Abstract
1. In an attempt to obtain information about structural changes related to electrical activity in Electrophorus electroplates, we have determined the size and time course of the changes in light scattering and in bire-fringence that occur during and after the discharge of the electric organ.
2. The changes in light intensity detected with a photomultiplier were never greater than 0·2% for a single discharge, and were often much smaller than this, but records with an acceptable ratio of signal to noise could be obtained by signal-averaging techniques.
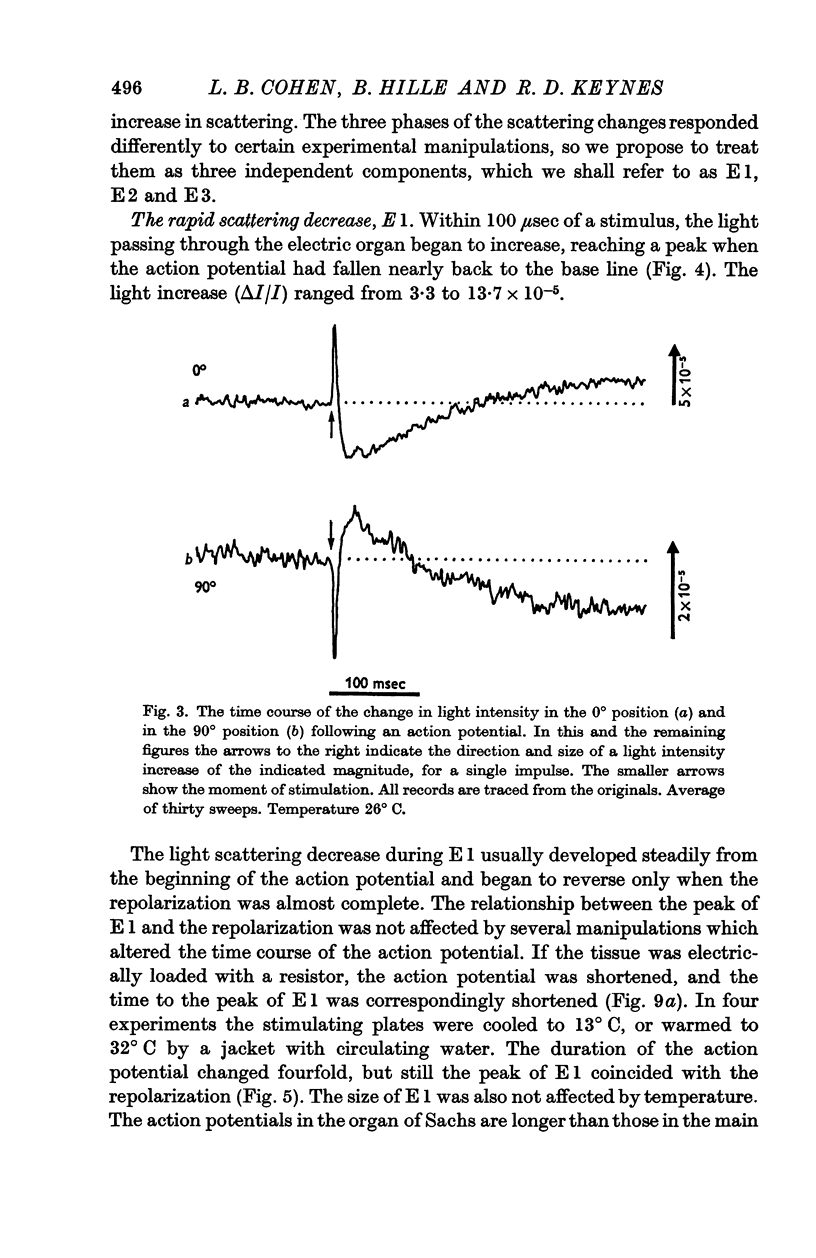
3. A single stimulus led to a decrease, then an increase, and finally another decrease in the light scattered by slices of the main electric organ. These three phases were designated E1, E2 and E3.
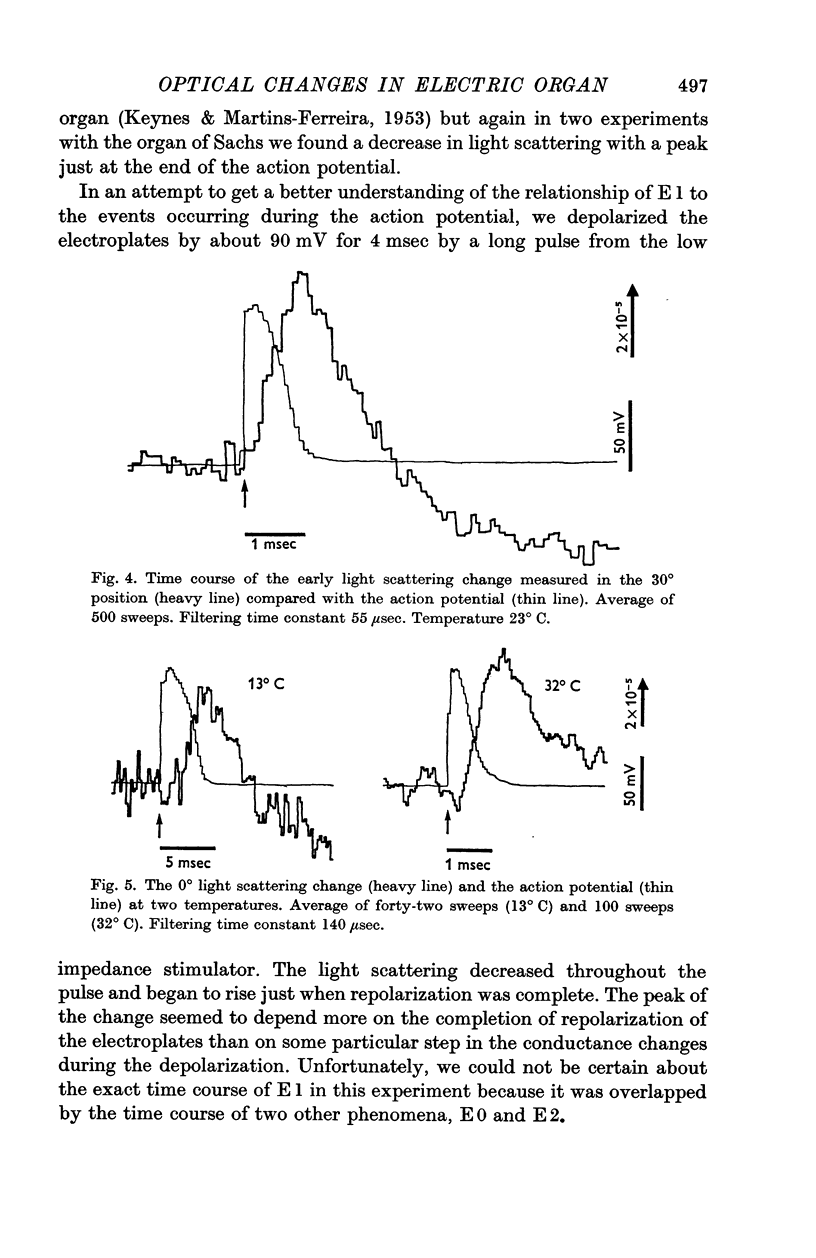
4. E1 started at the beginning of the action potential, and its peak was reached at the same time as the completion of repolarization, even when the repolarization was delayed by cooling or hastened by drawing larger currents from the tissue.
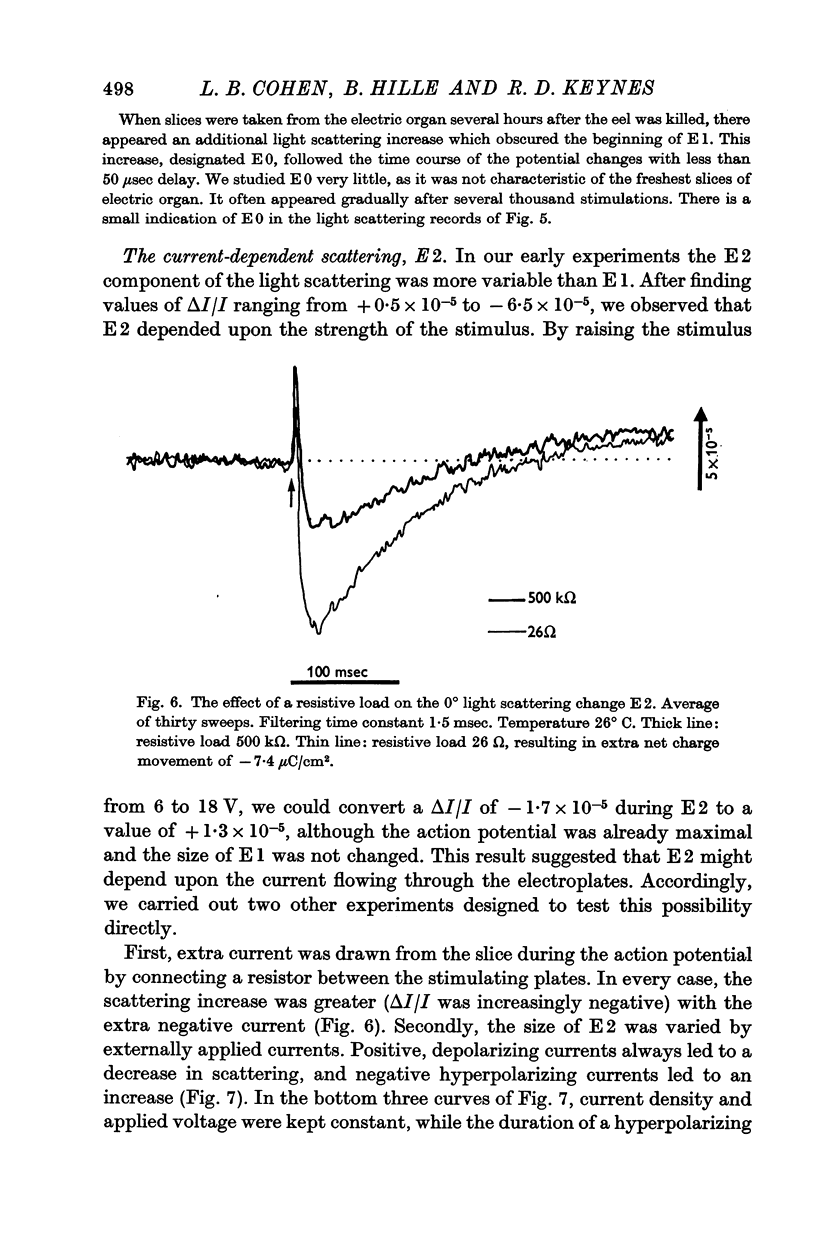
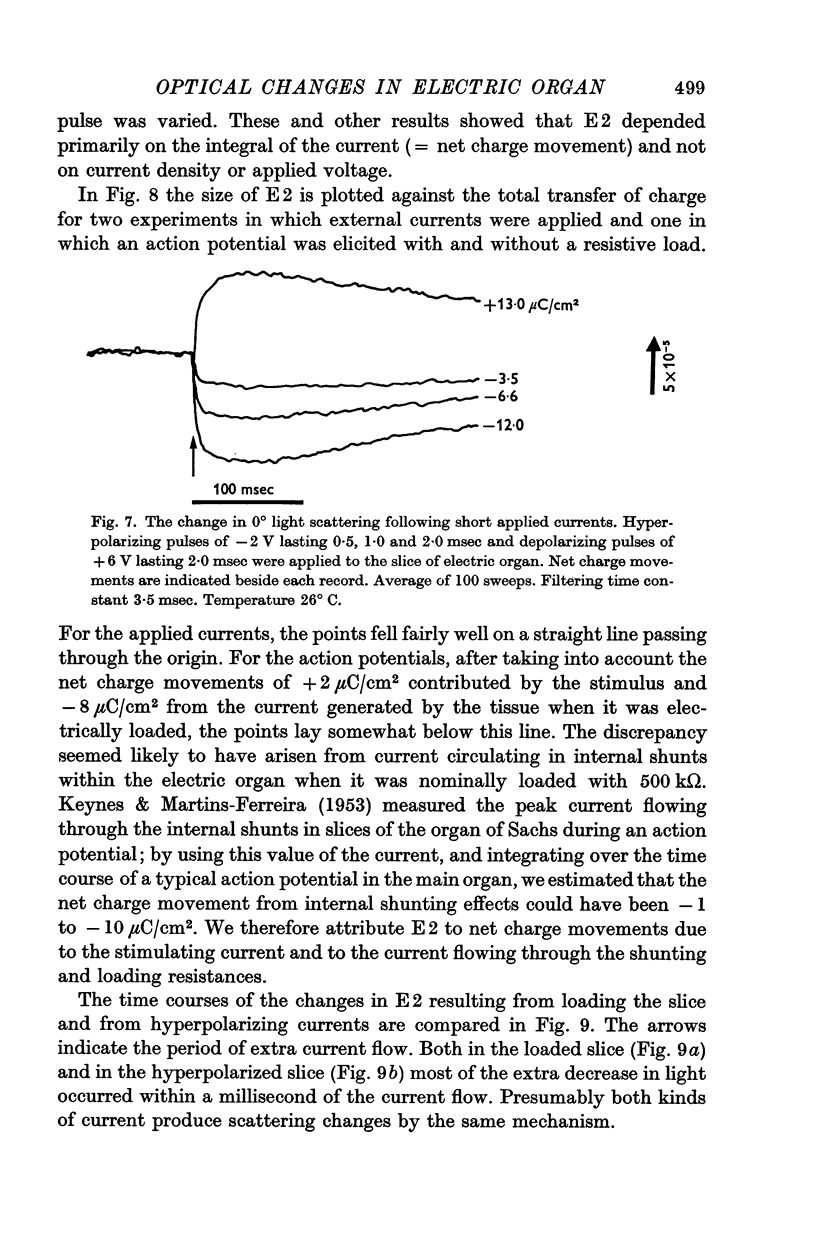
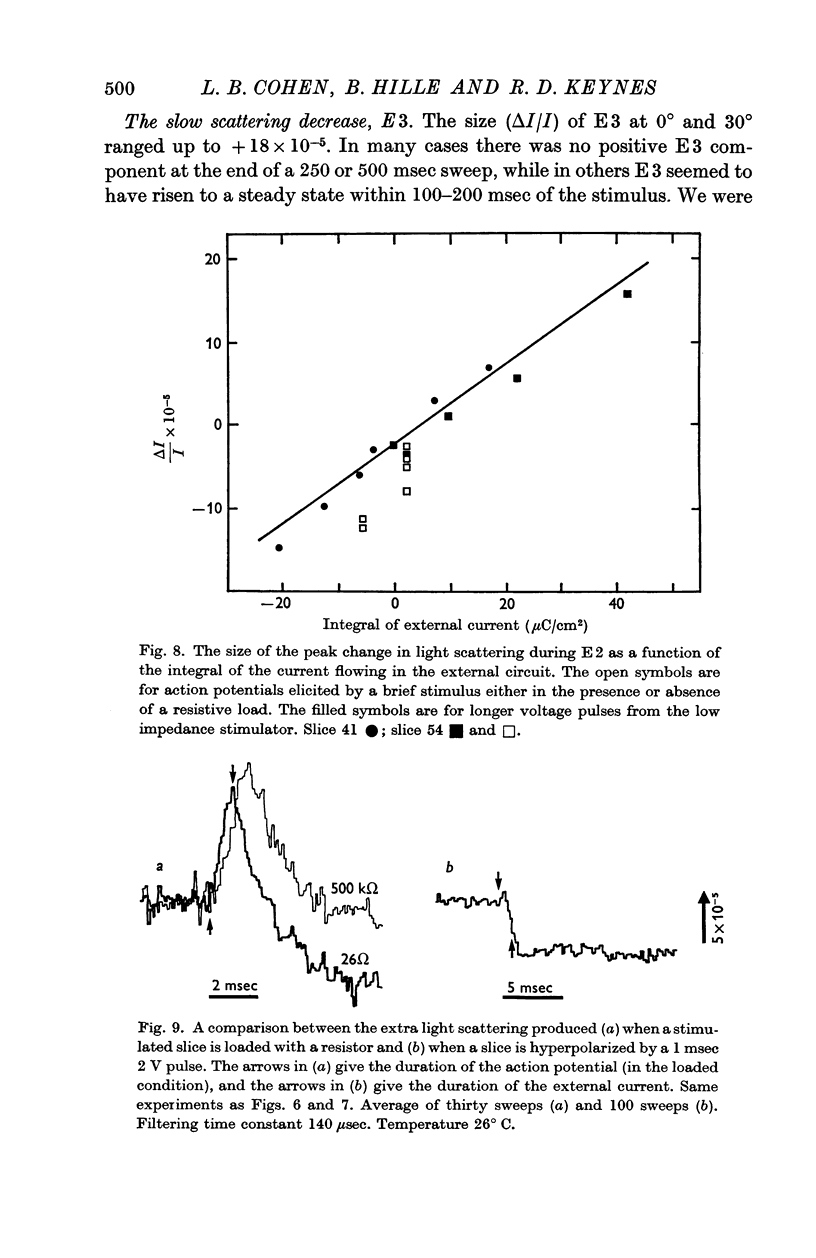
5. E2 was proportional to the integral of the current flowing through the slice of electric organ, and may arise from the swelling and shrinking of the tubules that stud the faces of the electroplates. It developed within a millisecond or two of the start of an applied current, and lasted for about 100 msec.
6. E3 was a variable decrease in scattering that lasted for some seconds.
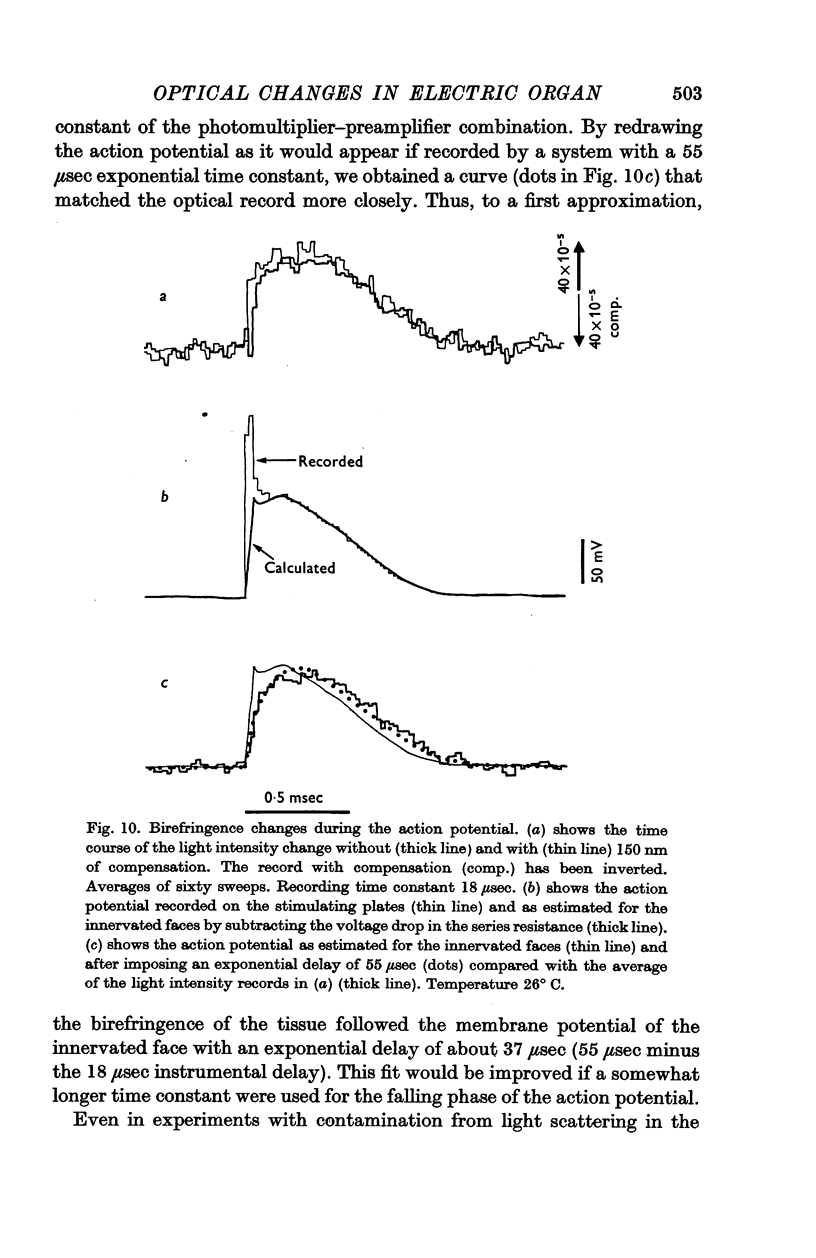
7. A stimulus also led to a transient increase in the birefringence of the electric organ. The optical change followed the change in electrical potential across the innervated faces of the electroplates with a delay of somewhat under 50 μsec.
8. This voltage-dependent change in birefringence may arise from a Kerr effect (electric birefringence) in the membrane or from compression of the membrane.
Full text
PDF


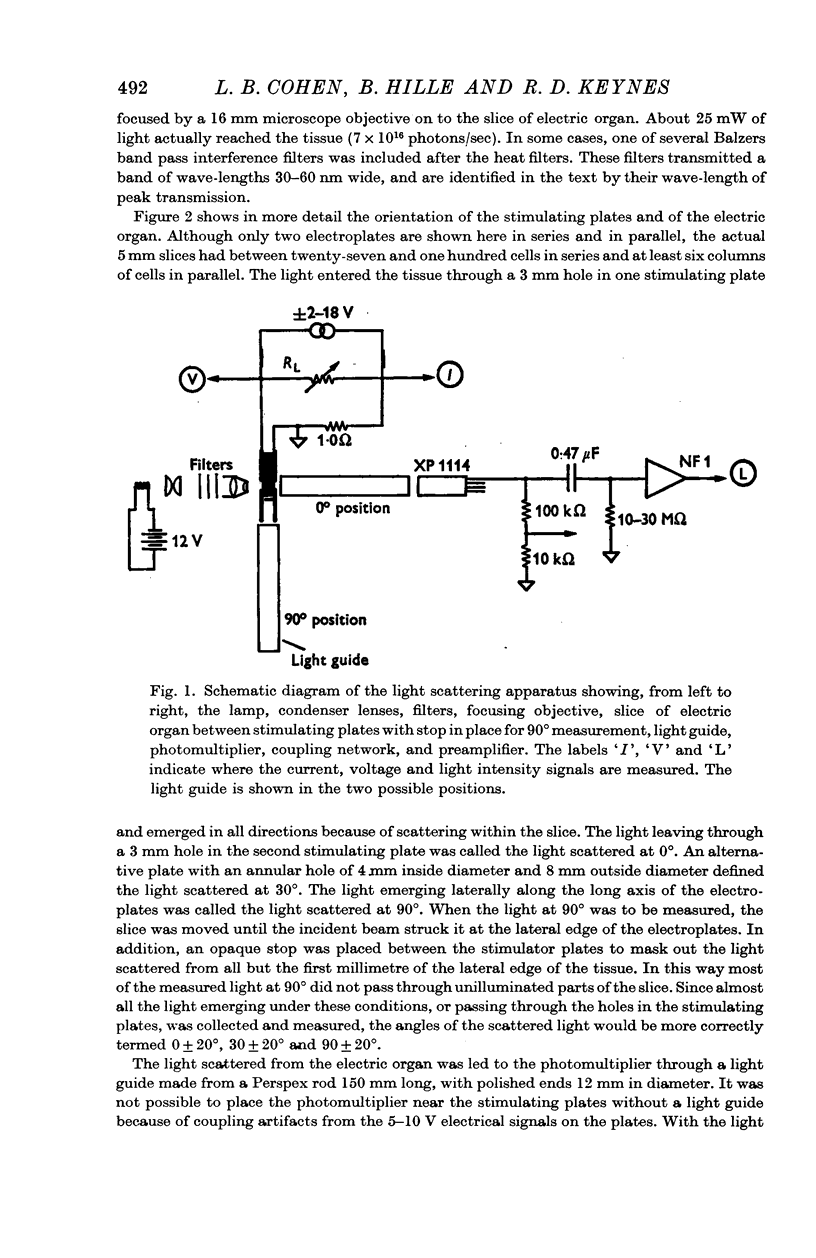
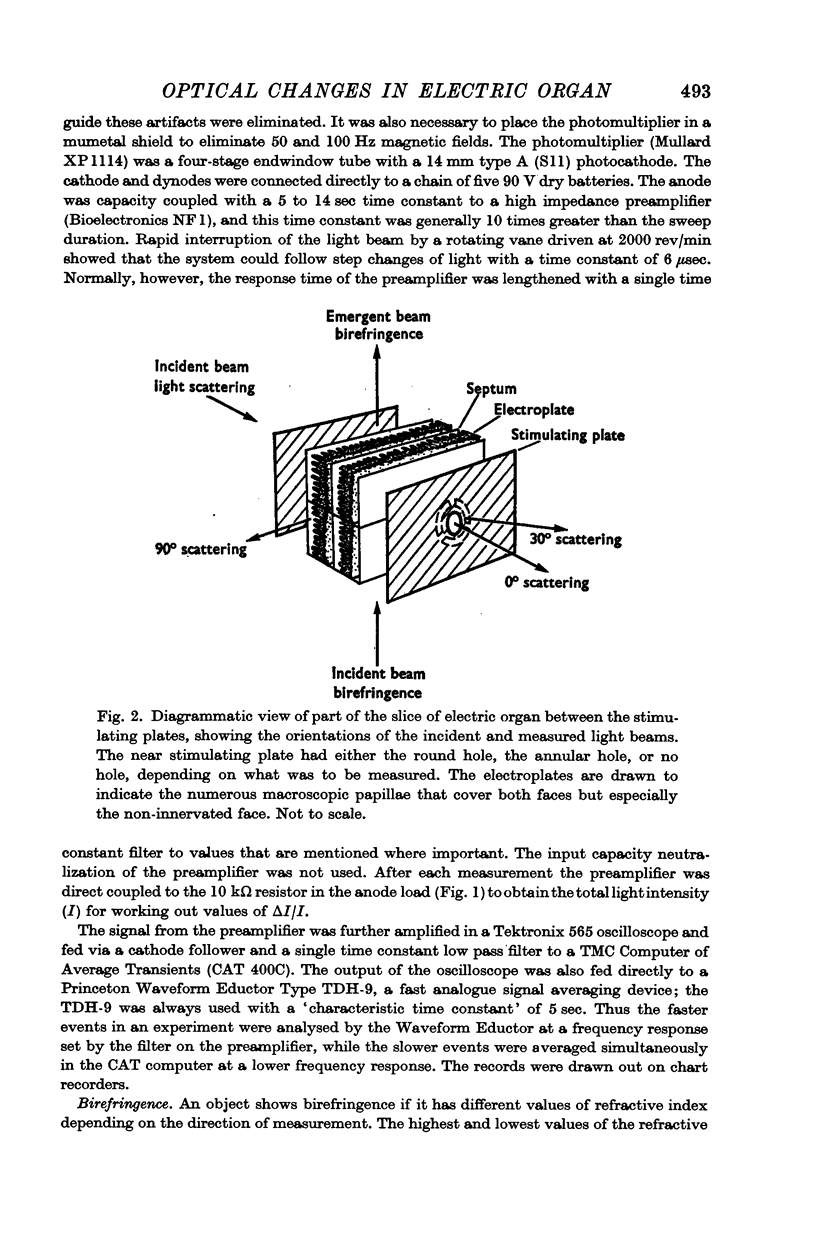










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubert X., Keynes R. D. The temperature changes during and after the discharge of the electric organ in Electrophorus electricus. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):241–263. doi: 10.1098/rspb.1968.0009. [DOI] [PubMed] [Google Scholar]
- Babakov A. V., Ermishkin L. N., Liberman E. A. Influence of electric field on the capacity of phospholipid membranes. Nature. 1966 May 28;210(5039):953–955. doi: 10.1038/210953b0. [DOI] [PubMed] [Google Scholar]
- Bloom F. E., Barrnett R. J. Fine structural localization of acetylcholinesterase in electroplaque of the electric eel. J Cell Biol. 1966 Jun;29(3):475–495. doi: 10.1083/jcb.29.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Hille B. Light scattering and birefringence changes during nerve activity. Nature. 1968 May 4;218(5140):438–441. doi: 10.1038/218438a0. [DOI] [PubMed] [Google Scholar]
- GIRARDIER L., REUBEN J. P., BRANDT P. W., GRUNDFEST H. EVIDENCE FOR ANION-PERMSELECTIVE MEMBRANE IN CRAYFISH MUSCLE FIBERS AND ITS POSSIBLE ROLE IN EXCITATION-CONTRACTION COUPLING. J Gen Physiol. 1963 Sep;47:189–214. doi: 10.1085/jgp.47.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., MARTINS-FERREIRA H. Membrane potentials in the electroplates of the electric eel. J Physiol. 1953 Feb 27;119(2-3):315–351. doi: 10.1113/jphysiol.1953.sp004849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. The fine structure of electric tissue. Exp Cell Res. 1958;14(Suppl 5):168–182. [PubMed] [Google Scholar]
- Rosen D., Sutton A. M. The effects of a direct current potential bias on the electrical properties of bimolecular lipid membranes. Biochim Biophys Acta. 1968 Sep 17;163(2):226–233. doi: 10.1016/0005-2736(68)90101-6. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Sandlin R., Carnay L. Changes in fluorescence, turbidity, and birefringence associated with nerve excitation. Proc Natl Acad Sci U S A. 1968 Nov;61(3):883–888. doi: 10.1073/pnas.61.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de ALMEIDA D., de CASTRO G. O., MIRANDA M., CHAGAS C. A study of the isolated posterior half of the electroplate of Electrophorus electricus (L). Exp Cell Res. 1963 Jan;29:42–49. doi: 10.1016/0014-4827(63)90355-0. [DOI] [PubMed] [Google Scholar]


