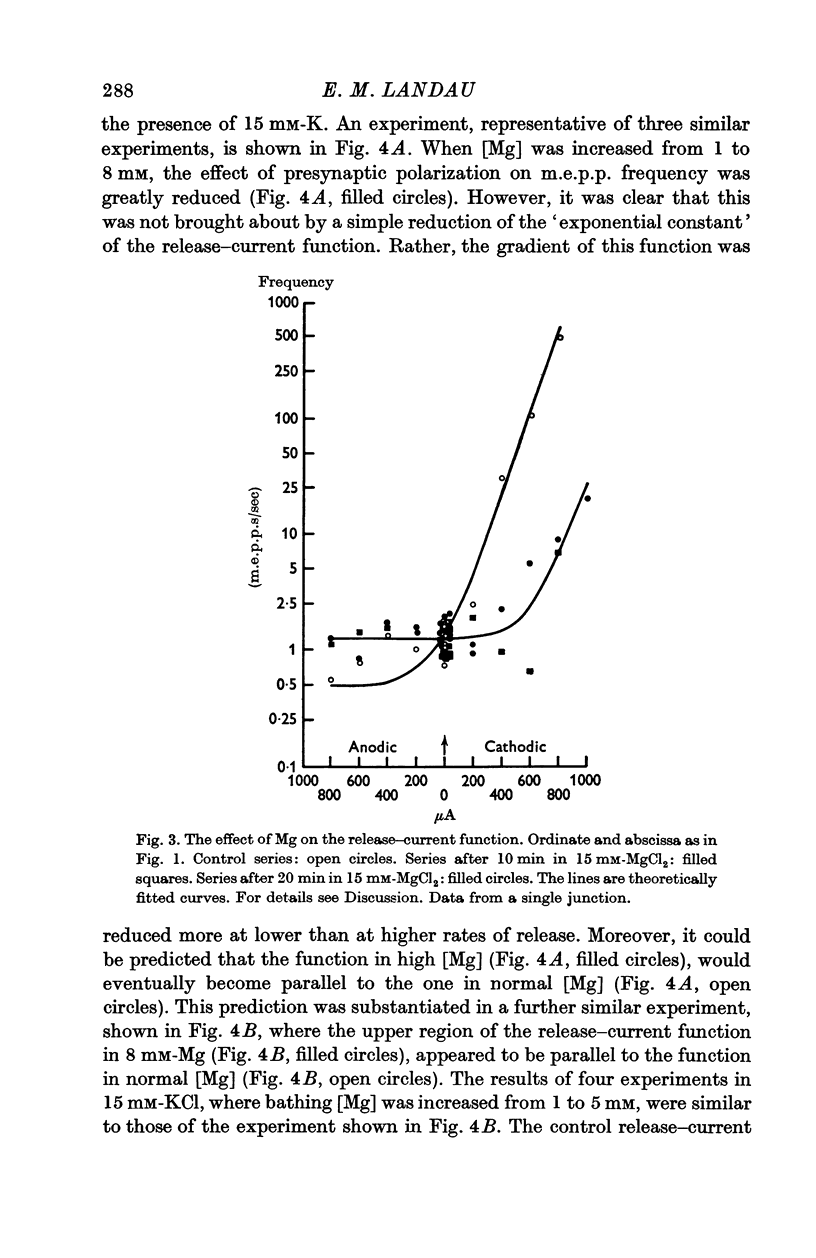
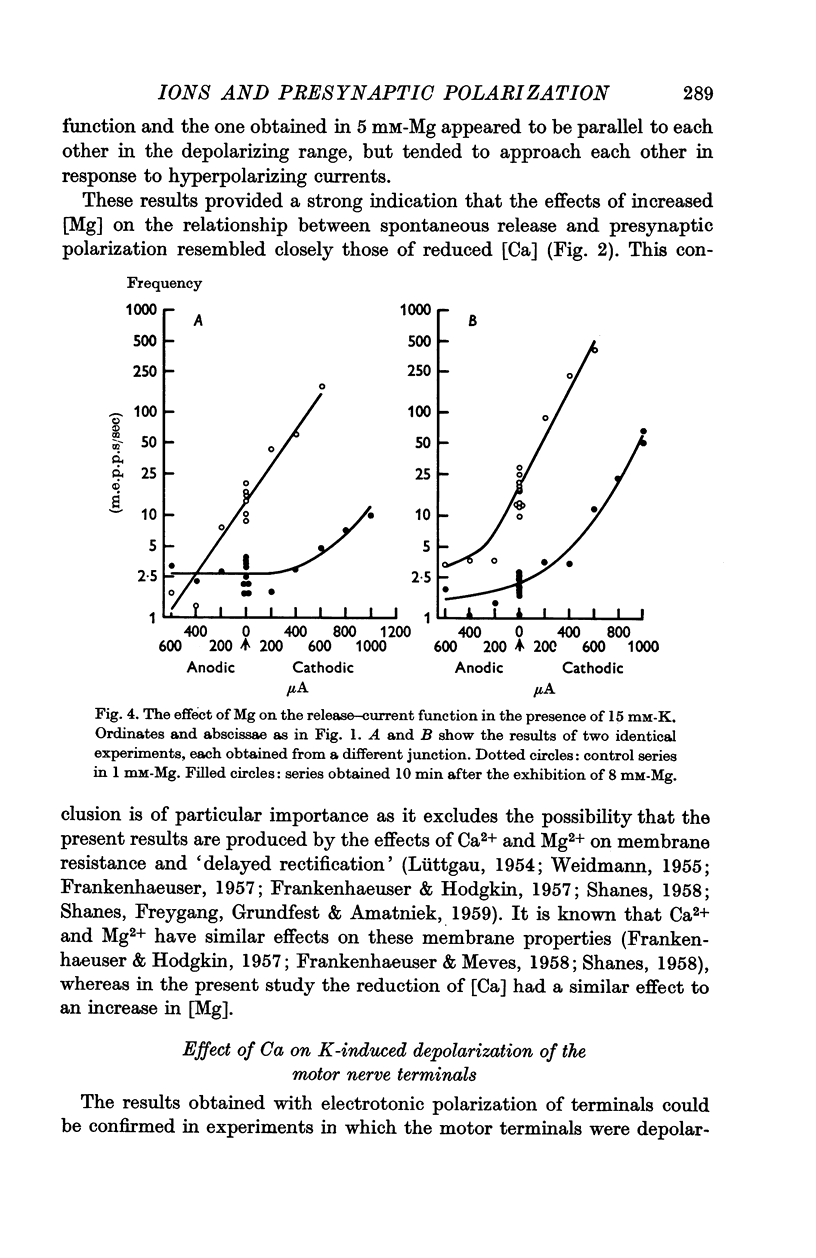
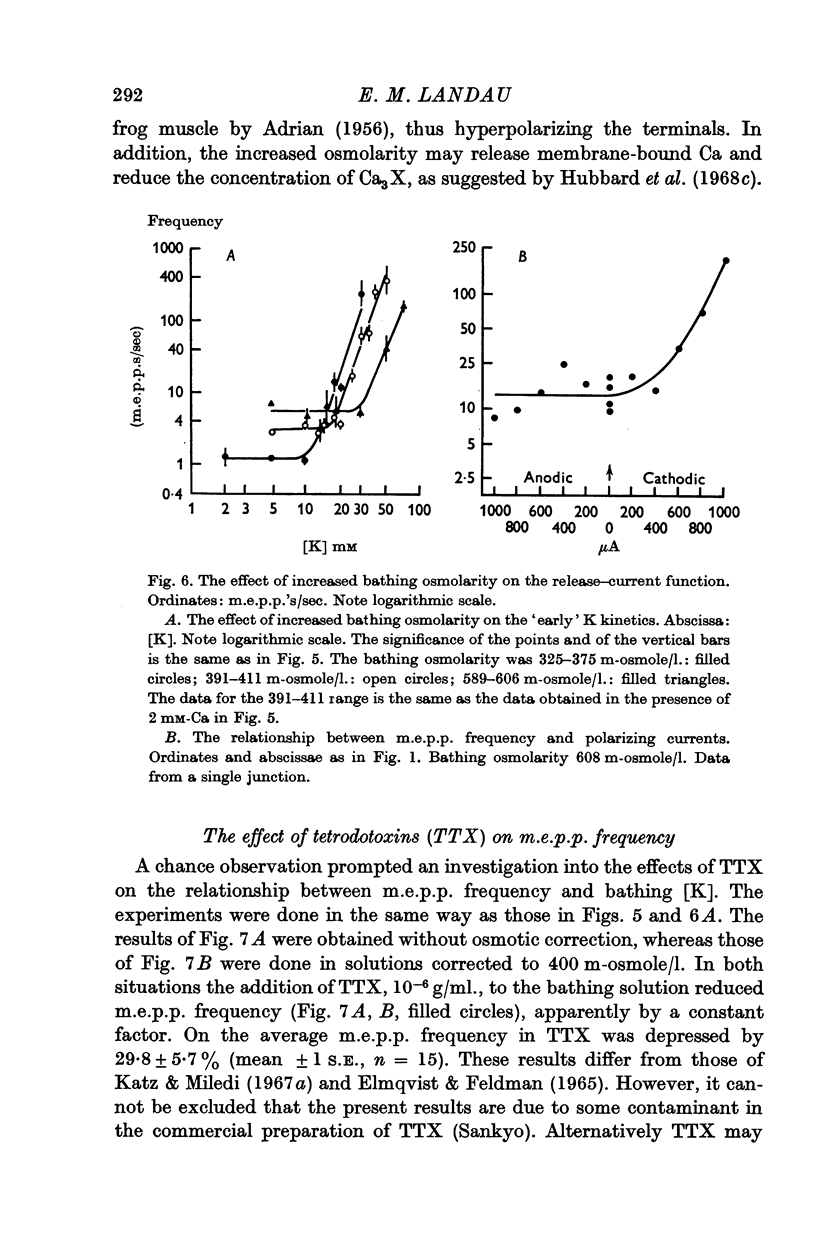
5. An increase in bathing [Mg] or osmolarity had a similar effect to a reduction of [Ca].
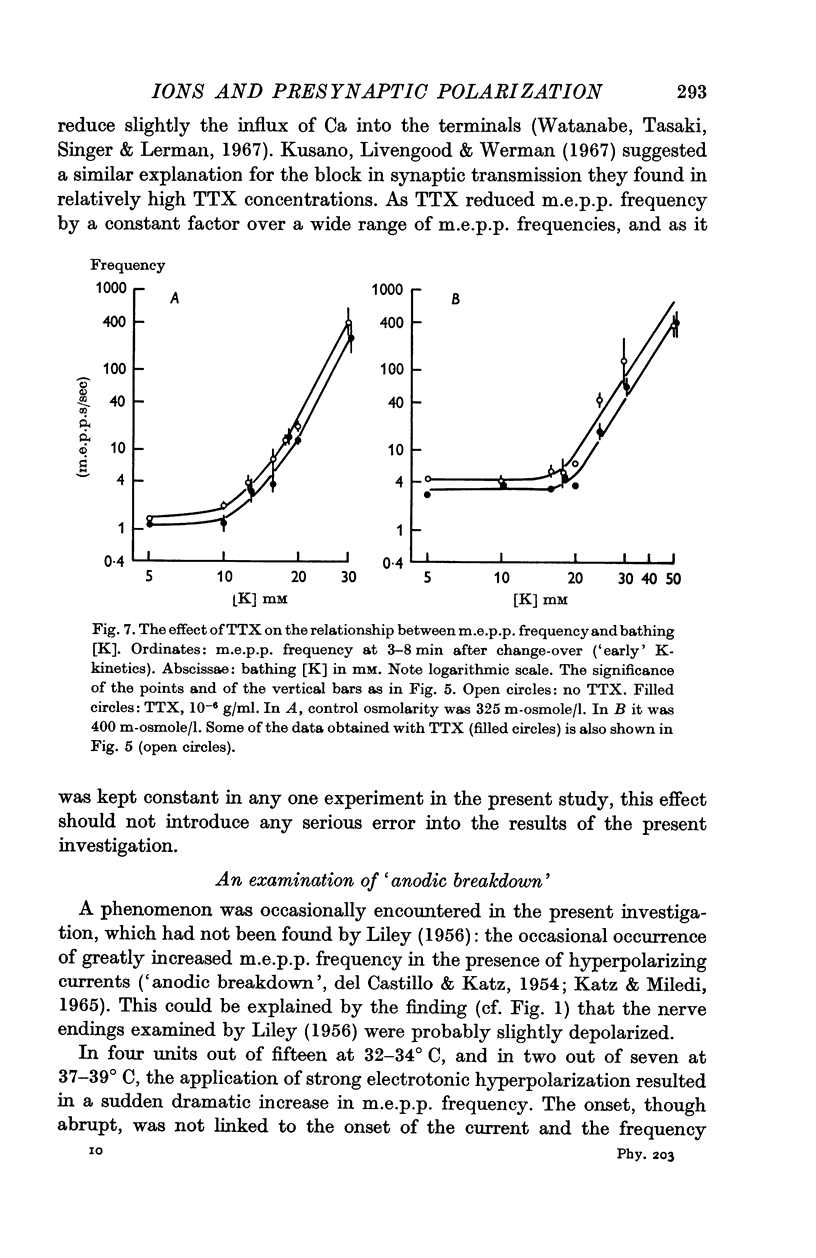
6. Tetrodotoxin (TTX) at a concentration of 10-6 g/ml. was found to reduce m.e.p.p. frequency, at various [K], by a constant fraction of about 30%.
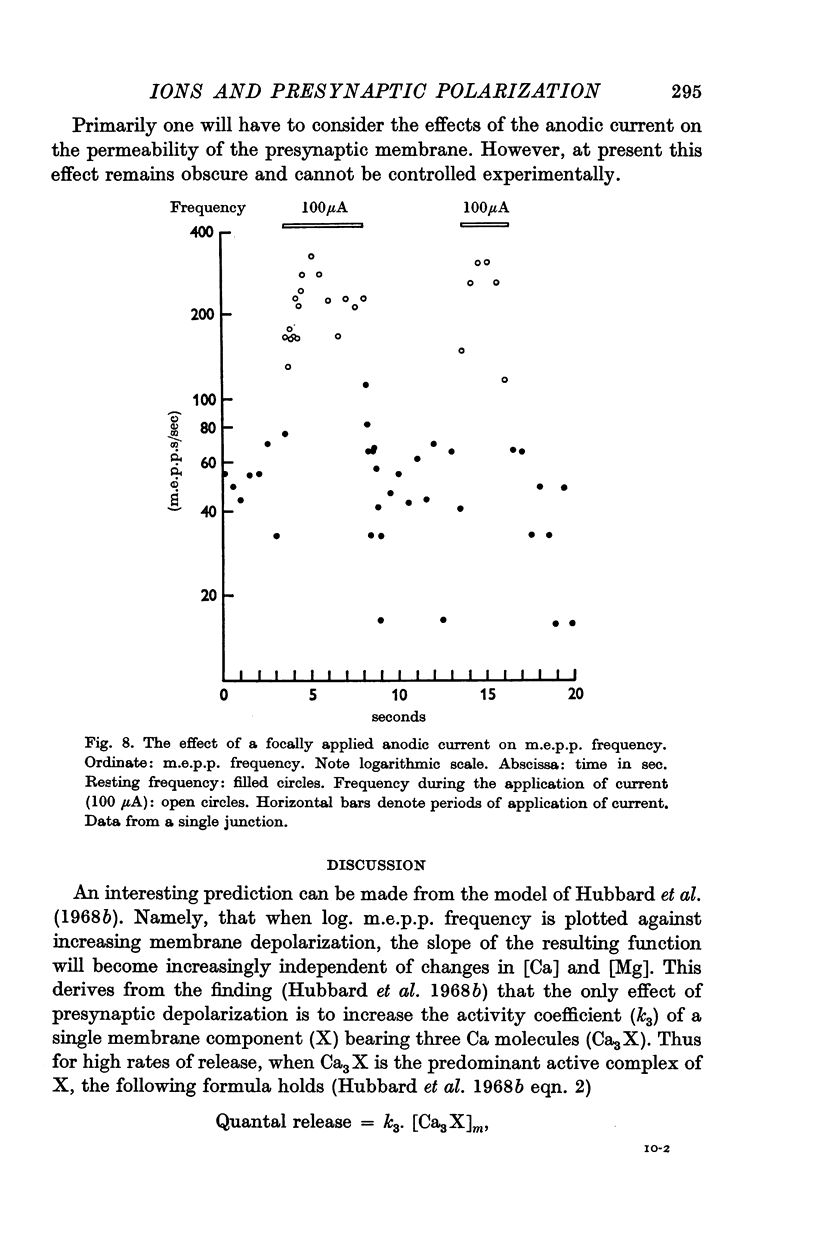
7. In some of the junctions `anodic break-down' was observed. An examination of this phenomenon with focal polarizing currents disclosed an unusual type of `anodic break-down', with rapid `on' and `off' responses. This phenomenon may indicate that release depends on the influx of positively charged particles into the nerve terminals.
8. It is concluded that nerve terminal depolarization accelerates exponentially the activity of a membrane component bearing three Ca molecules, the rate of acceleration being independent of bathing [Ca].
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSEN P., CURTIS D. R. THE EXCITATION OF THALAMIC NEURONES BY ACETYLCHOLINE. Acta Physiol Scand. 1964 May-Jun;61:85–99. doi: 10.1111/j.1748-1716.1964.tb02945.x. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Spontaneous activity at a mammalian neuromuscular junction in tetrodotoxin. Acta Physiol Scand. 1965 Aug;64(4):475–476. doi: 10.1111/j.1748-1716.1965.tb04206.x. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., MEVES H. The effect of magnesium and calcium on the frog myelinated nerve fibre. J Physiol. 1958 Jul 14;142(2):360–365. doi: 10.1113/jphysiol.1958.sp006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. The effect of calcium on the myelinated nerve fibre. J Physiol. 1957 Jul 11;137(2):245–260. doi: 10.1113/jphysiol.1957.sp005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Dual effect of potassium on transmitter release. Nature. 1965 May 8;206(984):625–626. doi: 10.1038/206625a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARTH J. V. The behaviour of frog muscle in hypertonic solutions. J Physiol. 1958 Nov 10;144(1):167–175. doi: 10.1113/jphysiol.1958.sp006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., WILLIS W. D. Hyperpolarization of mammalian motor nerve terminals. J Physiol. 1962 Aug;163:115–137. doi: 10.1113/jphysiol.1962.sp006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., TAYLOR R. E. Local activation of striated muscle fibres. J Physiol. 1958 Dec 30;144(3):426–441. doi: 10.1113/jphysiol.1958.sp006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. An examination of the effects of osmotic pressure changes upon transmitter release from mammalian motor nerve terminals. J Physiol. 1968 Aug;197(3):639–657. doi: 10.1113/jphysiol.1968.sp008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. The relationship between the state of nerve-terminal polarization and liberation of acetylcholine. Ann N Y Acad Sci. 1967 Oct 31;144(2):459–470. doi: 10.1111/j.1749-6632.1967.tb53787.x. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Willis W. D. The effects of depolarization of motor nerve terminals upon the release of transmitter by nerve impulses. J Physiol. 1968 Feb;194(2):381–405. doi: 10.1113/jphysiol.1968.sp008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin and neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):8–22. doi: 10.1098/rspb.1967.0010. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The release of acetylcholine from nerve endings by graded electric pulses. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):23–38. doi: 10.1098/rspb.1967.0011. [DOI] [PubMed] [Google Scholar]
- Kusano K. Further study of the relationship between pre- and postsynaptic potentials in the squid giant synapse. J Gen Physiol. 1968 Aug;52(2):326–345. doi: 10.1085/jgp.52.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Livengood D. R., Werman R. Correlation of transmitter release with membrane properties of the presynaptic fiber of the squid giant synapse. J Gen Physiol. 1967 Dec;50(11):2579–2601. doi: 10.1085/jgp.50.11.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. Spontaneous release of transmitter substance in multiquantal units. J Physiol. 1957 May 23;136(3):595–605. doi: 10.1113/jphysiol.1957.sp005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUTTGAU H. C. Analyse der Wirkung von Barium-Ionen an der isolierten markhaltigen Nervenfaser. Pflugers Arch. 1954;260(2):141–147. [PubMed] [Google Scholar]
- Lucas K. On the rate of development of the excitatory process in muscle and nerve. J Physiol. 1908 Dec 15;37(5-6):459–480. doi: 10.1113/jphysiol.1908.sp001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANES A. M. Electrochemical aspects of physiological and pharmacological action in excitable cells. II. The action potential and excitation. Pharmacol Rev. 1958 Jun;10(2):165–273. [PubMed] [Google Scholar]
- SHANES A. M., FREYGANG W. H., GRUNDFEST H., AMATNIEK E. Anesthetic and calcium action in the voltage-clamped squid giant axon. J Gen Physiol. 1959 Mar 20;42(4):793–802. doi: 10.1085/jgp.42.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDMANN S. Effects of calcium ions and local anesthetics on electrical properties of Purkinje fibres. J Physiol. 1955 Sep 28;129(3):568–582. doi: 10.1113/jphysiol.1955.sp005379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A., Tasaki I., Singer I., Lerman L. Effects of tetrodotoxin on excitability of squid giant axons in sodium-free media. Science. 1967 Jan 6;155(3758):95–97. doi: 10.1126/science.155.3758.95. [DOI] [PubMed] [Google Scholar]


