Abstract
1. This paper presents accurate increment threshold data for human rod vision for a small number of experimental parameters. The test is small and brief and the large background is either steady or transient.
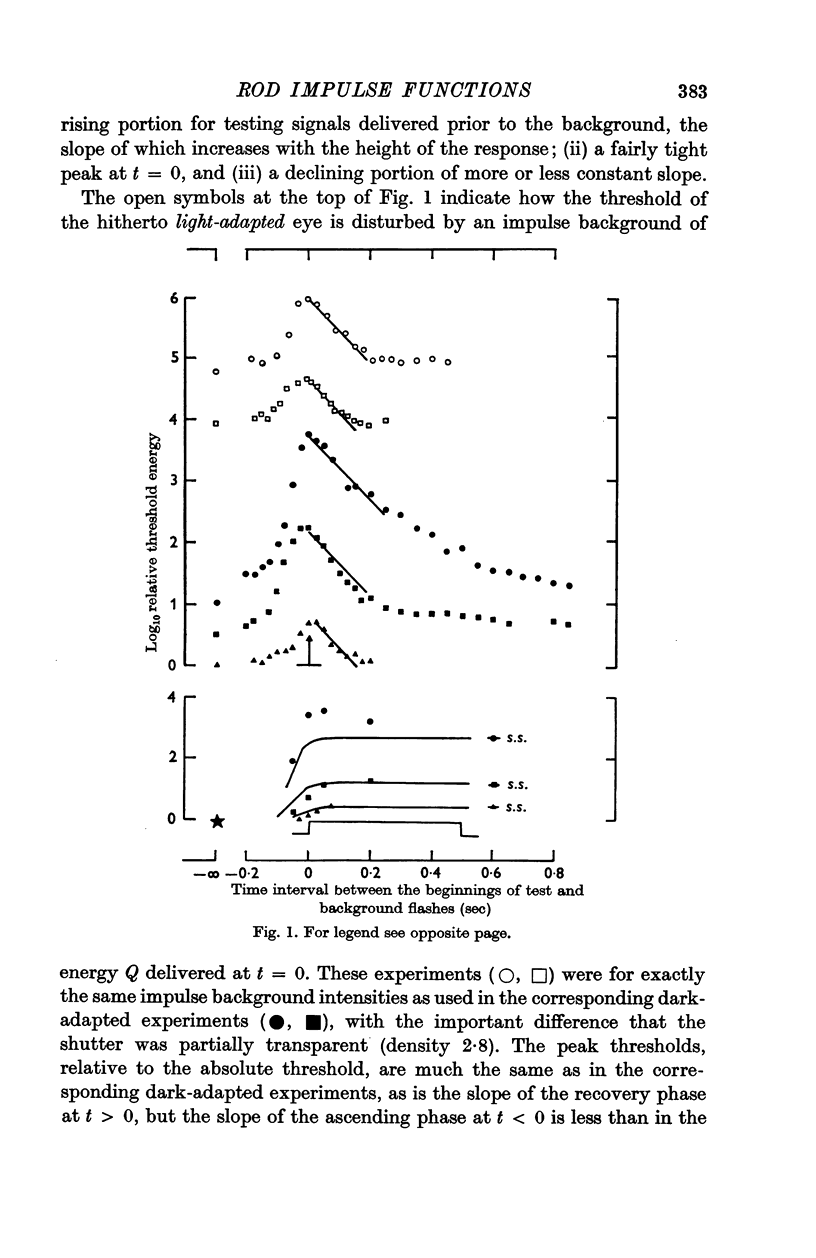
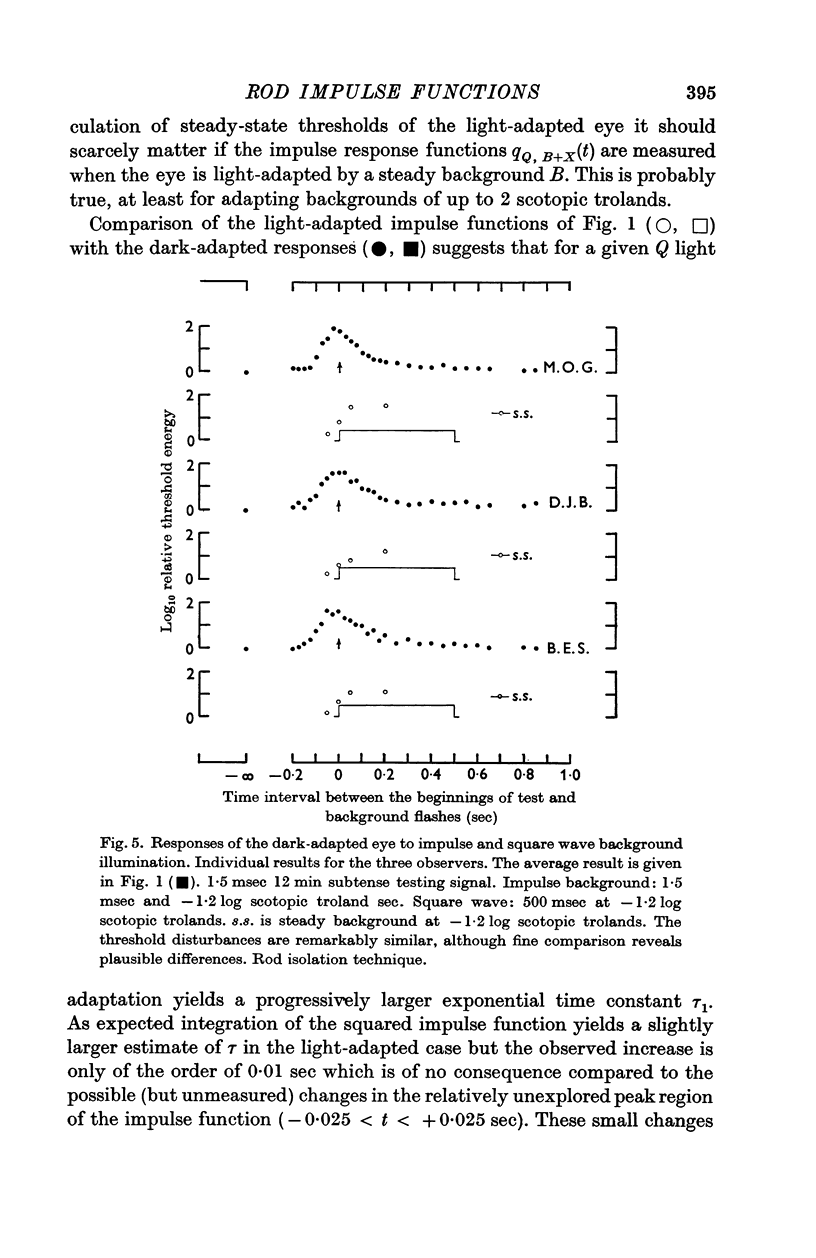
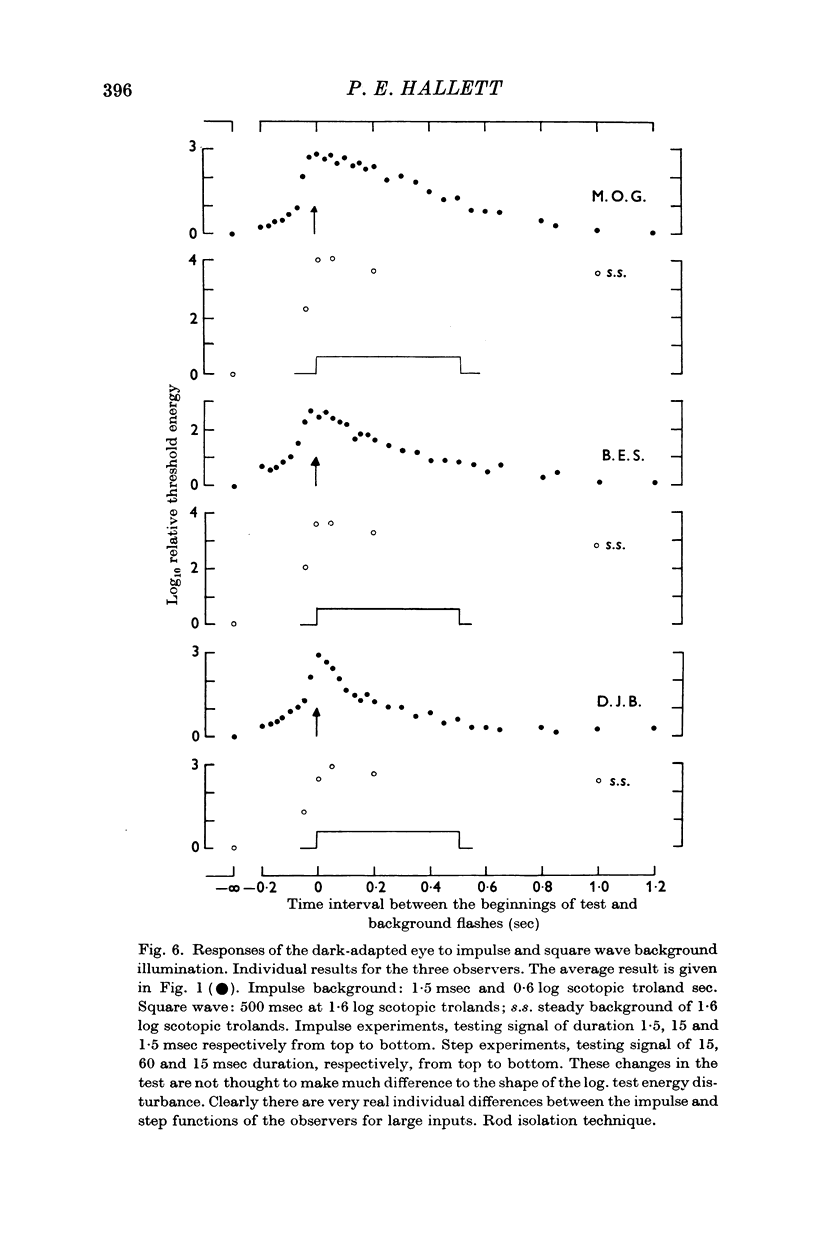
2. The linear threshold disturbance due to an impulse background consists of an input dependent exponential growth phase and an exponential recovery phase of more or less fixed time constant (ca. 0·08 sec).
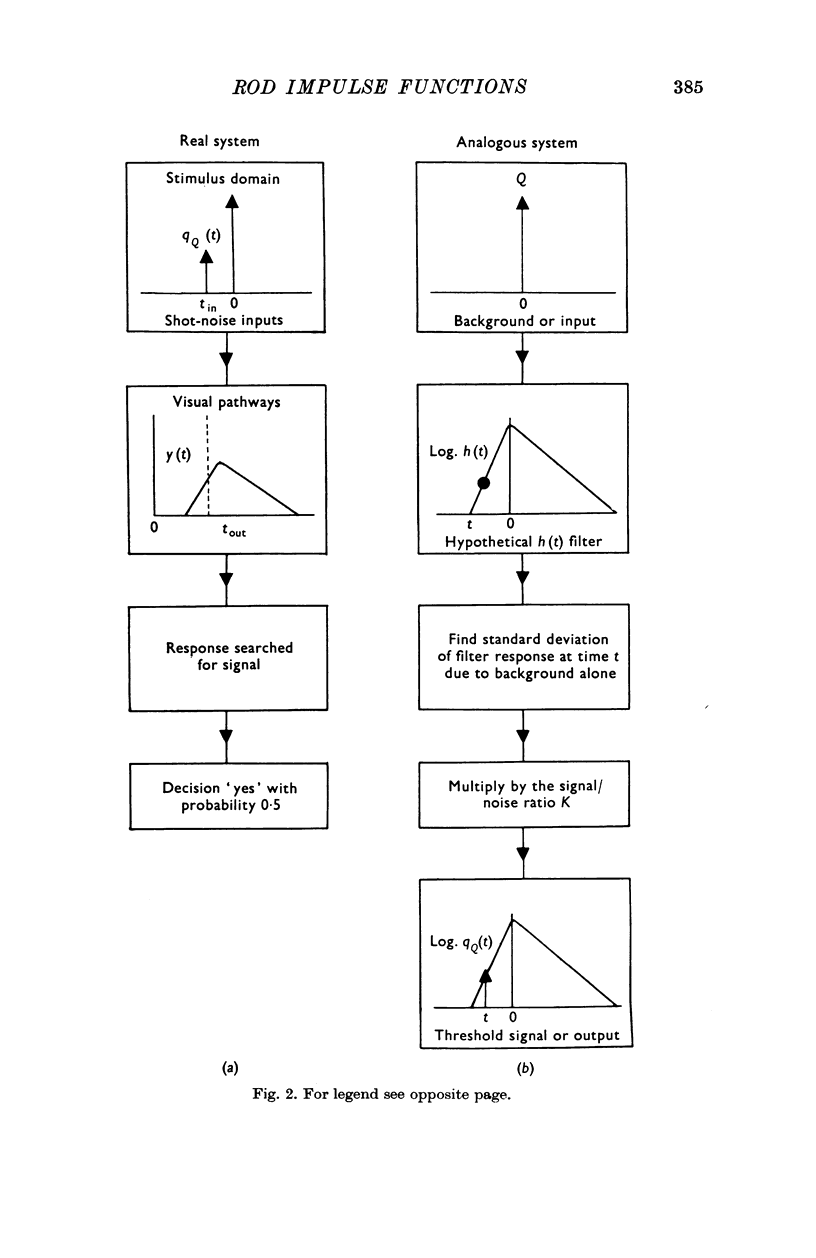
3. The data are treated by applying signal/noise decision theory to a hypothetical filter with two shot noise inputs, viz. the testing signal and the background. The gain and time course of the impulse function of the filter are slightly affected by the magnitude of the input.
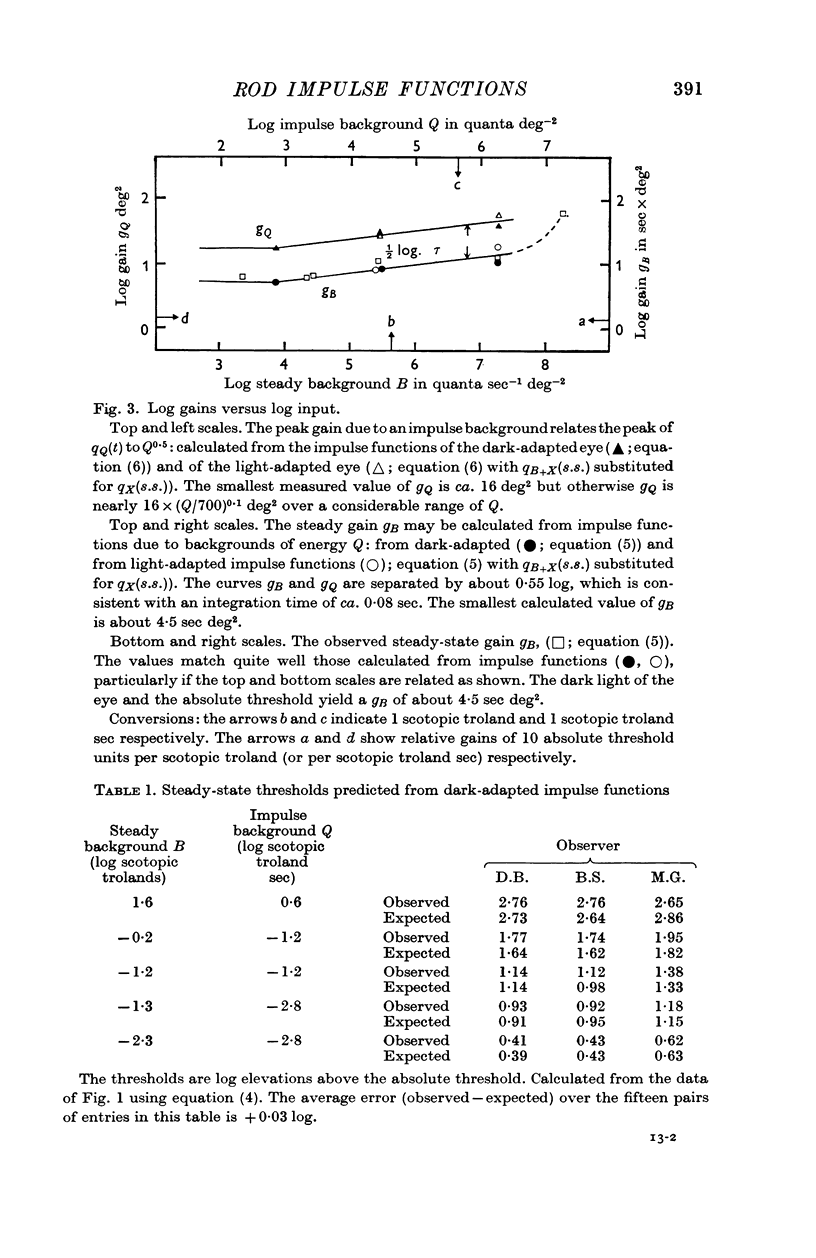
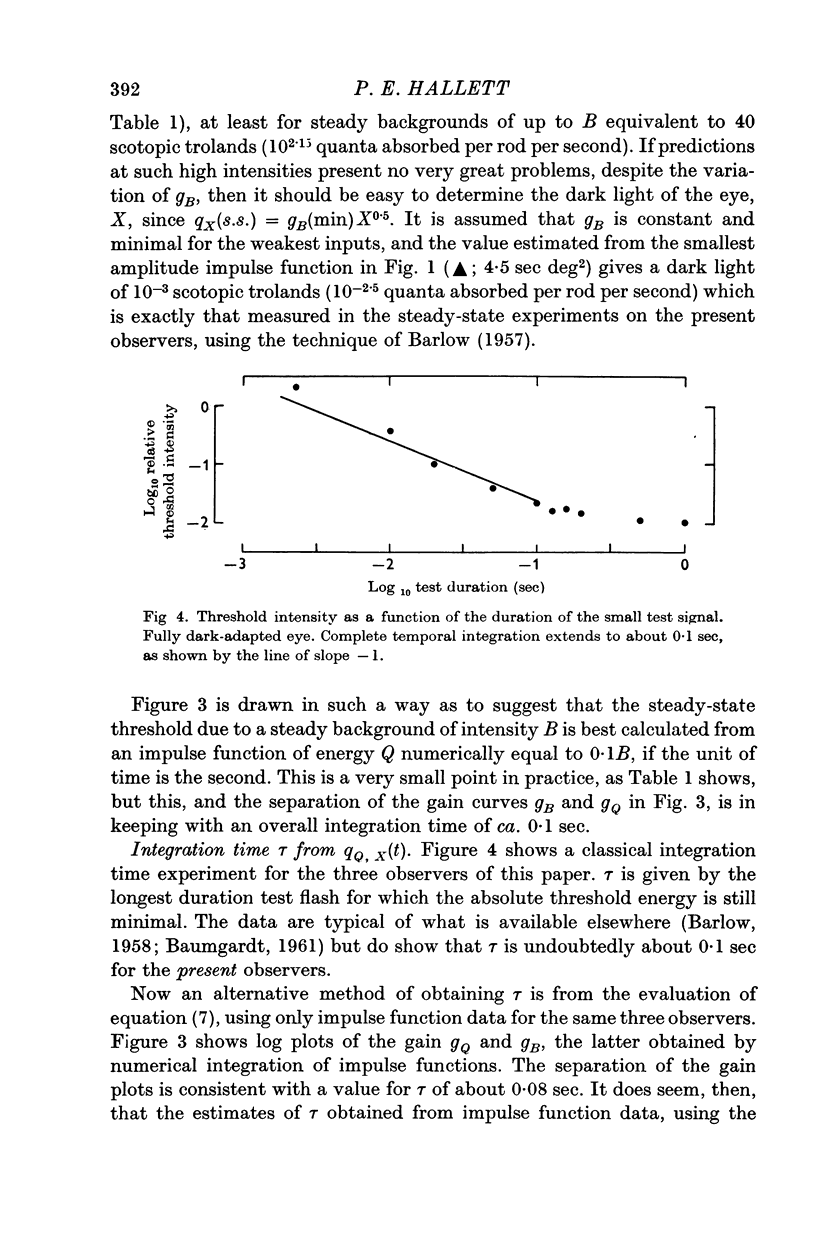
4. A linear approach is useful since the impulse functions for dark or light-adapted rod vision yield independent information about quantities which have previously only been used to describe the increment thresholds for small tests on steady backgrounds, viz. the integration time and dark light of the fully dark-adapted eye and the gain changes (or changes in the signal/background ratio) which occur on progressive light adaptation.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER H. D. Initial stages of dark and light adaptation. J Opt Soc Am. 1963 Jan;53:98–103. doi: 10.1364/josa.53.000098. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B. A method of determining the over-all quantum efficiency of visual discriminations. J Physiol. 1962 Jan;160:155–168. doi: 10.1113/jphysiol.1962.sp006838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Increment thresholds at low intensities considered as signal/noise discriminations. J Physiol. 1957 May 23;136(3):469–488. doi: 10.1113/jphysiol.1957.sp005774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Temporal and spatial summation in human vision at different background intensities. J Physiol. 1958 Apr 30;141(2):337–350. doi: 10.1113/jphysiol.1958.sp005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUMGARDT E., HILLMANN B. Duration and size as determinants of peripheral retinal response. J Opt Soc Am. 1961 Mar;51:340–344. doi: 10.1364/josa.51.000340. [DOI] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLETT P. E., MARRIOTT F. H., RODGER F. C. The relationship of visual threshold to retinal position and area. J Physiol. 1962 Feb;160:364–373. doi: 10.1113/jphysiol.1962.sp006851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLETT P. E. Scotopic acuity and absolute threshold in brief flashes. J Physiol. 1962 Aug;163:175–189. doi: 10.1113/jphysiol.1962.sp006966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett P. E. Rod increment thresholds on steady and flashed backgrounds. J Physiol. 1969 Jun;202(2):355–377. doi: 10.1113/jphysiol.1969.sp008816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett P. E. The variations in visual threshold measurement. J Physiol. 1969 Jun;202(2):403–419. doi: 10.1113/jphysiol.1969.sp008818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marimont R. B. Numerical studies of the Fuortes-Hodgkin Limulus model. J Physiol. 1965 Aug;179(3):489–497. doi: 10.1113/jphysiol.1965.sp007675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATLIFF F., HARTLINE H. K., MILLER W. H. Spatial and temporal aspects of retinal inhibitory interaction. J Opt Soc Am. 1963 Jan;53:110–120. doi: 10.1364/josa.53.000110. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. The rhodopsin density in the human rods. J Physiol. 1956 Oct 29;134(1):30–46. doi: 10.1113/jphysiol.1956.sp005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. VISUAL ADAPTATION. Proc R Soc Lond B Biol Sci. 1965 Mar 16;162:20–46. doi: 10.1098/rspb.1965.0024. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A., WESTHEIMER G. The effect upon the rod threshold of bleaching neighbouring rods. J Physiol. 1962 Nov;164:318–329. doi: 10.1113/jphysiol.1962.sp007024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton W. A. Bleached rhodopson and visual adaptation. J Physiol. 1965 Dec;181(3):645–655. doi: 10.1113/jphysiol.1965.sp007789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling G., Sondhi M. M. Model for visual luminance discrimination and flicker detection. J Opt Soc Am. 1968 Aug;58(8):1133–1145. doi: 10.1364/josa.58.001133. [DOI] [PubMed] [Google Scholar]


