Abstract
1. The metabolic requirements for catecholamine secretion elicited by acetylcholine or by calcium plus high K+ were studied on acutely denervated perfused cat adrenal glands.
2. Glucose-deprivation plus anoxia caused an increase in the spontaneous catecholamine output from adrenal glands perfused with normal Locke solution, which was abolished by the removal of calcium from the perfusion medium.
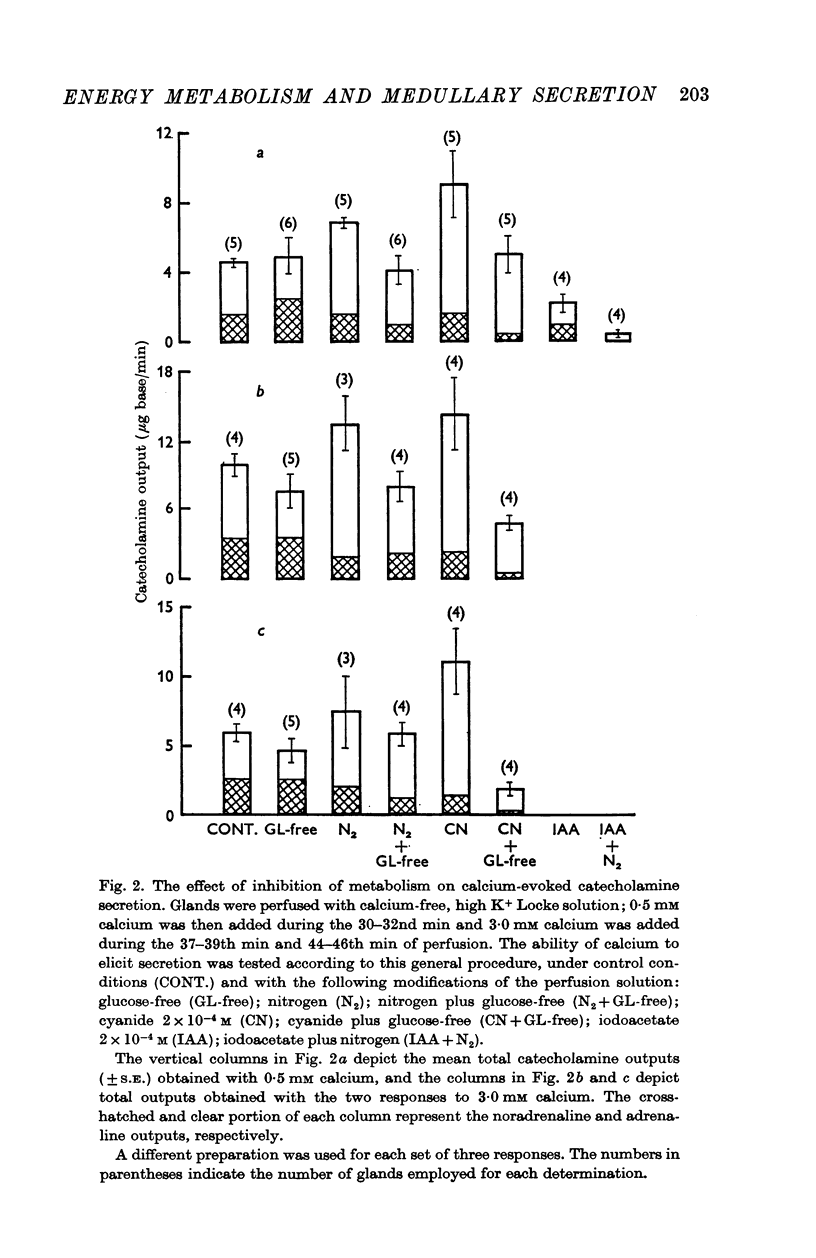
3. Anoxia plus glucose-deprivation did not depress the secretory response to repeated exposures of a low concentration of acetylcholine, but did depress the response to a higher concentration of acetylcholine. Glucose-deprivation and nitrogen, when imposed either separately or together, did not inhibit total catecholamine output in response to calcium. Differential analysis of the calcium-evoked secretion showed that during anoxia, catecholamine output was maintained primarily by adrenaline secretion.
4. Cyanide (0·2 mM) potentiated the secretory response to calcium in the presence of glucose, but when glucose was omitted from the perfusion medium, cyanide caused a gradual decline in calcium-evoked secretion. Iodoacetic acid (IAA) (0·2 mM) depressed the response to calcium by about 50% under aerobic conditions and by 90% under anaerobic conditions.
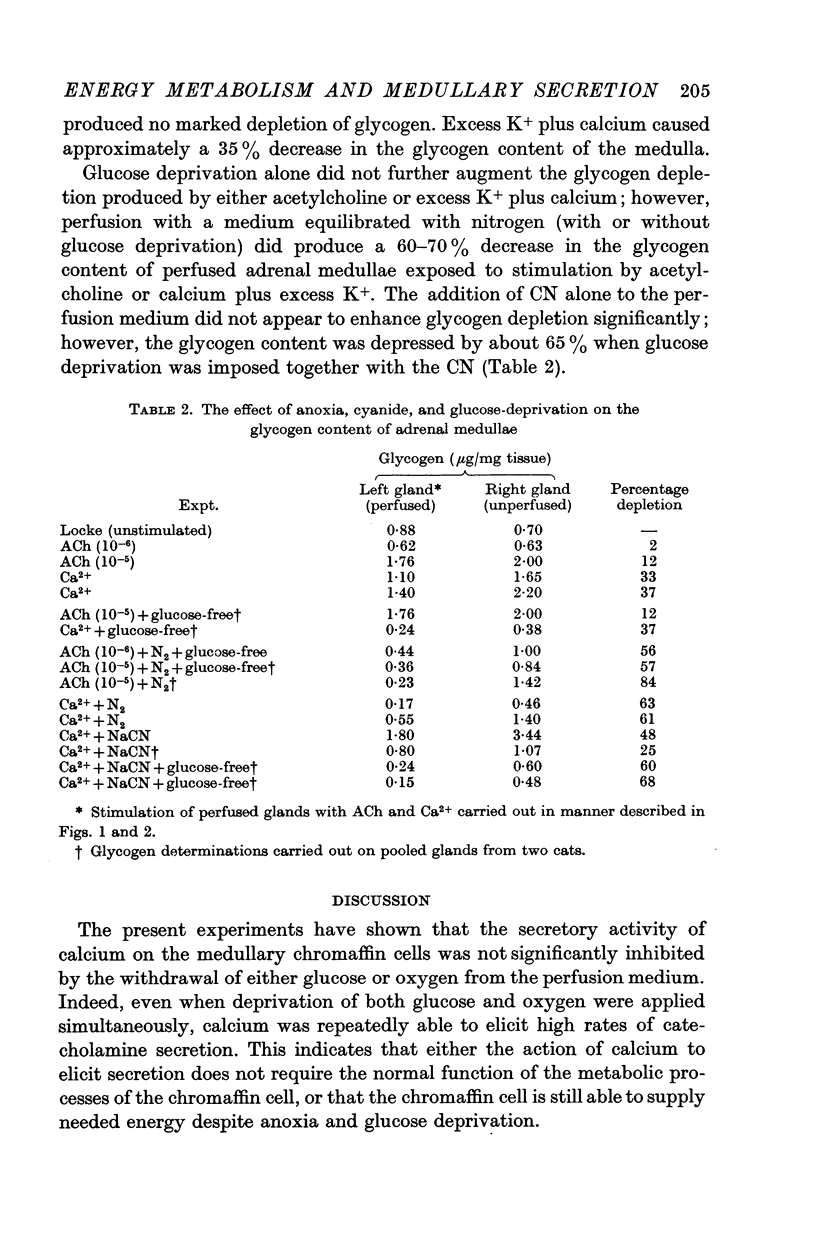
5. The glycogen content of medullae was profoundly depleted under anoxic conditions.
6. It is concluded that energy is required for the secretory action of calcium on medullary chromaffin cells. The energy may be derived from glycolysis or oxidative metabolism. A possible interaction between calcium and adenosine triphosphate acid (ATP) in eliciting catecholamine secretion is discussed.
7. The alteration in the percent adrenaline and noradrenaline secreted during anoxia indicates that anoxia may regulate medullary catecholamine secretion through a peripheral, as well as a central mechanism.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- BLASCHKO H., HAGEN P., WELCH A. D. Observations on the intracellular granules of the adrenal medulla. J Physiol. 1955 Jul 28;129(1):27–49. doi: 10.1113/jphysiol.1955.sp005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschko H., Comline R. S., Schneider F. H., Silver M., Smith A. D. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature. 1967 Jul 1;215(5096):58–59. doi: 10.1038/215058a0. [DOI] [PubMed] [Google Scholar]
- Comline R. S., Silver M. The development of the adrenal medulla of the foetal and new-born calf. J Physiol. 1966 Mar;183(2):305–340. doi: 10.1113/jphysiol.1966.sp007868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit P. K., Lazarow A. Effect of blood sugar levels on glycogen content of hypophysis and adrenal glands. Proc Soc Exp Biol Med. 1967 Mar;124(3):719–724. doi: 10.3181/00379727-124-31835. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M., Rubin R. P. Efflux of adenine nucleotides from perfused adrenal glands exposed to nicotine and other chromaffin cell stimulants. J Physiol. 1965 Jul;179(1):130–137. doi: 10.1113/jphysiol.1965.sp007652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshner N., Sage H. J., Smith W. J. Mechanism of secretion from the adrenal medulla. II. Release of catecholamines and storage vesicle protein in response to chemical stimulation. Mol Pharmacol. 1967 May;3(3):254–265. [PubMed] [Google Scholar]
- LARRABEE M. G., BRONK D. W. [Metabolic requirements of sympathetic neurons]. Cold Spring Harb Symp Quant Biol. 1952;17:245–266. doi: 10.1101/sqb.1952.017.01.023. [DOI] [PubMed] [Google Scholar]
- Poisner A. M., Trifaró J. M. The role of ATP and ATPase in the release of catecholamines from the adrenal medulla. I. ATP-evoked release of catecholamines, ATP, and protein from isolated chromaffin granules. Mol Pharmacol. 1967 Nov;3(6):561–571. [PubMed] [Google Scholar]
- Rubin R. P., Cohen M. S., Harman S. M., Roer E. M. The localization of adrenaline-rich medullary chromaffin cells adjacent to the adrenal cortex. J Endocrinol. 1968 Aug;41(4):541–545. doi: 10.1677/joe.0.0410541. [DOI] [PubMed] [Google Scholar]
- Rubin R. P., Feinstein M. B., Jaanus S. D., Paimre M. Inhibition of catecholamine secretion and calcium exchange in perfused cat adrenal glands by tetracaine and magnesium. J Pharmacol Exp Ther. 1967 Mar;155(3):463–471. [PubMed] [Google Scholar]
- Rubin R. P., Jaanus S. D. A study of the release of catecholamines from the adrenal medulla by indirectly acting sympathomimetic amines. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol. 1966;254(2):125–137. doi: 10.1007/BF00535900. [DOI] [PubMed] [Google Scholar]
- Rubin R. P., Miele E. A study of the differential secretion of epinephrine and norepinephrine from the perfused cat adrenal gland. J Pharmacol Exp Ther. 1968 Nov;164(1):115–121. [PubMed] [Google Scholar]
- Rubin R. P., Miele E. The relation between the chemical structure of local anesthetics and inhibition of calcium-evoked secretion from the adrenal medulla. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;260(4):298–308. doi: 10.1007/BF00537635. [DOI] [PubMed] [Google Scholar]
- SEIFTER S., DAYTON S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950 Jan;25(1):191–200. [PubMed] [Google Scholar]
- VOGT M. The secretion of the denervated adrenal medulla of the cat. Br J Pharmacol Chemother. 1952 Jun;7(2):325–330. doi: 10.1111/j.1476-5381.1952.tb01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


