Abstract
1. The patterns of nerve impulses in the afferent fibres from muscle spindles have been studied using the soleus muscle of the decerebrate cat. Impulses from up to five single units were recorded simultaneously on magnetic tape, while the muscle was stretched to a series of different lengths. Various statistics were later determined by computer analysis.
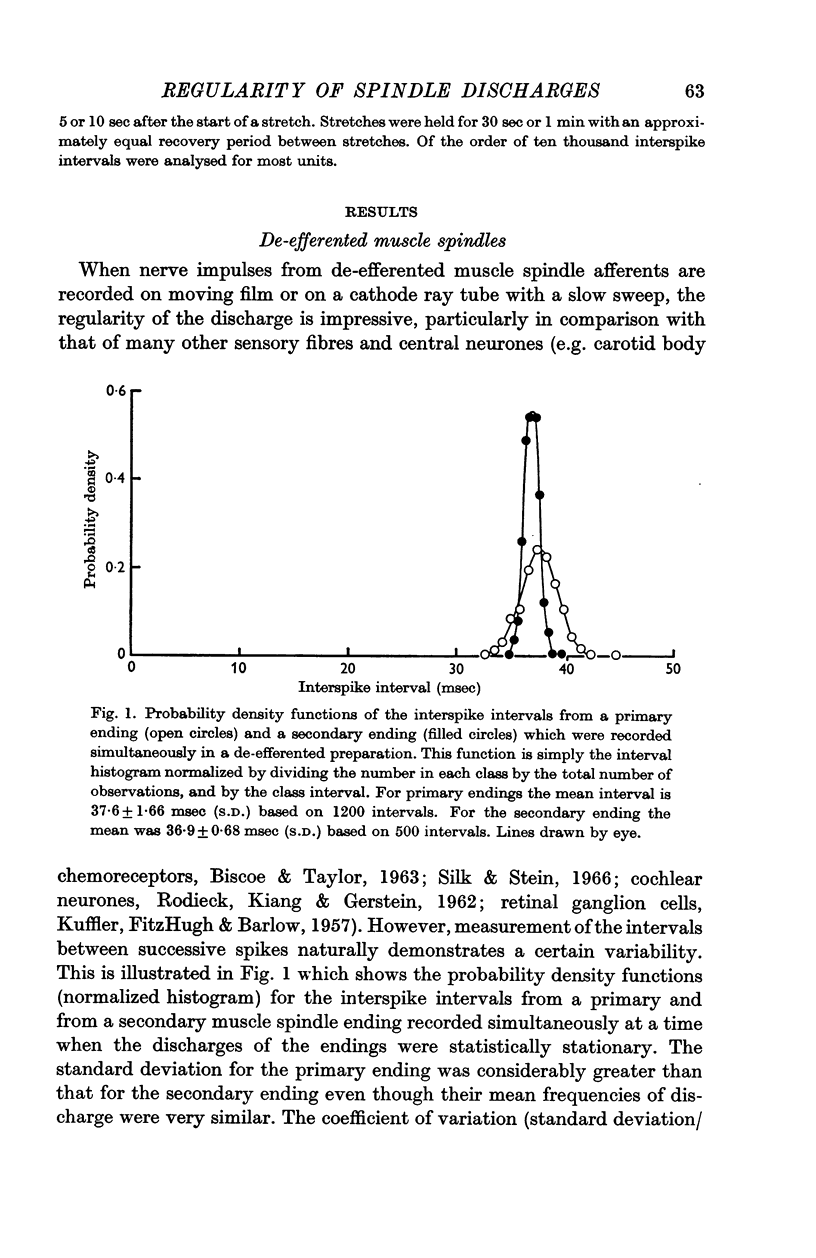
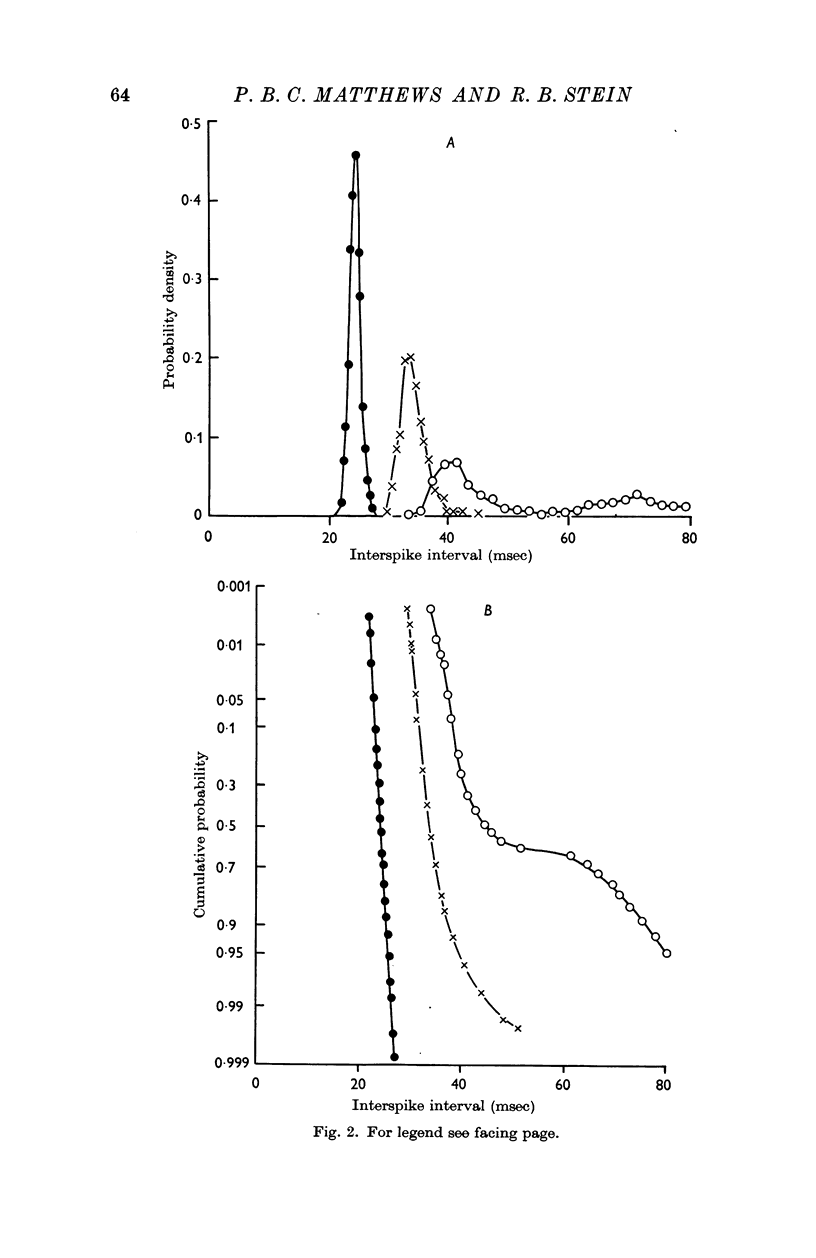
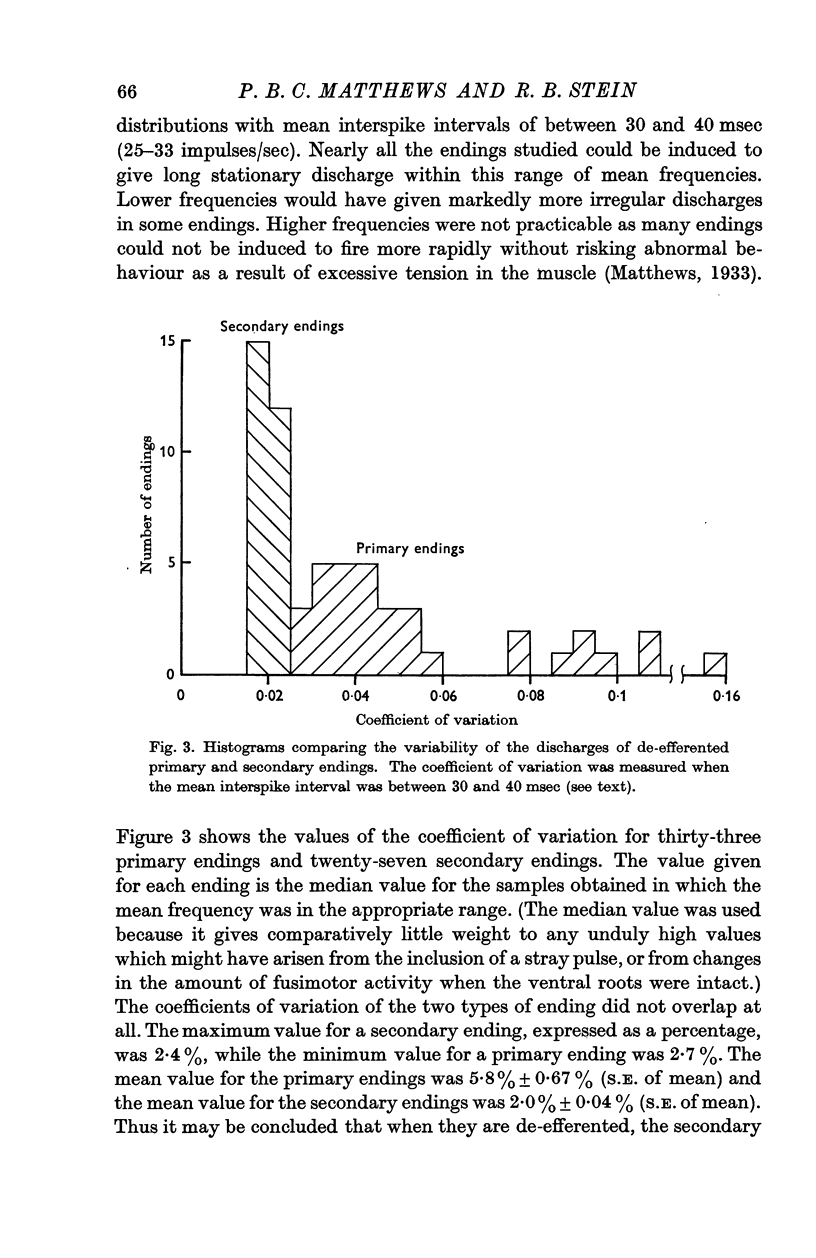
2. After the ventral roots were cut to eliminate any motor outflow to the muscle spindles, both primary and secondary spindle endings discharged very regularly. At frequencies around 30 impulses/sec the coefficient of variation of the interspike interval distributions had a mean value of only 0·02 for the secondary endings and 0·058 for the primary endings. The values obtained for the two kinds of ending did not overlap.
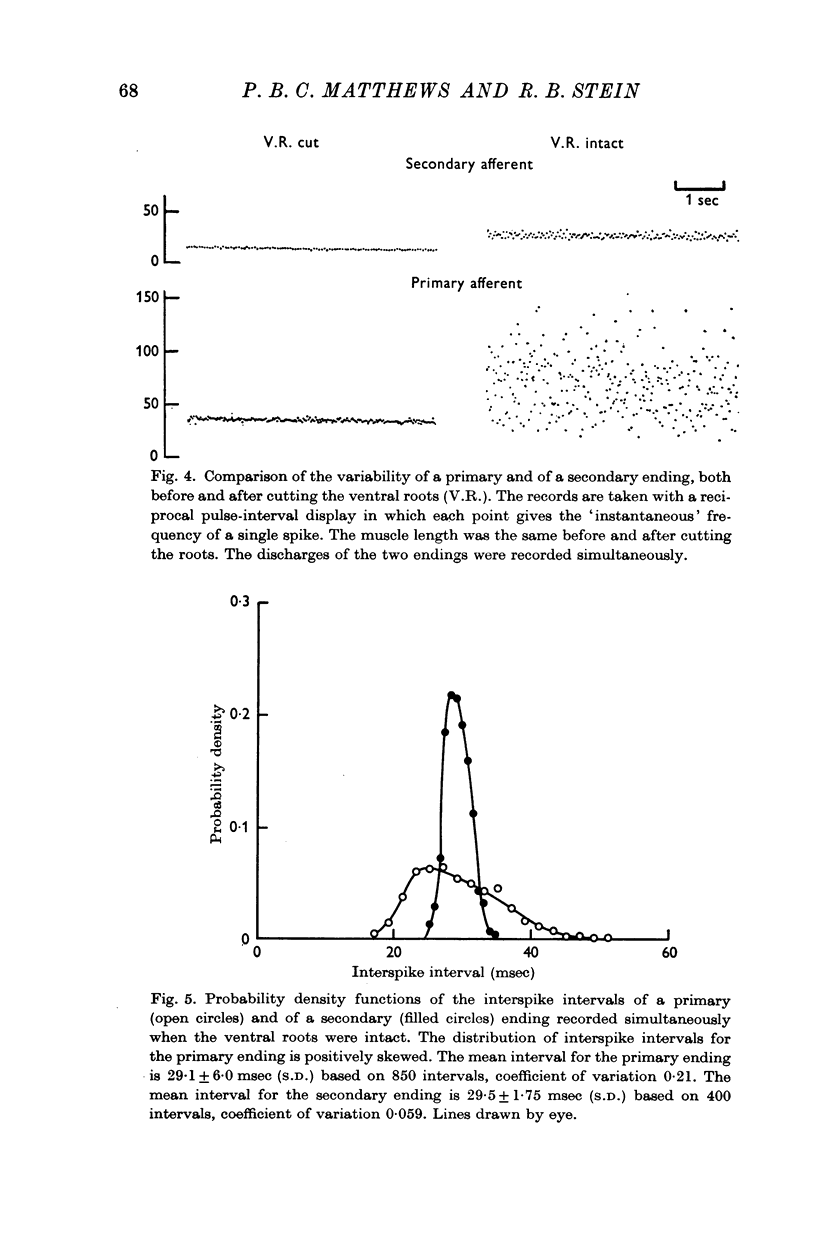
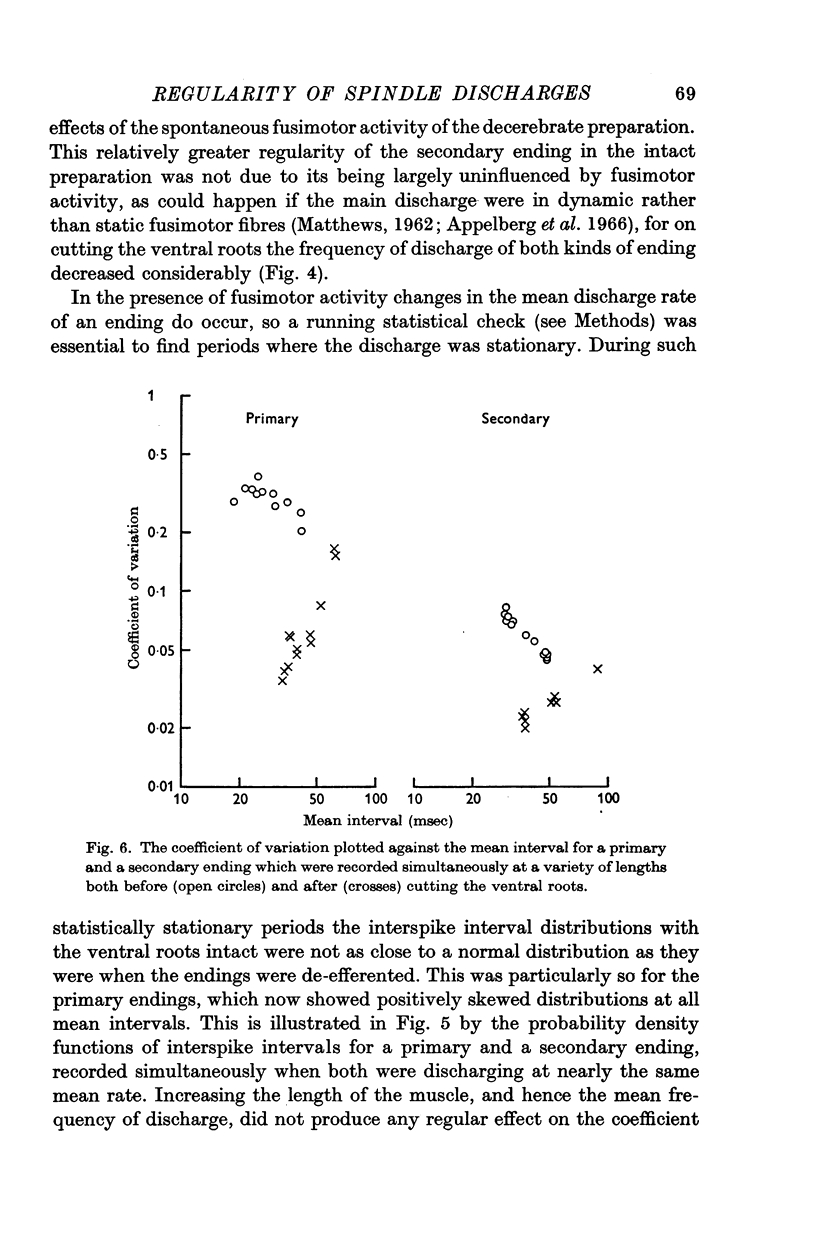
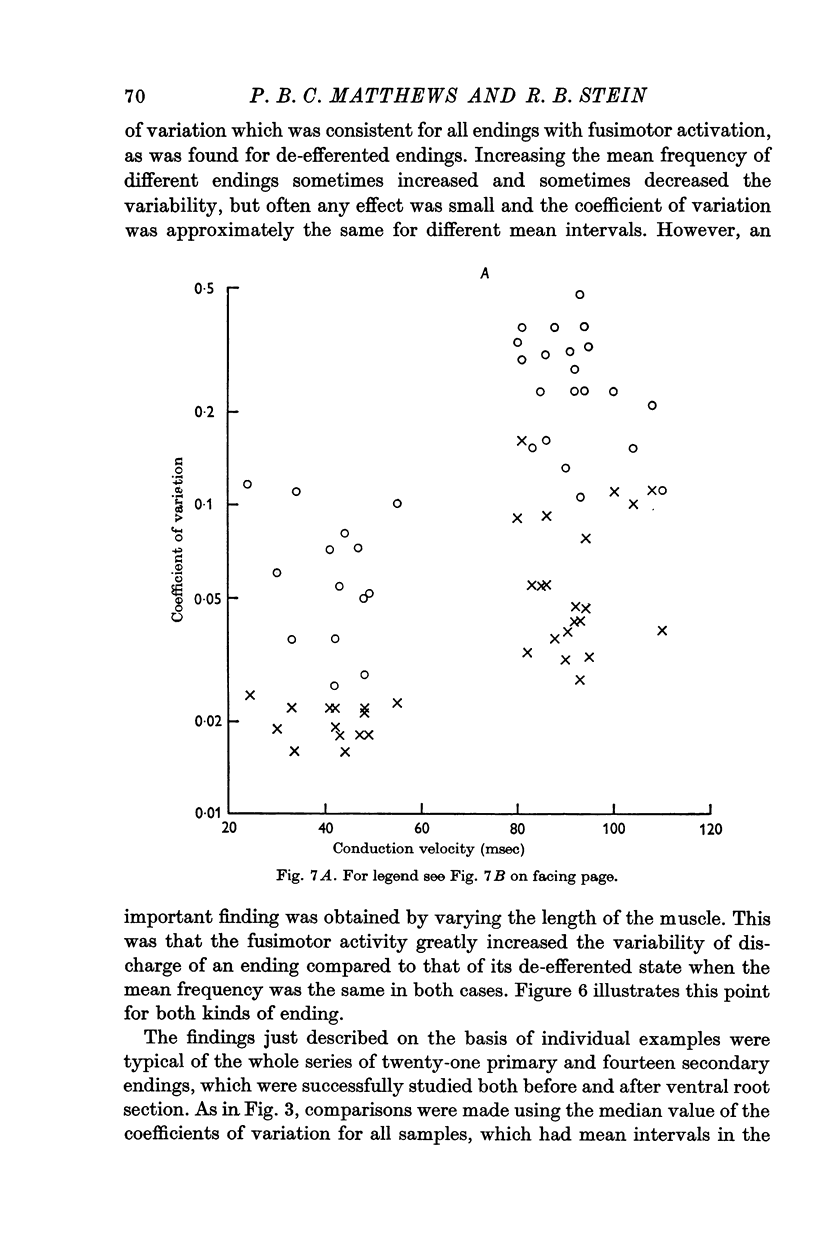
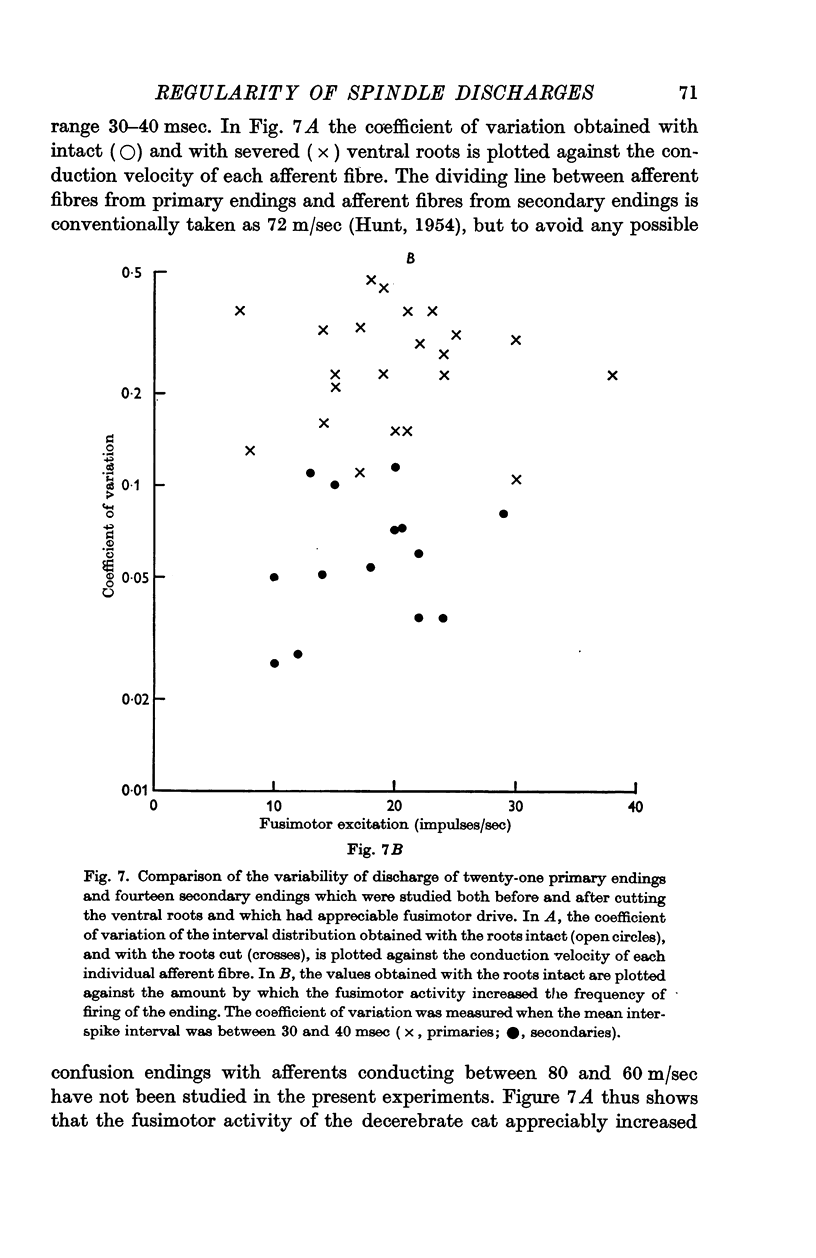
3. When the ventral roots were intact, the `spontaneous' fusimotor activity considerably increased the variability of both kinds of endings. Secondary endings still discharged much more regularly than primary endings, even when the fusimotor activity increased the frequency of firing equally for the two kinds of endings. At frequencies around 30/sec the average coefficient of variation of the interval distributions was then 0·064 for the secondary endings and 0·25 for the primary endings.
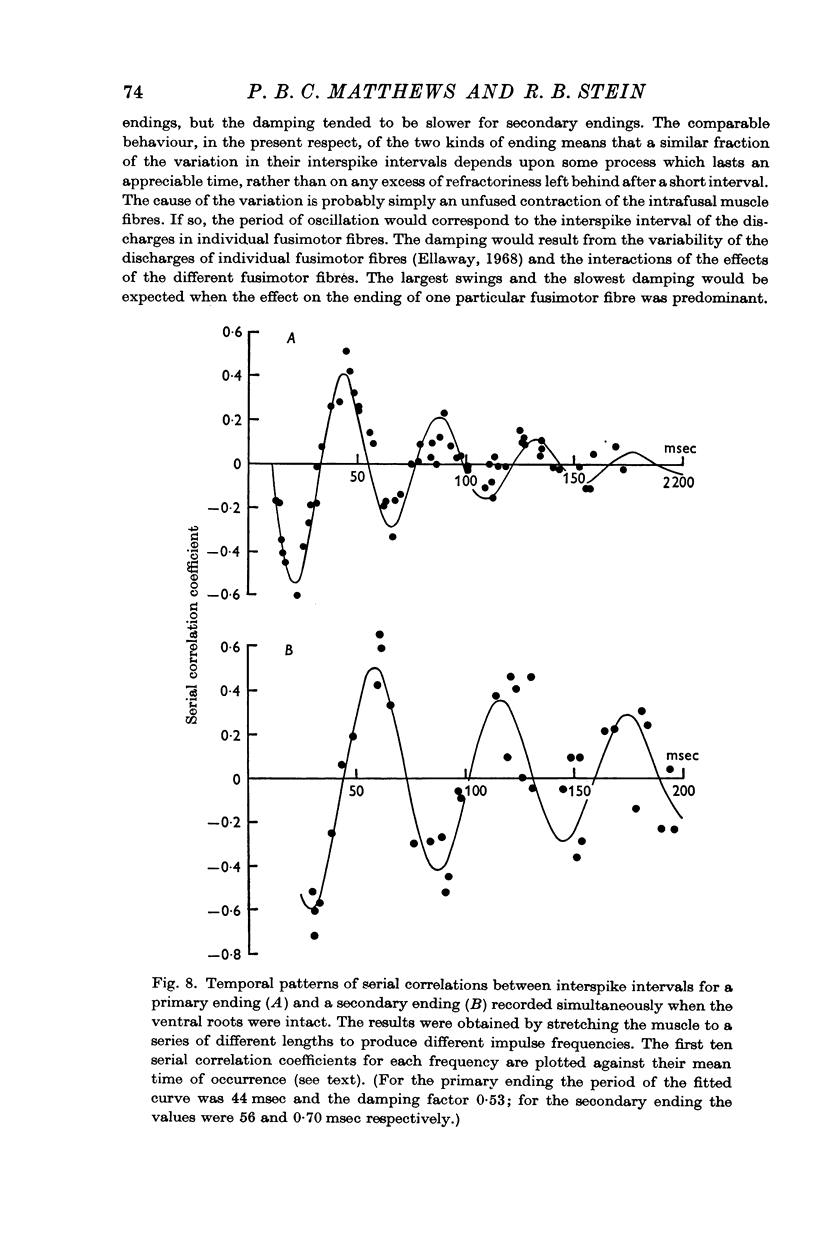
4. When the ventral roots were intact there was usually an inverse relation between the values of successive interspike intervals. The first serial correlation coefficient often had values down to - 0·6 for both kinds of ending. Higher order serial correlation coefficients were also computed.
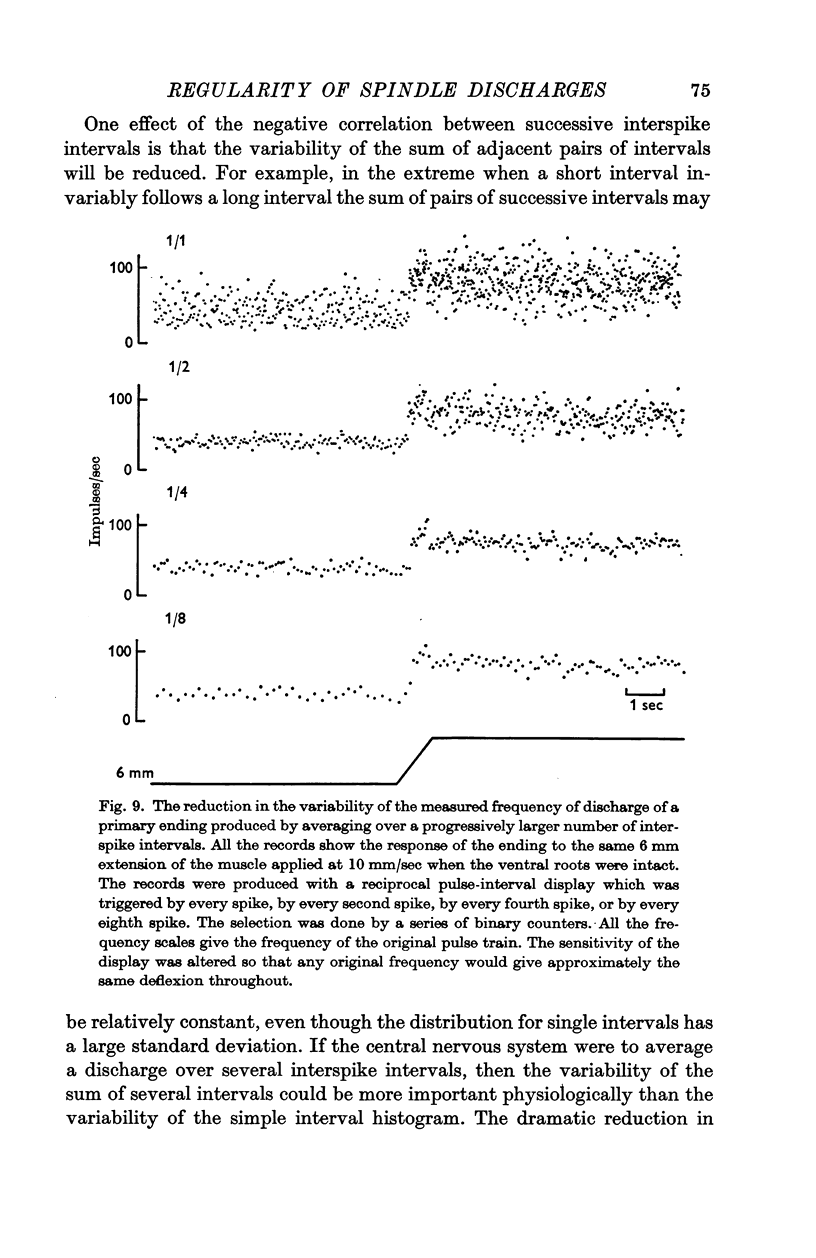
5. Approximate calculations, based on the variability observed when the ventral roots were intact, suggested that when the length of the muscle was constant an observer analysing a 1 sec period of discharge from a single primary ending would only be able to distinguish about six different lengths of the muscle. The corresponding figure for a secondary ending was twenty-five lengths.
6. The increase in variability with fusimotor activity, and the pattern of serial correlations, were probably caused by static fusimotor fibres firing at rates below the fusion frequency of the intrafusal muscle fibres that they supply.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg B., Bessou P., Laporte Y. Action of static and dynamic fusimotor fibres on secondary endings of cat's spindles. J Physiol. 1966 Jul;185(1):160–171. doi: 10.1113/jphysiol.1966.sp007978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISCOE T. J., TAYLOR A. THE DISCHARGE PATTERN RECORDED IN CHEMORECEPTOR AFFERENT FIBRES FROM THE CAT CAROTID BODY WITH NORMAL CIRCULATION AND DURING PERFUSION. J Physiol. 1963 Sep;168:332–344. doi: 10.1113/jphysiol.1963.sp007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P., Laporte Y., Pagès B. Frequencygrams of spindle primary endings elicited by stimulation of static and dynamic fusimotor fibres. J Physiol. 1968 May;196(1):47–63. doi: 10.1113/jphysiol.1968.sp008493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P., Laporte Y., Pagés B. A method of analysing the responses of spindle primary endings to fusimotor stimulation. J Physiol. 1968 May;196(1):37–45. doi: 10.1113/jphysiol.1968.sp008492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd I. A. The behaviour of isolated mammalian muscle spindles with intact innervation. J Physiol. 1966 Oct;186(2):109P–110P. [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. FURTHER STUDIES OF STATIC AND DYNAMIC FUSIMOTOR FIBRES. J Physiol. 1964 Oct;174:132–151. doi: 10.1113/jphysiol.1964.sp007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith I., Rowe M. J., Sessle B. J. "Tactile" stimulus intensity: information transmission by relay neurons in different trigeminal nuclei. Science. 1968 May 17;160(3829):791–794. doi: 10.1126/science.160.3829.791. [DOI] [PubMed] [Google Scholar]
- ELDRED E., GRANIT R., MERTON P. A. Supraspinal control of the muscle spindles and its significance. J Physiol. 1953 Dec 29;122(3):498–523. doi: 10.1113/jphysiol.1953.sp005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., KELLERTH J. O., WILLIAMS T. D. INTRACELLULAR ASPECTS OF STIMULATING MOTONEURONES BY MUSCLE STRETCH. J Physiol. 1964 Nov;174:435–452. doi: 10.1113/jphysiol.1964.sp007496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. Relation of function to diameter in afferent fibers of muscle nerves. J Gen Physiol. 1954 Sep 20;38(1):117–131. doi: 10.1085/jgp.38.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J. K., Nicolaysen K., Rudjord T. Discharge pattern of neurons of the dorsal spinocerebellar tract activated by static extension of primary endings of muscle spindles. J Neurophysiol. 1966 Nov;29(6):1061–1086. doi: 10.1152/jn.1966.29.6.1061. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Nicolaysen K., Rudjord T. On the firing pattern of spinal neurones activated from the secondary endings of muscle spindles. Acta Physiol Scand. 1967 Jun;70(2):188–193. doi: 10.1111/j.1748-1716.1967.tb03614.x. [DOI] [PubMed] [Google Scholar]
- KATZ B. Action potentials from a sensory nerve ending. J Physiol. 1950 Oct 16;111(3-4):248–260. doi: 10.1113/jphysiol.1950.sp004478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., FITZHUGH R., BARLOW H. B. Maintained activity in the cat's retina in light and darkness. J Gen Physiol. 1957 May 20;40(5):683–702. doi: 10.1085/jgp.40.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS P. B. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:324–333. doi: 10.1113/expphysiol.1962.sp001616. [DOI] [PubMed] [Google Scholar]
- Matthews B. H. Nerve endings in mammalian muscle. J Physiol. 1933 Apr 13;78(1):1–53. doi: 10.1113/jphysiol.1933.sp002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Stein R. B. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol. 1969 Feb;200(3):723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. The dependence of tension upon extension in the stretch reflex of the soleus muscle of the decerebrate cat. J Physiol. 1959 Oct;147(3):521–546. doi: 10.1113/jphysiol.1959.sp006260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe J. E. The effects of ethyl chloride on the muscle spindles of the triceps surae of the rabbit. J Physiol. 1965 Oct;180(4):673–683. doi: 10.1113/jphysiol.1965.sp007723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh P. M., Stone S. L. The effect of 2,4-dinitrophenol and related compounds on bile secretion. J Physiol. 1968 Sep;198(1):39–49. doi: 10.1113/jphysiol.1968.sp008592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODIECK R. W., KIANG N. Y., GERSTEIN G. L. Some quantitative methods for the study of spontaneous activity of single neurons. Biophys J. 1962 Jul;2:351–368. doi: 10.1016/s0006-3495(62)86860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN R. B. A THEORETICAL ANALYSIS OF NEURONAL VARIABILITY. Biophys J. 1965 Mar;5:173–194. doi: 10.1016/s0006-3495(65)86709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. B., Mattews P. B. Differences in variability of discharge frequency between primary and secondary muscle spindle afferent endings of the cat. Nature. 1965 Dec 18;208(5016):1217–1218. doi: 10.1038/2081217a0. [DOI] [PubMed] [Google Scholar]
- Stein R. B. Some models of neuronal variability. Biophys J. 2008 Dec 31;7(1):37–68. doi: 10.1016/S0006-3495(67)86574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. B. The information capacity of nerve cells using a frequency code. Biophys J. 2008 Dec 31;7(6):797–826. doi: 10.1016/S0006-3495(67)86623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERNER G., MOUNTCASTLE V. B. NEURAL ACTIVITY IN MECHANORECEPTIVE CUTANEOUS AFFERENTS: STIMULUS-RESPONSE RELATIONS, WEBER FUNCTIONS, AND INFORMATION TRANSMISSION. J Neurophysiol. 1965 Mar;28:359–397. doi: 10.1152/jn.1965.28.2.359. [DOI] [PubMed] [Google Scholar]


