Abstract
Yersinia enterocolitica organisms secrete Yop proteins via the type III pathway. Translational fusion of yop genes to ubiquitin or dihydrofolate reductase results in hybrid proteins that cannot be secreted. The folding of hybrids prevents their own transport, but it does not hinder the type III secretion of other Yops.
The type III secretion machinery of pathogenic yersiniae (Yersinia pestis, Y. pseudotuberculosis, and Y. enterocolitica) provides for bacterial escape from phagocytic killing and is encoded by a 70-kb virulence plasmid (15). Twenty-one ysc (mnemonic for Yop secretion) genes encode secretion machinery components that promote transport of 14 Yops (mnemonic for Yersinia outer proteins) across the bacterial double-membrane envelope (15). The secretion signal of Yop proteins has been mapped by fusing the open reading frame of yop proteins to the 5′ end of coding sequence for reporter proteins, thereby generating translational hybrids (38). Fusion of yop coding sequences to the 5′ end of the adenylate cyclase-encoding domain of cya (23) results in the secretion of Yop-Cya fusions in a manner resembling that of the type III transport of native Yops (51). lacZ encodes β-galactosidase (29), a large (116-kDa) cytoplasmic polypeptide that assembles into a tetrameric structure (12). The type III secretion of yop fusions to full-length lacZ has not yet been examined. The first 92 codons of lacZ (lacZ′) specify the α-peptide, a module that unfolds readily but also provides for the alpha complementation phenotype of certain lacZ alleles (33). Fusion of lacZ′ to the 3′ end of Yersinia Yops results in the transport Yop-LacZ′ hybrids into the extracellular medium (38). npt encodes neomycin phosphotransferase, a cytoplasmic protein that confers bacterial resistance to aminoglycoside antibiotics (47). Similarly to Cya and LacZ′ fusions, Yop-Npt hybrids are also transported by the type III secretion machinery (3).
Fusion of the first 15 codons of yopE, yopH, yopN, or yopQ to the 5′ end of cya or npt leads to the type III secretion of hybrid Yop proteins (1, 3, 5, 49, 50). Sory and Cornelis proposed that the amino acid sequence generated by the first 15 codons functions as a signal peptide and mediates substrate recognition by the type III machinery (51). Lloyd et al. developed this model further, predicting an amphipathic helical structure as a common substrate property of all Yop signal peptides (35, 36). yop secretion signals (codons 1 to 15) have been altered by frameshift mutations immediately following the AUG start codon, while reporter expression was restored by suppressor mutations at the fusion site. Several of these frameshift mutations do not affect secretion signaling, albeit the peptide sequence is completely altered (1-3, 5). yopQ signal mutations with a defect in secretion in which the mRNA sequence is changed without affecting the codon specificity (synonymous mutations) have been described (44). Thus, it appears that yop mRNA may function as a signal for the type III secretion of Yop proteins (4). The two models vary considerably in predicting the mode of substrate recognition. Future work will need to provide more definitive proof to reveal the mechanisms of type III secretion.
Much of the thought on type III secretion is influenced by observations that were previously made with other protein secretion pathways (9). Signal peptides are hydrophobic sequences at the N terminus of precursor proteins that initiate polypeptides into the secretory pathway, a mechanism that translocates proteins across membranes (10). Signal peptides provide for substrate recognition by interacting with the SecYEG translocon in the plasma membranes of bacterial cells (7, 21). Once the precursor protein is translocated across the membrane, the signal peptide is removed by signal peptidase (leader peptidase) and the mature polypeptide is released from the secretion machinery (17). Transport by the Sec machinery occurs only when proteins assume an unfolded state and often requires the association of substrate with chaperones (32). Chaperones bind to the hydrophobic core residues of unfolded globular proteins (45) and are dissociated from the secretion substrate when a mobile machinery component, the ATPase SecA, moves precursor proteins across the plasma membrane (18, 24). A second transport pathway that involves the recognition of signal peptides in nascent polypeptides has been revealed (55). The signal recognition particle (SRP) binds to the signal peptide and the ribosome (42, 56). The complex between SRP, signal peptide of nascent polypeptide, and ribosome docks on the SRP receptor (34, 37). The ribosome is subsequently lodged onto the SecYEG secretion channel to promote cotranslational secretion of the remainder of the polypeptide chain (43, 57).
To identify bacterial machinery components that interact with signal peptides, previous work used several different strategies. Translational fusions between lamB and malE, encoding two secreted (signal peptide-bearing) proteins whose expression is induced by maltose, and lacZ, specifying a cytoplasmic protein, cause the resulting hybrid proteins to jam the secretion pathway and to confer a maltose-sensitive growth phenotype (27, 41). Selection for maltose resistance led to the isolation of mutations that abolished the signal peptide function of lamB (20, 30). Mutations in secretion genes could be identified as conditional lethal mutations that confer increased LacZ activity due to the decreased initiation of the hybrids into the secretory pathway (8, 41, 48). The genetic strategies of identifying secretion machinery components are based on a single principle: signal peptide-bearing polypeptides are by default initiated into the secretion pathway and cannot be discarded unless the signal peptide is removed. Mutations in one secretion gene, secB, encoding the secretion chaperone, represent an important exception to this rule. Escherichia coli lacking the secB gene allows premature folding of the MalE precursor without initiating this polypeptide into the secretion pathway (46). This report examines whether the principle of a default mechanism for the initiation of polypeptides into the secretion machinery is applicable to the type III pathway of Y. enterocolitica.
Ubiquitin (Ub), a 76-residue polypeptide, folds rapidly into a compact, protease-resistant structure of five β-strands and four turns of α-helix (54). When fused to the C terminus of signal peptide-bearing precursor protein, the Sec pathway of Saccharomyces cerevisiae cannot translocate folded ubiquitin in a posttranslational manner across the endoplasmic reticulum (ER) membrane (28). Nevertheless, the SRP pathway of yeast does promote cotranslational transport of Ub if a sufficiently long spacer (more than 33 residues) separates the signal peptide and the Ub domain (28). Presumably, synthesis and folding of Ub fusion precursors in the cytoplasm are prevented by SRP-mediated arrest of translation. Once ribosomes are docked on the secretion channel in the ER membrane, cotranslational secretion proceeds at efficient speed and within space constraints that prevent Ub folding.
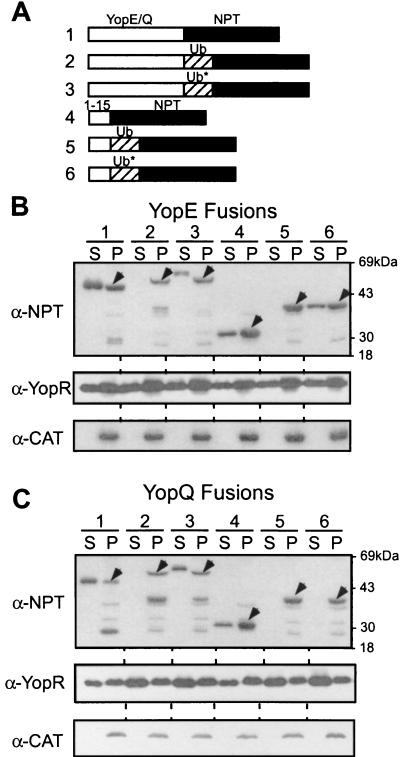
The Ub-coding sequence was cloned into the middle of reporter genes flanked by 5′ yop and 3′ npt sequences, inserted into the low-copy-number plasmid pHSG576 (53), and transformed into yersiniae (13) (Table 1 lists the properties of plasmids and strains used here). Overnight cultures of Yersinia were diluted 1:50 into fresh tryptic soy broth, supplemented with 5 mM EGTA, grown for 2 h at 26°C, and induced at 37°C for 3 h. Cultures were centrifuged at 15,000 × g for 15 min, and the supernatant (S) was separated from the cell pellet (P). Proteins in both fractions were precipitated with trichloroacetic acid (TCA), washed in acetone, and suspended in sample buffer. Proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting. Expression of yopE1-220-Ub gene-npt in Y. enterocolitica led to the accumulation of the polypeptide in bacterial cells but did not result in type III secretion of YopE1-220-Ub-Npt (full-length YopE fusion) (Fig. 1A and B). In contrast, YopE1-220-Npt was secreted efficiently into the extracellular medium (Fig. 1B). A similar result was observed for YopQ-Ub fusions. YopQ1-198-Npt (full-length YopQ fusion) was secreted via the type III pathway, whereas YopQ1-198-Ub-Npt was not (Fig. 1C). To test our conjecture that the defect in type III secretion is caused by the folding of Ub domains, we analyzed Ub fusions carrying amino acid substitutions. UbGly3,13 cannot assume the tightly folded structure of native Ub and does not act as a substrate for Ub proteases (6). Because of their folding defects, UbGly3,13 fusions are translocated across the Sec channel in the yeast ER membrane in both co- and posttranslational manners (28). Expression of YopE1-220-UbGly3,13-Npt and YopQ1-198-UbGly3,13-Npt in Y. enterocolitica resulted in their secretion, indicating that it is indeed the tight folding of the Ub domain that prevents the type III transport of YopE1-220-Ub-Npt and YopQ1-198-Ub-Npt (Fig. 1).
TABLE 1.
List of strains and plasmids used in this study
| Strain or plasmid | Property | Reference or source |
|---|---|---|
| Y. enterocolitica W22703 | Human clinical isolate, wild type | 16 |
| Y. enterocolitica LC2 | Isogenic W22703 variant, Δ(sycE) | 13 |
| pDA14 | yopE promoter and full-length ORF fused to 5′ end of lacZ | This study |
| pDA36 | yopE promoter and full-length ORF fused to 5′ end of npt | 3 |
| pDA46 | yopE promoter and codons 1 to 15 fused to 5′ end of npt | 3 |
| pDA182 | yopQ promoter and full-length ORF fused to 5′ end of npt | 5 |
| pDA184 | yopQ promoter and codons 1 to 15 fused to 5′ end of npt | 5 |
| pVL9 | yopE promoter and full-length ORF fused to 5′ end of Ub gene-npt | This study |
| pVL13 | yopE promoter and codons 1 to 15 fused to 5′ end of Ub gene-npt | This study |
| pVL14 | yopE promoter and full-length ORF fused to 5′ end of UbGly3,13 gene-npt | This study |
| pVL15 | yopE promoter and codons 1 to 15 fused to 5′ end of UbGly3,13 gene-npt | This study |
| pVL16 | yopQ promoter and full-length ORF fused to 5′ end of Ub gene-npt | This study |
| pVL17 | yopQ promoter and codons 1 to 15 fused to 5′ end of Ub gene-npt | This study |
| pVL18 | yopQ promoter and full-length ORF fused to 5′ end of UbGly3,13 gene-npt | This study |
| pVL19 | yopQ promoter and codons 1 to 15 fused to 5′ end of UbGly3,13 gene-npt | This study |
| pVL20 | yopE promoter and full-length ORF fused to 5′ end of DHFR gene | This study |
| pVL21 | yopE promoter and codons 1 to 15 fused to 5′ end of DHFR gene | This study |
FIG. 1.
Ubiquitin fusions are not secreted by the type III machinery of Y. enterocolitica. (A) The drawing depicts the primary structures of hybrid polypeptides as follows: line 1, YopE1-220-Npt (YopQ1-198-Npt), full-length yop fusion; line 2, YopE1-220-Ub-Npt (YopQ1-198-Ub-Npt), full-length yop ubiquitin fusion; line 3, YopE1-220-UbGly3,13-Npt (YopQ1-198-UbGly3,13-Npt), full-length yop fusion to ubiquitin mutant; line 4, YopE1-15-Npt (YopQ1-15-Npt), yop codons 1 to 15 fused; line 5, YopE1-15-Ub-Npt (YopQ1-15-Ub-Npt), yop codons 1 to 15 fused; line 6, YopE1-15-UbGly3,13-Npt (YopQ1-15-UbGly3,13-Npt), yop codons 1 to 15 fused. Ub represents wild-type ubiquitin, whereas UbGly3,13 carries two mutations that substitute codons 3 (isoleucine) and 13 (isoleucine) with glycine codons, causing destabilization of the folded polypeptide. Npt, neomycin phosphotransferase. (B) Y. enterocolitica strain W22703 (wild type) was transformed with plasmids listed in Table 1, grown in tryptic soy broth supplemented with 5 mM EGTA, and induced for type III secretion by a temperature shift to 37°C. Cultures were centrifuged, and the extracellular medium was separated with the supernatant (S) from the bacterial pellet (P). Proteins were precipitated with TCA, suspended in sample buffer, separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and analyzed by immunoblotting with specific antibody (α-Npt, α-YopR, or α-Cat). (C) Y. enterocolitica strain W22703 (wild type) was transformed with plasmids encoding the yopQ hybrids described for panel A and analyzed as described for panel B.
YopE can be initiated into the type III pathway in one of two ways. The secretion signal in the first 15 codons promotes secretion of the polypeptide by the type III machinery (3). SycE is a small homodimeric polypeptide that binds to YopE amino acid residues 15 to 100 in the bacterial cytoplasm (13). It is not yet certain whether SycE binding functions as a secretion chaperone in maintaining protein substrates in an unfolded or export-competent conformation (13, 14, 58). Nevertheless, binding of SycE to amino acid residues 15 to 100 also allows the initiation of YopE transport, even in the absence of codons 1 to 15 (13). To test whether fusions of the yop secretion signal in the first 15 codons are capable of promoting type III secretion of Ub fusions, we expressed YopE1-15-Ub-Npt and YopQ1-15-Ub-Npt in Y. enterocolitica. Neither YopE1-15-Ub-Npt nor YopQ1-15-Ub-Npt was transported by the type III pathway (Fig. 1). In contrast, YopE1-15-Npt and YopQ1-15-Npt as well as YopE1-15-UbGly3,13-Npt were efficiently secreted, suggesting that the folding of the Ub domain interfered with transport by the type III machinery. We observed only small amounts of secretion (less than 10%) for YopQ1-15-UbGly3,13-Npt (Fig. 1C). It is conceivable that fusion of the Ub domain affects signaling of the yopQ1-15 secretion signal. Nevertheless, we cannot yet provide a definitive explanation for the observed differences in secretion between YopE1-15-UbGly3,13-Npt and YopQ1-15-UbGly3,13-Npt.
As YopE1-220-Ub-Npt, YopE1-15-Ub-Npt, YopQ1-198-Ub-Npt, and YopQ1-15-Ub-Npt cannot be transported by the type III machinery because of their fused Ub domains, we expected these hybrids to interfere with the secretion of other Yop proteins. We were surprised to find that the secretions of YopE, YopQ, and YopR were not affected by the expression of hybrid Ub fusions (Fig. 1; also data not shown). The results suggested that the type III secretion pathway, a presumed channel within the needle structure (25, 31), cannot be occluded by folded proteins carrying Yop secretion signals. It should be emphasized that this property of the Yersinia type III machinery is distinct from that of other secretion pathways in which recognition of signal peptides leads to a productive and irrevocable interaction between a protein substrate and its translocation machinery.
To test the generality of this conjecture, we examined the secretion of YopE fusions to mouse dihydrofolate reductase (DHFR). Translational fusion of genes encoding mitochondrial precursor proteins to DHFR results in hybrid proteins that can be transported into mitochondria, because secretion chaperones unravel the folded precursor prior to translocation (26). Incubation of the in vitro translocation substrates with methotrexate, an active-site ligand of DHFR, irreversibly arrests the fused DHFR domain in a fully folded conformation and jams the import machinery (19). Fusion of an N-terminal signal peptide to DHFR causes degradation of the hybrid protein in the cytoplasm of E. coli (22). No secretion of the signal peptide DHFR hybrid was observed; however, it was also not clear whether the hybrid protein is capable of jamming the secretory pathway (22). Fusion of the DHFR C terminal to the secretion signal of E. coli hemolysin (HlyA) also did not lead to efficient secretion (39). Thus, although DHFR can be transported across membranes by the mitochondrial import machinery, not all membrane translocators can achieve the unfolding of DHFR.
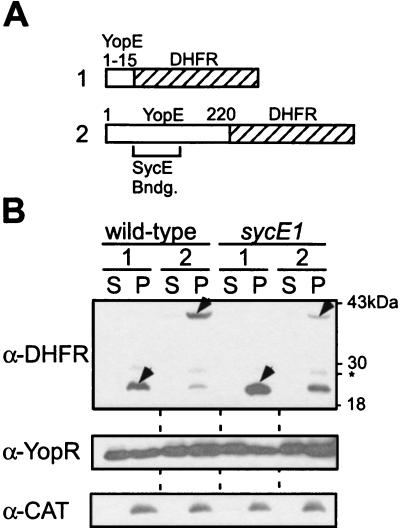
To explore the fate of DHFR in the Yersinia type III pathway, YopE-DHFR hybrids were expressed in Y. enterocolitica W22703 (wild type) (Fig. 2). Even in the absence of methotrexate, yersiniae secreted only very small amounts of YopE1-220-DHFR (less than 5%) into the medium and altogether failed to transport YopE1-15-DHFR (Fig. 2). Expression of YopE1-220-DHFR or YopE1-15-DHFR in Δ(yopE) or Δ(sycE) mutant strains did not alter the ability of yersiniae to transport either native Yops or the hybrid proteins (Fig. 2; also data not shown). As previously reported, the Δ(sycE) mutant strains contain less YopE or YopE fusion than do wild-type yersiniae (Fig. 2; also data not shown). Together these data suggest that SycE cannot unfold the fused Ub or DHFR domains and that the function of SycE is limited to the substrate recognition of YopE.
FIG. 2.
Dihydrofolate reductase fusions are not secreted by the type III machinery of Y. enterocolitica. (A) The drawing depicts the primary structures of hybrid polypeptides as follows: line 1, YopE1-15-DHFR, yop codons 1 to 15 fused to DHFR; line 2, YopE1-220-DHFR, yop codons 1 to 220 (full-length) fused to dihydrofolate reductase (DHFR). (B) Y. enterocolitica strains W22703 (wild type) and LC1 Δ(sycE1) were transformed with plasmids described above, and type III secretion was analyzed by immunoblotting with specific antibody (α-DHFR, α-YopR, or α-Cat) as described in the legend to Fig. 1.
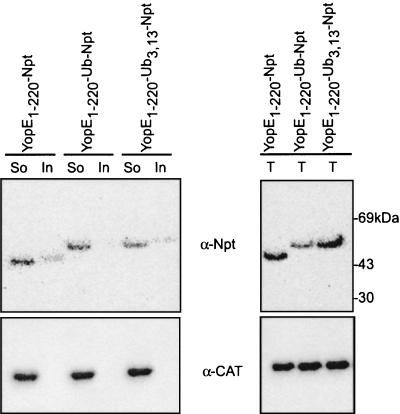
One simple explanation for a failure of fusion proteins to block the type III pathway could be their aggregation in the bacterial cytoplasm. To test whether fusion proteins are soluble in the cytoplasm, bacterial extracts were generated in a French pressure cell (14,000 lb/in2) and unbroken cells were removed by slow-speed centrifugation (4,000 × g for 15 min). The supernatant was removed, and lysates were subjected to ultracentrifugation at 100,000 × g for 30 min. Soluble supernatant and insoluble sediment were separated and analyzed by immunoblotting. Most of YopE1-220-Npt, YopE1-220-Ub-Npt, and YopE1-220-Ub3,13-Npt did not sediment at 100,000 × g and remained in the supernatant (Fig. 3). Thus, it appears that the fusion proteins are soluble in the bacterial cytoplasm and do not form aggregates that are hindered in their interaction with the type III secretion machinery.
FIG. 3.
YopE1-220-Npt, YopE1-220-Ub-Npt, and YopE1-220-UbGly3,13-Npt are soluble proteins. Y. enterocolitica W22703 expressing either YopE1-220-Npt, YopE1-220-Ub-Npt, or YopE1-220-UbGly3,13-Npt was lysed in a French pressure cell. Crude extracts were subjected to ultracentrifugation at 100,000 × g. The soluble supernatant (So) and insoluble sediment (In) were separated, and proteins were precipitated with TCA and analyzed by immunoblotting (α-Npt or α-CAT). In the right panel, the signal intensities of Yop fusions in cell extracts are compared with the signal intensities in samples obtained by TCA precipitation of total cultures (T).
We wish to propose a model that accounts for all of the observations reported here. Yop fusions to folded domains are not permanently engaged by the type III machinery but are rejected from the pathway (presumably once and for all) shortly after the synthesis of the secretion signal has been completed. If the secretion signal is recognized and the substrate is accommodated by the type III machinery, transport will be initiated and completed. If, however, the secretion signal is recognized and the substrate cannot be accommodated, the protein will be rejected and cannot be reconsidered (or reinitiated) for transport by the type III machinery. Are this model and the data reported here compatible with the signal peptide hypothesis whereby an amphipathic helical peptide initiates substrates into the type III pathway? We think the results argue against a signal peptide mechanism of type III secretion, as this model would predict the initiation of signal peptide-bearing substrates as well as the block of a pathway charged with substrates that cannot be transported. In contrast, the RNA signal hypothesis may account for the observed substrate rejection within the type III secretion pathway. Assuming that the RNA signals of yop transcripts couple translation to secretion of the polypeptide, one could predict that the N-terminal portion of Yops is initiated into the type III pathway. Once the polypeptide has been completed and is folded, the rejected Yop-Ub or Yop-DHFR hybrids cannot reenter the pathway because the protein product has been separated from the RNA signal.
We think it unlikely that an entire Yop protein is transported in a cotranslational, unfolded manner, as the needle structures are longer than a linear polypeptide of 220 residues (3). Yop fusions to small (76-residue ubiquitin, 8-kDa) or medium-sized (187-residue DHFR, 21-kDa) domains are obviously permitted to fold and can be rejected at an undefined step along the pathway. We wondered whether the size of the type III machinery is incompatible with the size of folded ubiquitin or DHFR. By using the crystallographic coordinates and computer simulation (40, 54), conservative estimates for the smallest diameters of folded ubiquitin and DHFR are 22 and 27 Å, respectively (data not shown). The diameter of the lumen of type III needle, a conduit through which Yop proteins are presumed to travel, is measured at about 20 Å (11, 25, 31). Thus, it seems that folded Yop proteins (52) or Yop fusions to folded ubiquitin or DHFR are too large to be transported through the lumen of the needle structure. In summary, we conclude that substrate recognition of type III machines occurs by unique mechanisms with governing principles that are distinct from those of co- and posttranslational translocation by the Sec pathway.
Acknowledgments
We thank Anthony Kossiakoff (University of Chicago) for help with computer simulation of crystallographic data.
V.T.L. acknowledges support by a fellowship from the National Science Foundation and the Warsaw Family Fellowship. This work was supported by U.S. Public Health Service Grant AI42797 from the NIH-NIAID, Infectious Diseases Branch.
REFERENCES
- 1.Anderson, D. M., D. Fouts, A. Collmer, and O. Schneewind. 1999. Reciprocal secretion of proteins by the bacterial type III machines of plant and animal pathogens suggests universal recognition of mRNA targeting signals. Proc. Natl. Acad. Sci. USA 96:12839-12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, D. M., K. S. Ramamurthi, C. Tam, and O. Schneewind. 2002. YopD and LcrH regulate the expression of Yersinia enterocolitica YopQ at a post-transcriptional step and bind to yopQ mRNA. J. Bacteriol. 184:1287-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, D. M., and O. Schneewind. 1997. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science 278:1140-1143. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, D. M., and O. Schneewind. 1999. Type III machines of Gram-negative pathogens: injecting virulence factors into host cells and more. Curr. Opin. Microbiol. 2:18-24. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, D. M., and O. Schneewind. 1999. Yersinia enterocolitica type III secretion: an mRNA signal that couples translation and secretion of YopQ. Mol. Microbiol. 31:1139-1148. [DOI] [PubMed] [Google Scholar]
- 6.Baker, R. T., J. T. Tobias, and A. Varshavsky. 1992. Ubiquitin-specific proteases of Saccharomyces cerevisiae. J. Biol. Chem. 267:23364-23375. [PubMed] [Google Scholar]
- 7.Benson, S. A., M. N. Hall, and T. J. Silhavy. 1985. Genetic analysis of protein export in Escherichia coli K12. Annu. Rev. Biochem. 54:101-134. [DOI] [PubMed] [Google Scholar]
- 8.Bieker, K. L., and T. J. Silhavy. 1990. PrlA (SecY) and PrlG (SecE) interact directly and function sequentially during protein translocation in E. coli. Cell 61:833-842. [DOI] [PubMed] [Google Scholar]
- 9.Blobel, G. 1980. Intracellular protein topogenesis. Proc. Natl. Acad. Sci. USA 77:1496-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blobel, G., and B. Dobberstein. 1975. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J. Cell Biol. 67:835-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blocker, A., N. Jouihri, E. Larquet, P. Gounon, F. Ebel, C. Parsot, P. Sansonetti, and A. Allaoui. 2001. Structure and composition of the Shigella flexneri ‘needle complex’, a part of its type III secreton. Mol. Microbiol. 39:652-663. [DOI] [PubMed] [Google Scholar]
- 12.Brown, J. L., D. M. Brown, and I. Zabin. 1967. Thiogalactoside transacetylase. Physical and chemical studies of subunit structure. J. Biol. Chem. 242:4254-4258. [PubMed] [Google Scholar]
- 13.Cheng, L. W., D. M. Anderson, and O. Schneewind. 1997. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol. Microbiol. 24:757-765. [DOI] [PubMed] [Google Scholar]
- 14.Cheng, L. W., and O. Schneewind. 1999. Yersinia enterocolitica type III secretion: on the role of SycE in targeting YopE into HeLa cells. J. Biol. Chem. 274:22102-22108. [DOI] [PubMed] [Google Scholar]
- 15.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M.-P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelis, G. R., and C. Colson. 1975. Restriction of DNA in Yersinia enterocolitica detected by the recipient ability for a derepressed R factor from Escherichia coli. J. Gen. Microbiol. 87:285-291. [DOI] [PubMed] [Google Scholar]
- 17.Dalbey, R. E., and W. Wickner. 1985. Leader peptidase catalyzes the release of exported proteins from the outer surface of the Escherichia coli plasma membrane. J. Biol. Chem. 260:15925-15931. [PubMed] [Google Scholar]
- 18.Economou, A., J. A. Pogliano, J. Beckwith, D. B. Oliver, and W. Wickner. 1995. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell 83:1171-1181. [DOI] [PubMed] [Google Scholar]
- 19.Eilers, M., and G. Schatz. 1986. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature 322:228-232. [DOI] [PubMed] [Google Scholar]
- 20.Emr, S., and T. J. Silhavy. 1980. Mutations affecting localization of an Escherichia coli outer membrane protein, the bacteriophage λ receptor. J. Mol. Biol. 141:63-90. [DOI] [PubMed] [Google Scholar]
- 21.Emr, S. D., S. Hanley-Way, and T. J. Silhavy. 1981. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell 23:79-88. [DOI] [PubMed] [Google Scholar]
- 22.Gentz, R., Y. Kuys, C. Zwieb, D. Taatjes, H. Taatjes, W. Bannwarth, D. Stueber, and I. Ibrahimi. 1988. Association of degradation and secretion of three chimeric polypeptides in Escherichia coli. J. Bacteriol. 170:2212-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glaser, P., H. Sakamoto, J. Bellalou, A. Ullmann, and A. Danchin. 1988. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-hemolysin bifunctional protein of Bordetella pertussis. EMBO J. 7:3997-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartl, F. U., S. Lecker, E. Schiebel, J. P. Hendrick, and W. Wickner. 1990. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell 63:269-279. [DOI] [PubMed] [Google Scholar]
- 25.Hoiczyk, E., and G. Blobel. 2001. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc. Natl. Acad. Sci. USA 98:4669-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurt, E. C., B. Pesold-Hurt, and G. Schatz. 1984. The amino-terminal region of an imported mitochondrial precursor polypeptide can direct cytoplasmic dihydrofolate reductase into the mitochondrial matrix. EMBO J. 3:3149-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito, K., P. J. J. Bassford, and J. Beckwith. 1981. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer membrane proteins? Cell 24:707-717. [DOI] [PubMed] [Google Scholar]
- 28.Johnsson, N., and A. Varshavsky. 1994. Ubiquitin-assisted dissection of protein transport across membranes. EMBO J. 13:2686-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalnins, A., K. Otto, U. Ruether, and B. Mueller-Hill. 1983. Sequence of the lacZ gene of Escherichia coli. EMBO J. 2:593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiino, D. R., and T. J. Silhavy. 1984. Mutation in prlF1 relieves the lethality associated with export of beta-galactosidase hybrid proteins in Escherichia coli. J. Bacteriol. 158:878-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galan, and S.-I. Aizawa. 1998. Supermolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280:602-605. [DOI] [PubMed] [Google Scholar]
- 32.Kumamoto, C. A. 1989. Escherichia coli SecB protein associates with exported protein precursors in vivo. Proc. Natl. Acad. Sci. USA 86:5320-5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langley, K. E., M. R. Villarejo, A. V. Fowler, P. J. Zamenhof, and I. Zabin. 1975. Molecular basis of beta galactosidase alpha-complementation. Proc. Natl. Acad. Sci. USA 72:1254-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipp, J., B. Dobberstein, and M. T. Haeuptle. 1987. Signal recognition particle arrests elongation of nascent secretory and membrane proteins at multiple sites in a transient manner. J. Biol. Chem. 262:1680-1684. [PubMed] [Google Scholar]
- 35.Lloyd, S. A., M. Norman, R. Rosqvist, and H. Wolf-Watz. 2001. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol. Microbiol. 39:520-531. [DOI] [PubMed] [Google Scholar]
- 36.Lloyd, S. A., M. Sjostrom, S. Andersson, and H. Wolf-Watz. 2002. Molecular characterization of the type III secretion signals via analysis of synthetic N-terminal amino acid sequences. Mol. Microbiol. 43:51-59. [DOI] [PubMed] [Google Scholar]
- 37.Meyer, D. I., E. Krause, and B. Dobberstein. 1982. Secretory protein translocation across membranes—the role of the “docking protein.” Nature 297:647-650. [DOI] [PubMed] [Google Scholar]
- 38.Michiels, T., and G. R. Cornelis. 1991. Secretion of hybrid proteins by the Yersinia Yop export system. J. Bacteriol. 173:1677-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakano, H., Y. Kawakami, and H. Nishimura. 1992. Secretion of genetically-engineered dihydrofolate reductase from Escherichia coli using an E. coli alpha-hemolysin membrane translocation system. Appl. Microbiol. Biotechnol. 37:765-771. [DOI] [PubMed] [Google Scholar]
- 40.Oefner, C., A. D'Arcy, and F. K. Winkler. 1988. Crystal structure of human dihydrofolate reductase complexed with folate. Eur. J. Biochem. 174:377-385. [DOI] [PubMed] [Google Scholar]
- 41.Oliver, D. B., and J. Beckwith. 1981. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell 25:765-772. [DOI] [PubMed] [Google Scholar]
- 42.Poritz, M. A., H. D. Bernstein, K. Strub, D. Zopf, H. Wilhelm, and P. Walter. 1990. An E. coli ribonucleoprotein containing 4.5S RNA resembles mammalian signal recognition particle. Science 250:1111-1117. [DOI] [PubMed] [Google Scholar]
- 43.Powers, T., and P. Walter. 1997. Co-translational protein targeting catalyzed by the Escherichia coli signal recognition particle and its receptor. EMBO J. 16:4880-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramamurthi, K. S., and O. Schneewind. 2002. Yersinia enterocolitica type III secretion: mutational analysis of the yopQ secretion signal. J. Bacteriol. 184:3321-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Randall, L. L. 1992. Peptide binding by chaperone SecB: implications for recognition of non-native structure. Science 257:241-245. [DOI] [PubMed] [Google Scholar]
- 46.Randall, L. L., and S. J. Hardy. 1986. Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli. Cell 46:921-928. [DOI] [PubMed] [Google Scholar]
- 47.Reiss, B., R. Sprengel, and H. Schaller. 1984. Protein fusions with the kanamycin resistance gene from transposon Tn5. EMBO J. 3:3317-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schatz, P. J., and J. Beckwith. 1990. Genetic analysis of protein export in Escherichia coli. Annu. Rev. Genet. 24:215-248. [DOI] [PubMed] [Google Scholar]
- 49.Schesser, K., E. Fritzh-Lindsten, and H. Wolf-Watz. 1996. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J. Bacteriol. 178:7227-7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sory, M.-P., A. Boland, I. Lambermont, and G. R. Cornelis. 2002. 1995. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl. Acad. Sci. USA 92:11998-12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sory, M.-P., and G. R. Cornelis. 1994. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 14:583-594. [DOI] [PubMed] [Google Scholar]
- 52.Stuckey, J. A., H. L. Schubert, E. B. Fauman, Z. Y. Zhang, J. E. Dixon, and M. A. Saper. 1994. Crystal structure of Yersinia protein tyrosine phosphatase at 2.5 Å and the complex with tungstate. Nature 370:571-575. [DOI] [PubMed] [Google Scholar]
- 53.Takeshita, S., M. Sato, M. Toba, W. Masahashi, and T. Hashimoto-Gotoh. 1987. High-copy-number and low-copy-number plasmid vectors for LacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63-74. [DOI] [PubMed] [Google Scholar]
- 54.Vijay-Kumar, S., C. E. Bugg, and W. J. Cook. 1987. Structure of ubiquitin refined at 1.8 Å resolution. J. Mol. Biol. 194:531-544. [DOI] [PubMed] [Google Scholar]
- 55.Walter, P., and G. Blobel. 1980. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 77:7112-7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walter, P., and G. Blobel. 1982. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature 299:691-698. [DOI] [PubMed] [Google Scholar]
- 57.Walter, P., R. Keenan, and U. Schmitz. 2000. SRP—where the RNA and membrane worlds meet. Science 287:1212-1213. [DOI] [PubMed] [Google Scholar]
- 58.Wattiau, P., and G. R. Cornelis. 1993. SycE, a chaperone-like protein of Yersinia enterocolitica involved in the secretion of YopE. Mol. Microbiol. 8:123-131. [DOI] [PubMed] [Google Scholar]