Abstract
1. The electrical properties of frog slow muscle fibres were investigated with intracellular micropipettes to determine their characteristic length (λ), specific membrane resistance (Rm) and specific membrane capacitance.
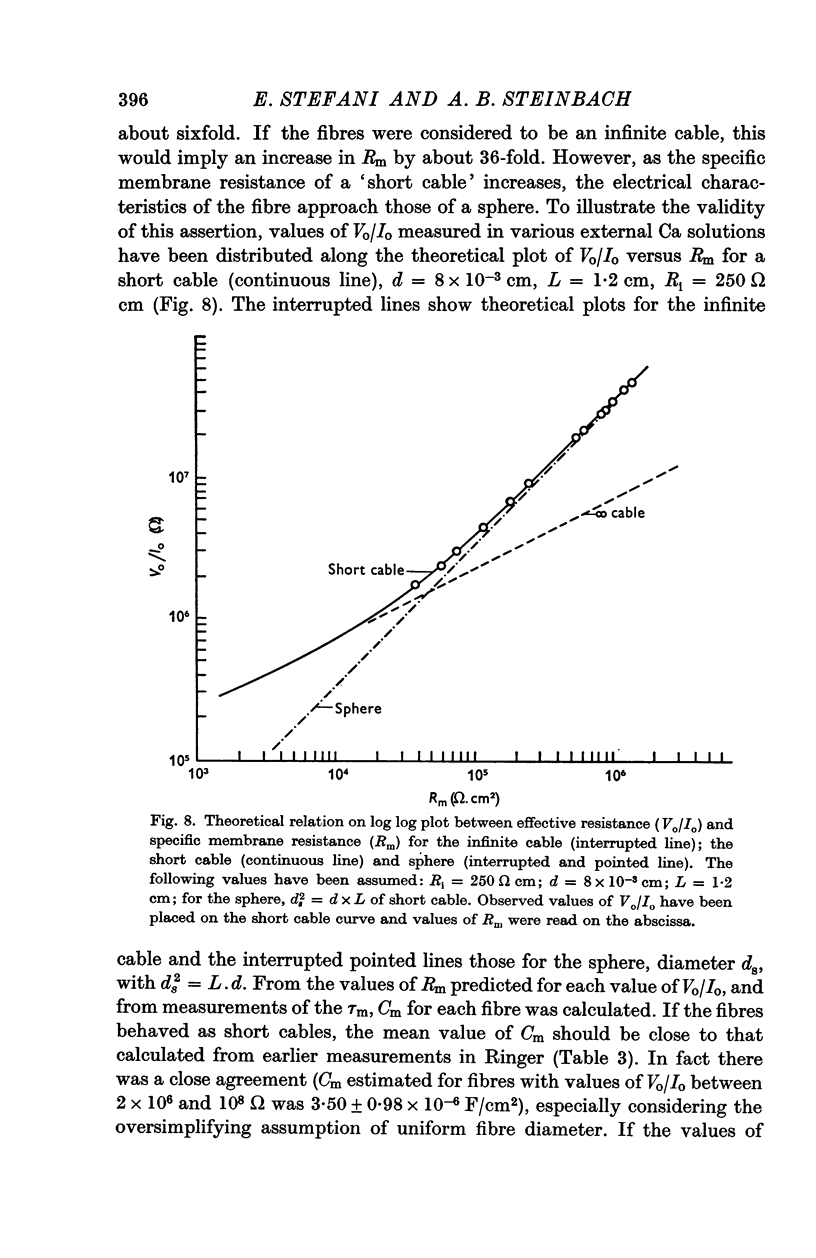
2. The value of λ was about 1 cm in fibres of 1·2 cm length. The `short cable model' was used to calculate Rm. Its mean value was 1·12 × 105 ohm cm2, about 10-20 times larger than the value for twitch fibres. The mean value for Cm was 3·24 × 10-6 F/cm2.
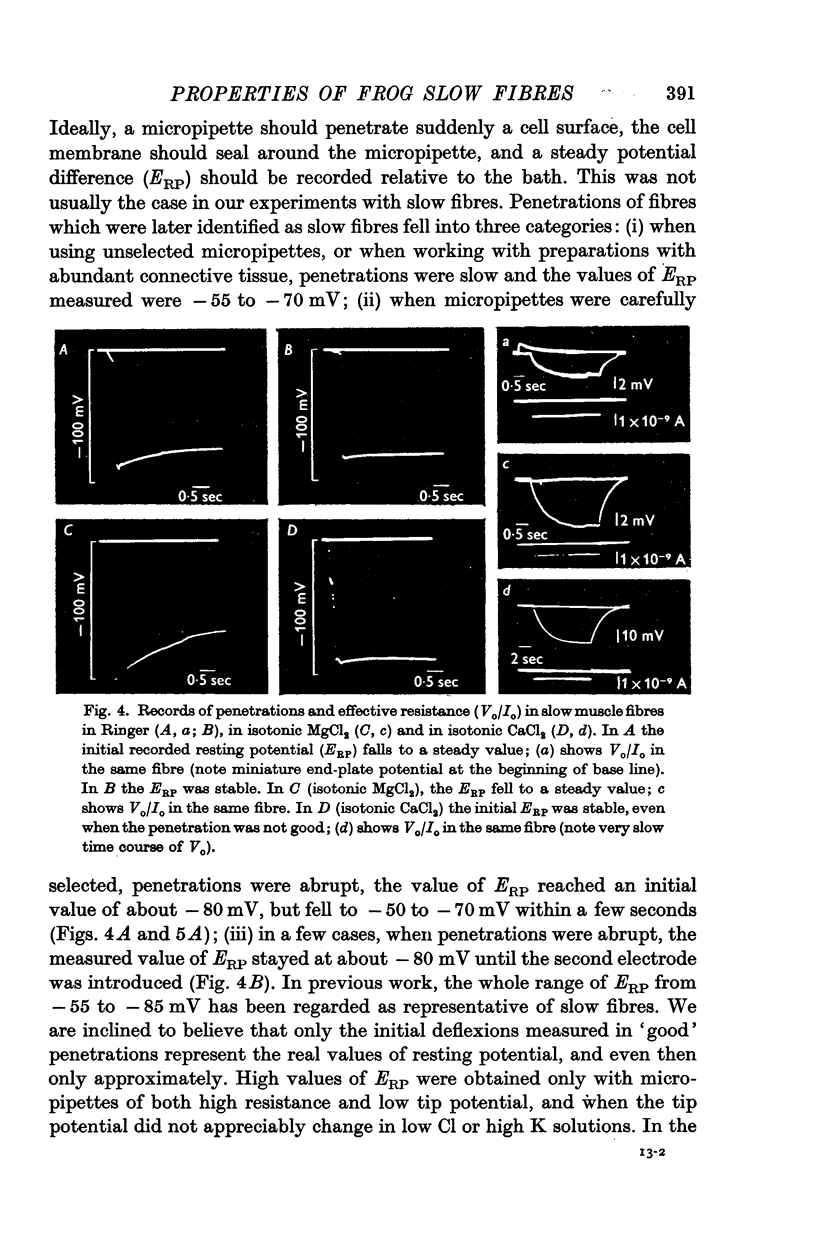
3. Resting potentials measured immediately after penetration with a single micropipette were about — 80 mV. Lower values can be attributed to the effects of damage or leakage produced by micropipette insertion.
4. Changes in external K concentration produced changes in the initially recorded resting potentials which follow the constant field theory using a ratio of Na: K permeabilities PNa/PK = 0·02. Changes in external Cl concentration produced little or no change in the resting potential or membrane resistance, indicating a low Cl permeability.
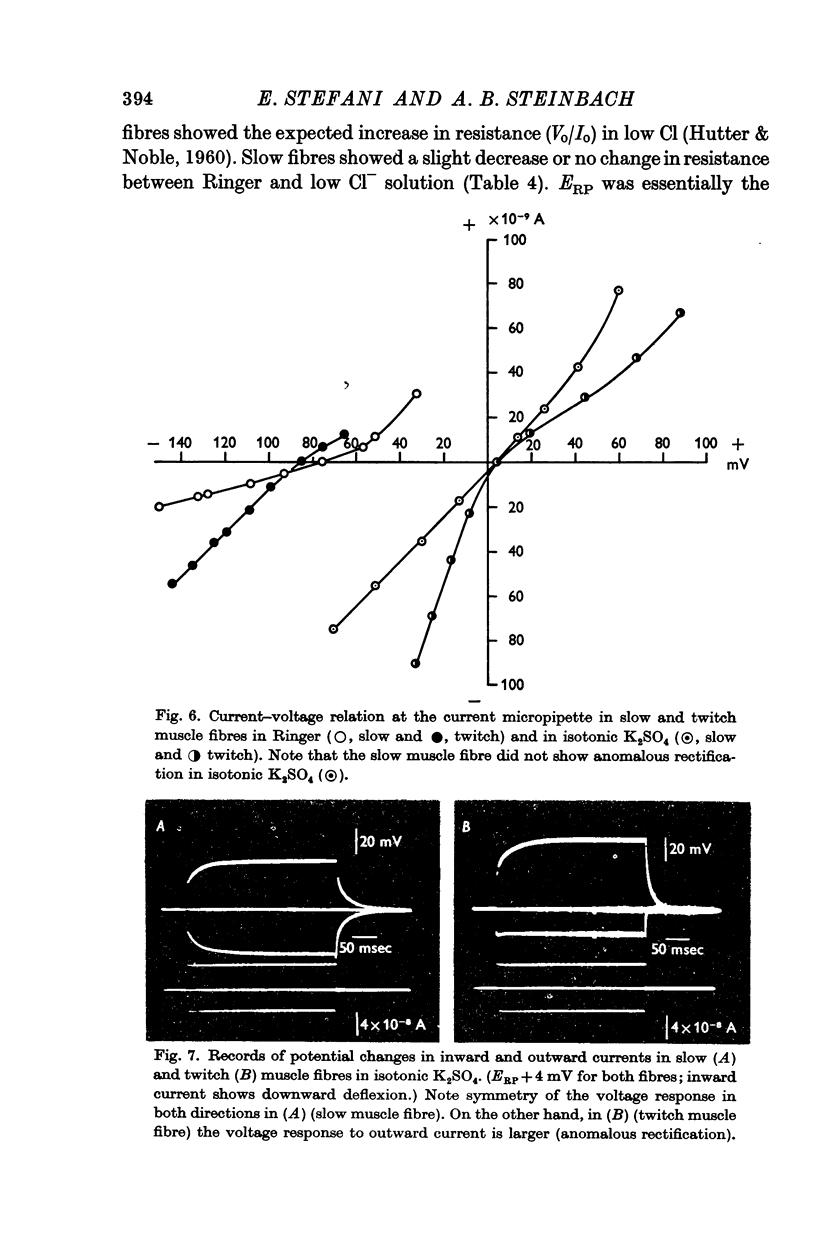
5. In agreement with previous work, slow fibres showed a time-dependent decrease in resistance (`delayed rectification') for membrane potentials more positive than — 60 mV. `Anomalous rectification' observed in twitch fibres was not seen in slow fibres. In high external K concentrations the resistance of slow fibres is almost unaffected by changes in membrane potential.
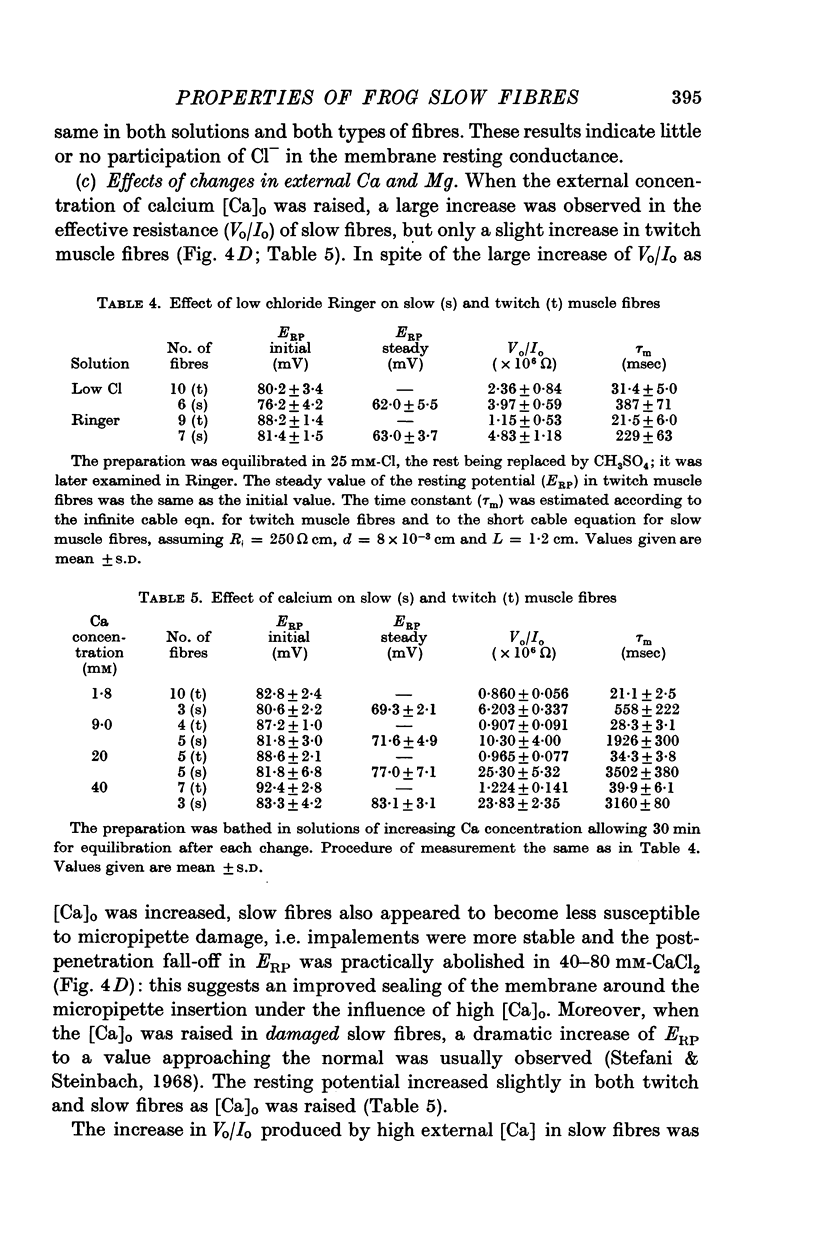
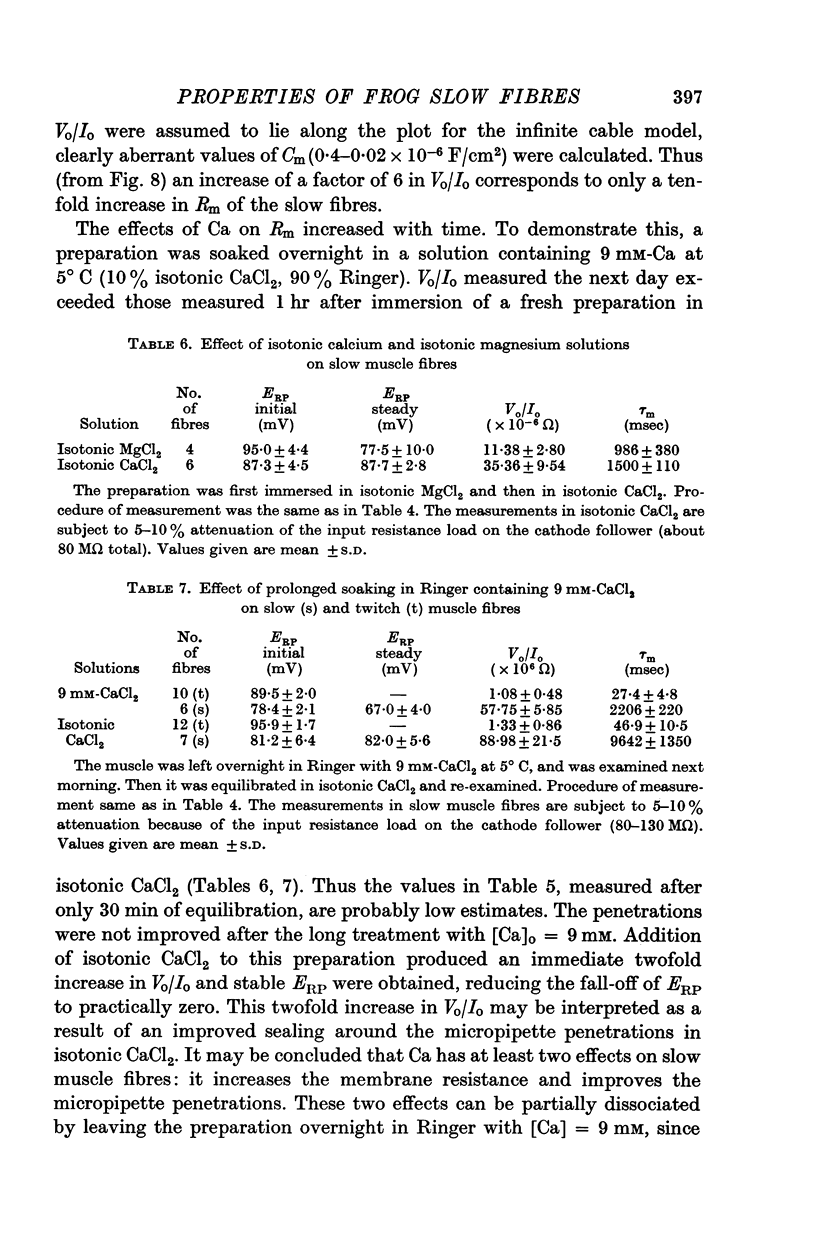
6. Increasing the concentration of external Ca (up to isotonic) has two distinct effects on slow fibres. It increases Rm up to ten times, and it improves the stability of trans-membrane recordings, probably by reducing the leakage due to micropipette penetrations. Magnesium does not appear to have either of these effects.
Full text
PDF

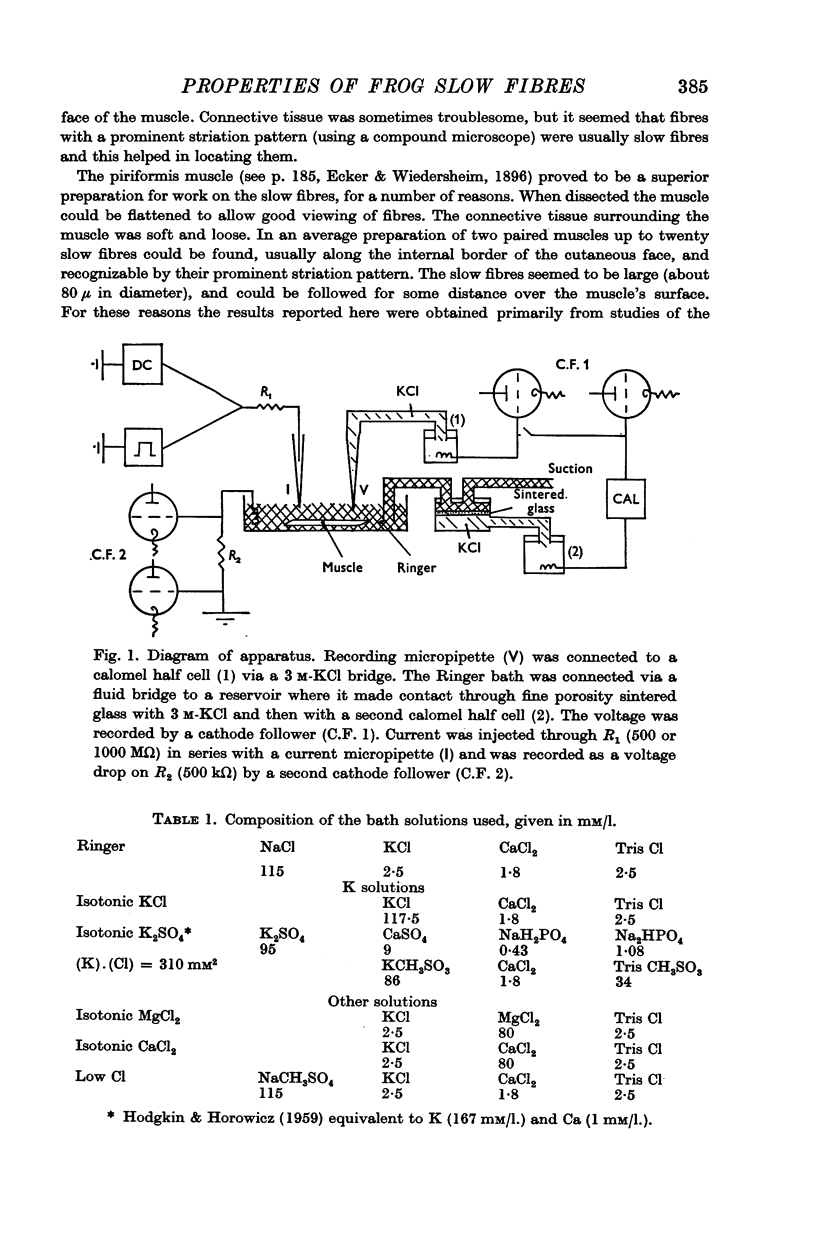

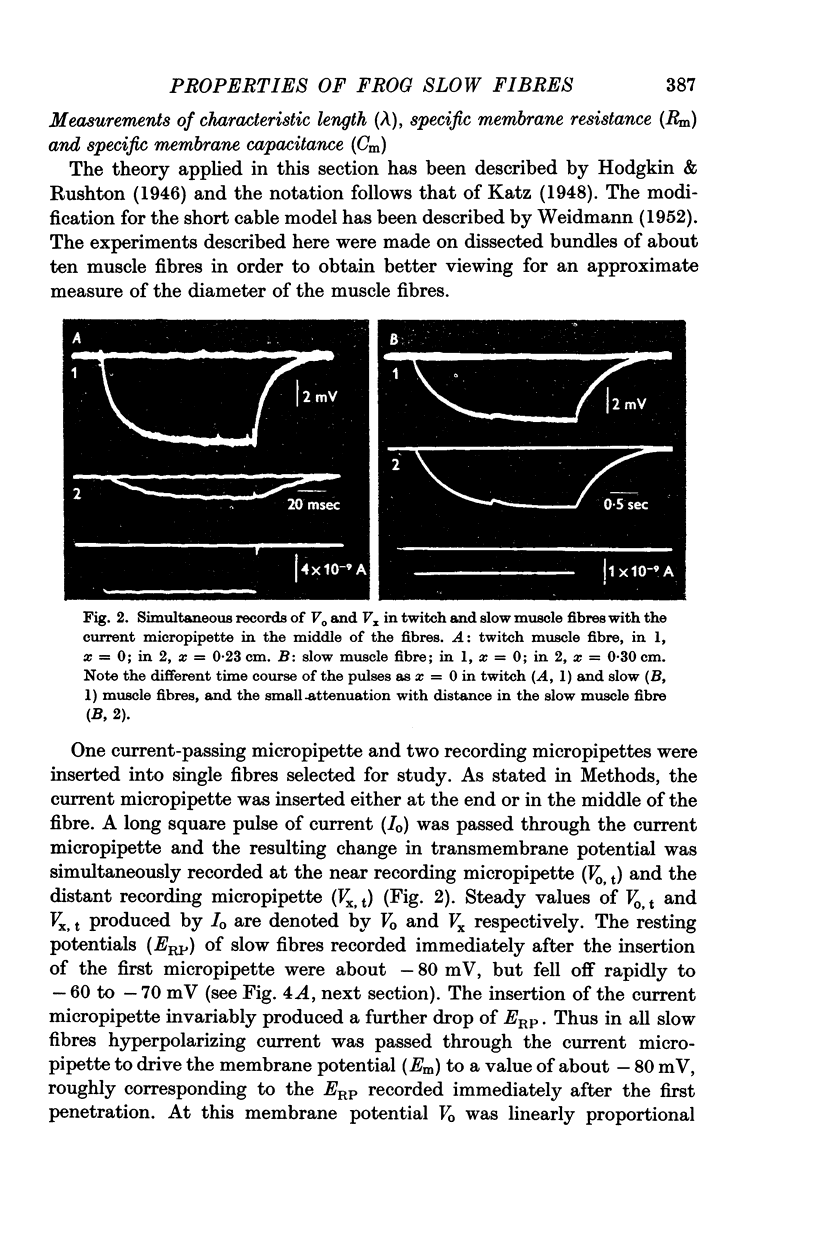

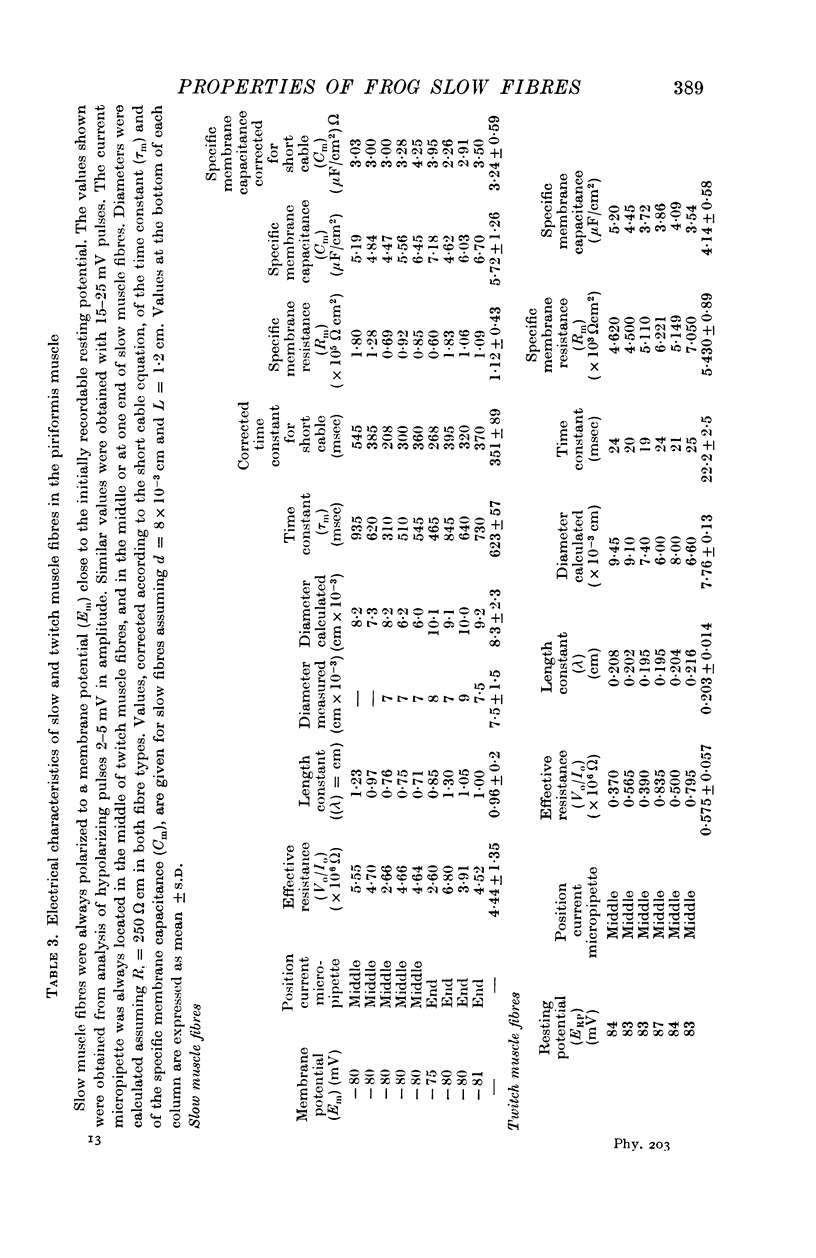
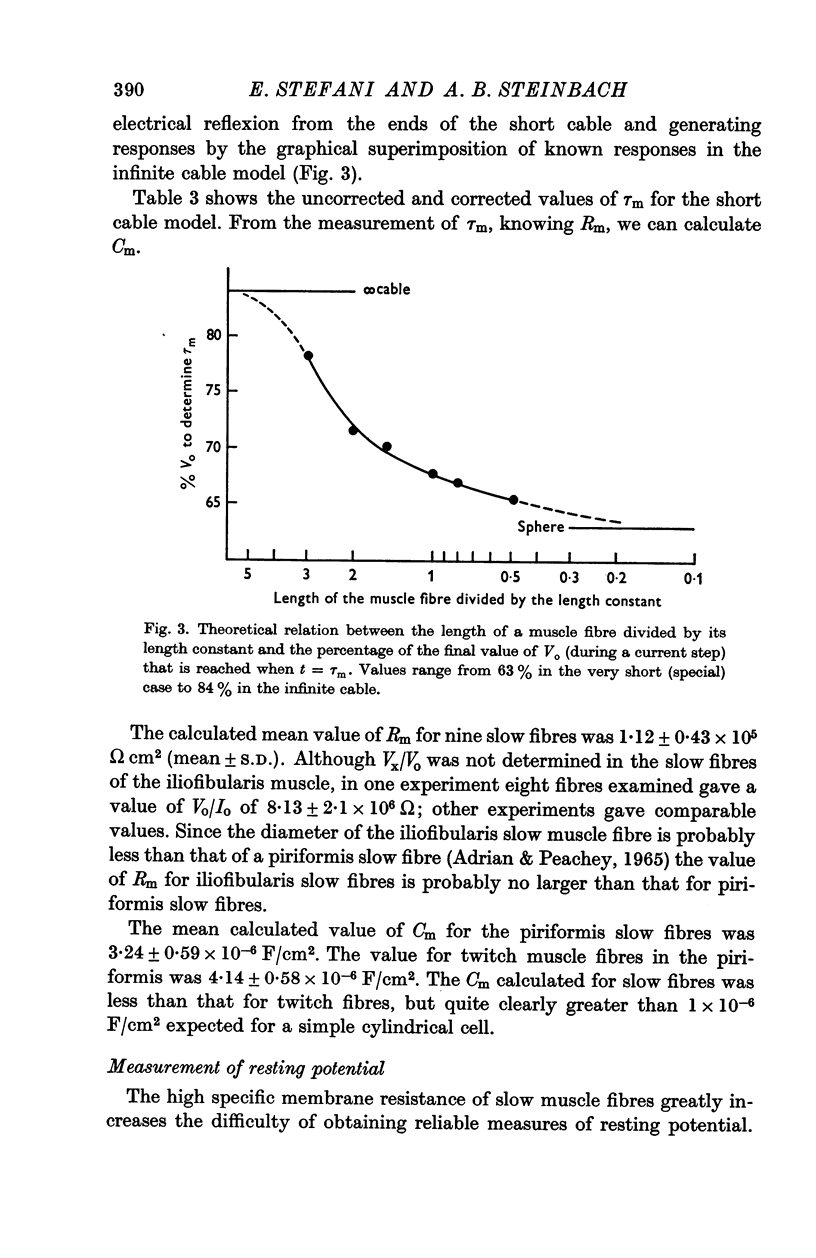






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Peachey L. D. The membrane capacity of frog twitch and slow muscle fibres. J Physiol. 1965 Nov;181(2):324–336. doi: 10.1113/jphysiol.1965.sp007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKE W., GINSBORG B. L. The electrical properties of the slow muscle fibre membrane. J Physiol. 1956 Jun 28;132(3):586–598. doi: 10.1113/jphysiol.1956.sp005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALK G., FATT P. LINEAR ELECTRICAL PROPERTIES OF STRIATED MUSCLE FIBRES OBSERVED WITH INTRACELLULAR ELECTRODES. Proc R Soc Lond B Biol Sci. 1964 Apr 14;160:69–123. doi: 10.1098/rspb.1964.0030. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUSCALDO K. E., JONES H. H. A method for the reconstruction of three-dimensional models from electron micrographs of serial sections. J Ultrastruct Res. 1959 Oct;3:1–10. doi: 10.1016/s0022-5320(59)80010-1. [DOI] [PubMed] [Google Scholar]
- Grundfest H. Comparative electrobiology of excitable membranes. Adv Comp Physiol Biochem. 1966;2:1–116. doi: 10.1016/b978-0-12-395511-1.50006-8. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. The chloride conductance of frog skeletal muscle. J Physiol. 1960 Apr;151:89–102. [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The pH sensitivity of the chloride conductance of frog skeletal muscle. J Physiol. 1967 Apr;189(3):403–425. doi: 10.1113/jphysiol.1967.sp008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIESSLING A. [The resting potential of "tonic" skeletal muscle fibers of the frog]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;271:124–138. [PubMed] [Google Scholar]
- KUFFLER S. W., VAUGHAN WILLIAMS E. M. Properties of the 'slow' skeletal muscles fibres of the frog. J Physiol. 1953 Aug;121(2):318–340. doi: 10.1113/jphysiol.1953.sp004949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., VAUGHAN WILLIAMS E. M. Small-nerve junctional potentials; the distribution of small motor nerves to frog skeletal muscle, and the membrane characteristics of the fibres they innervate. J Physiol. 1953 Aug;121(2):289–317. doi: 10.1113/jphysiol.1953.sp004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J. Contractures of single slow muscle fibres of Xenopus laevis elicited by potassium, acetylcholine or choline. Acta Physiol Scand. 1967 Apr;69(4):362–372. doi: 10.1111/j.1748-1716.1967.tb03533.x. [DOI] [PubMed] [Google Scholar]
- Nasledov G. A., Zachar J., Zacharová D. The ionic requirements for the development of contracture in isolated slow muscle fibres of the frog. Physiol Bohemoslov. 1966;15(4):293–306. [PubMed] [Google Scholar]
- PEACHEY L. D., HUXLEY A. F. Structural identification of twitch and slow striated muscle fibers of the frog. J Cell Biol. 1962 Apr;13:177–180. doi: 10.1083/jcb.13.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. G. A comparison of the fine structures of frog slow and twitch muscle fibers. J Cell Biol. 1965 Aug;26(2):477–497. doi: 10.1083/jcb.26.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. Muscle. Annu Rev Physiol. 1968;30:401–440. doi: 10.1146/annurev.ph.30.030168.002153. [DOI] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Stefani E., Steinbach A. B. Action potentials in denervated "slow" muscle fibres of the frog. J Physiol. 1968 Jul;197(1):4P–5P. [PubMed] [Google Scholar]
- WEIDMANN S. The electrical constants of Purkinje fibres. J Physiol. 1952 Nov;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Effects of pH and polyvalent cations on the selective permeability of gall-bladder epithelium to monovalent ions. Biochim Biophys Acta. 1968 Aug;163(1):57–74. doi: 10.1016/0005-2736(68)90033-3. [DOI] [PubMed] [Google Scholar]


