Abstract
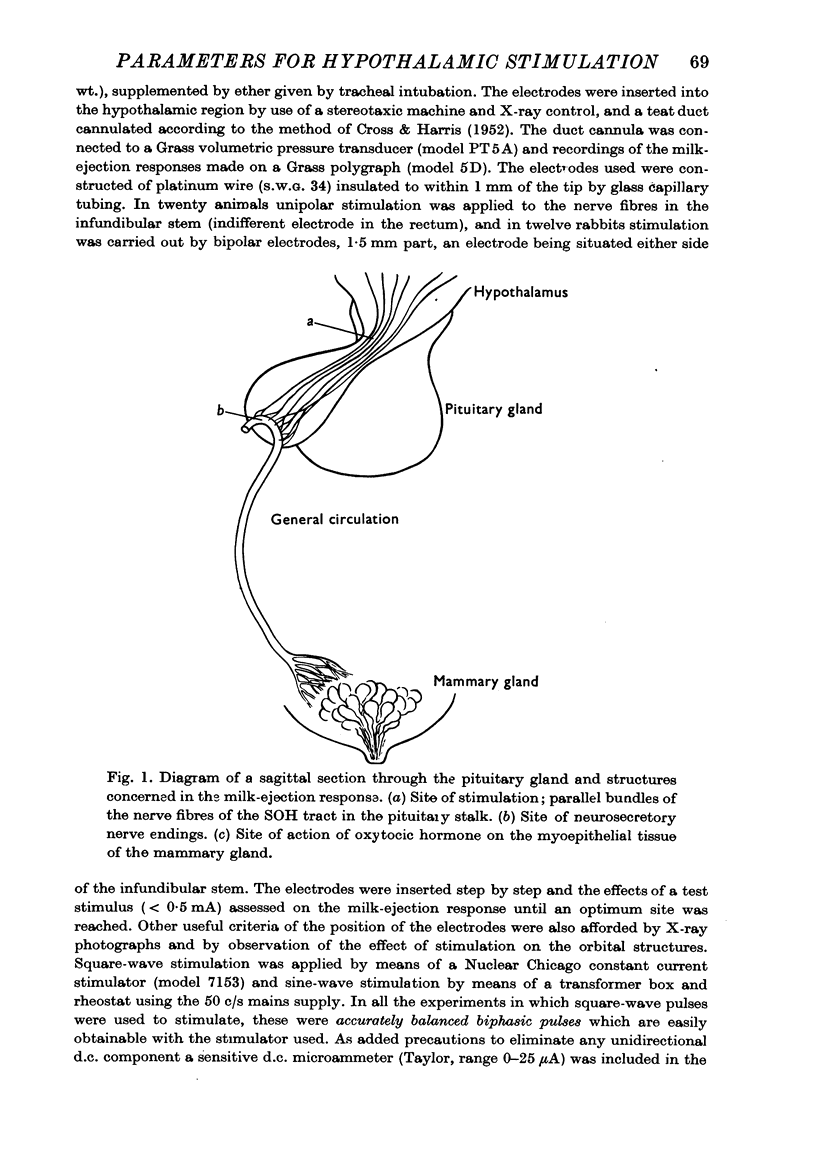
1. The milk-ejection response in lactating rabbits has been used to study the effect of electrical stimuli of different types applied to the supraopticohypophysial tract in the pituitary stalk.
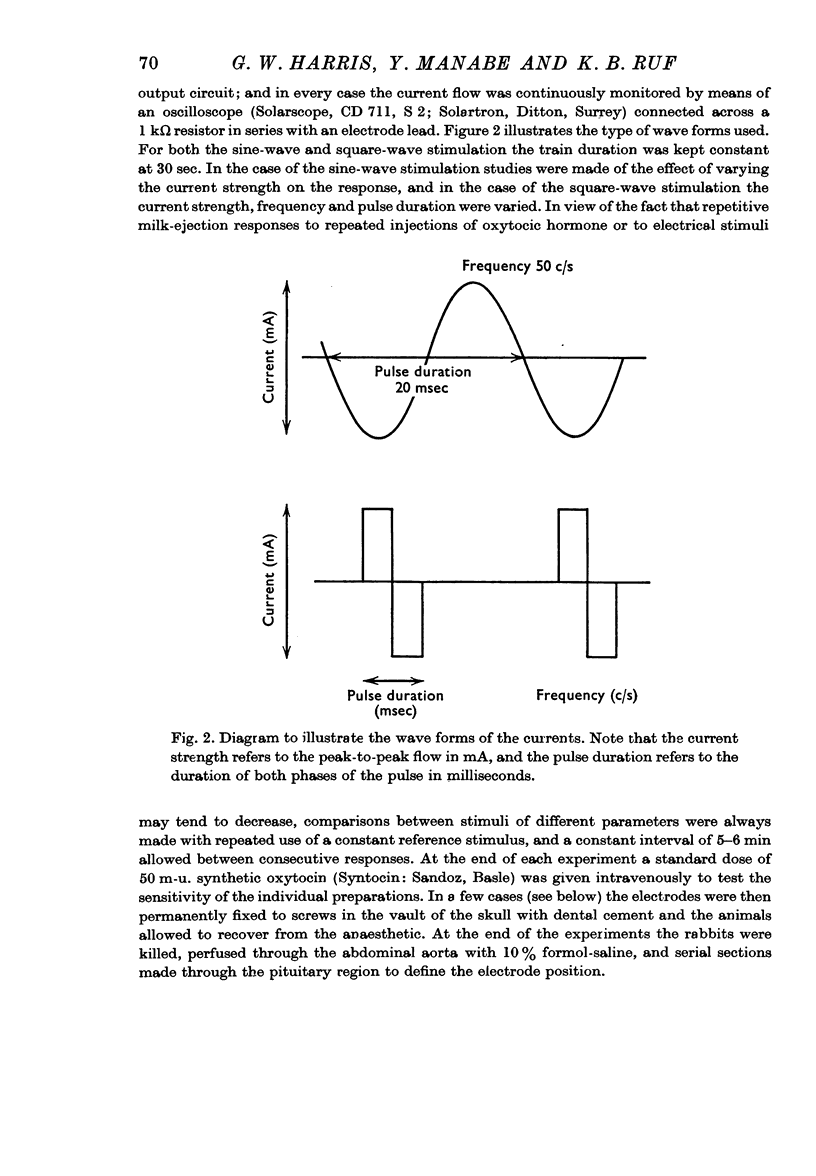
2. Sine-wave alternating-current pulses were compared with balanced biphasic square-wave pulses of the same frequency and peak-to-peak current strength. At a pulse duration of 2-4 msec the square-wave stimulation was less effective than the sine wave, but at a pulse duration of 8 msec and over, more effective.
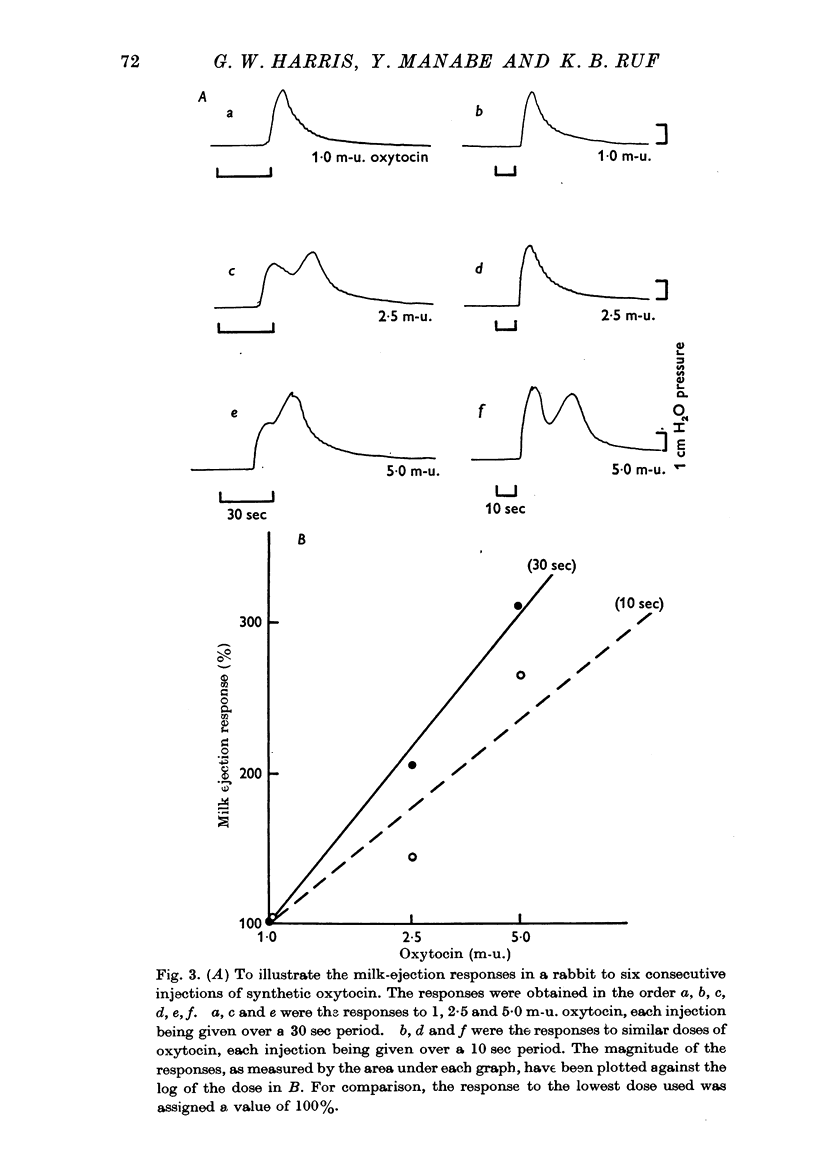
3. Above threshold levels of 0·12 mA for the current strength, and of 0·5 msec for the pulse duration, the response increased with increasing current strengths to 2·4 mA and increasing pulse durations to 10 msec.
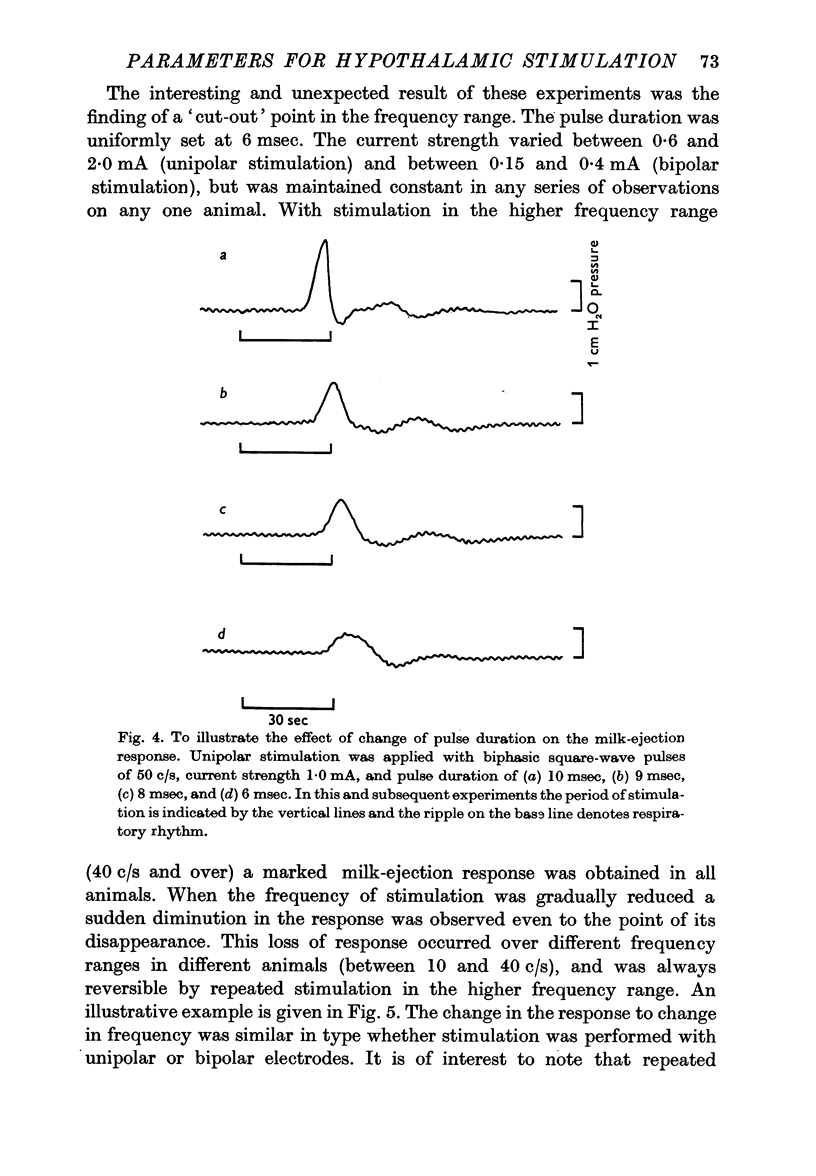
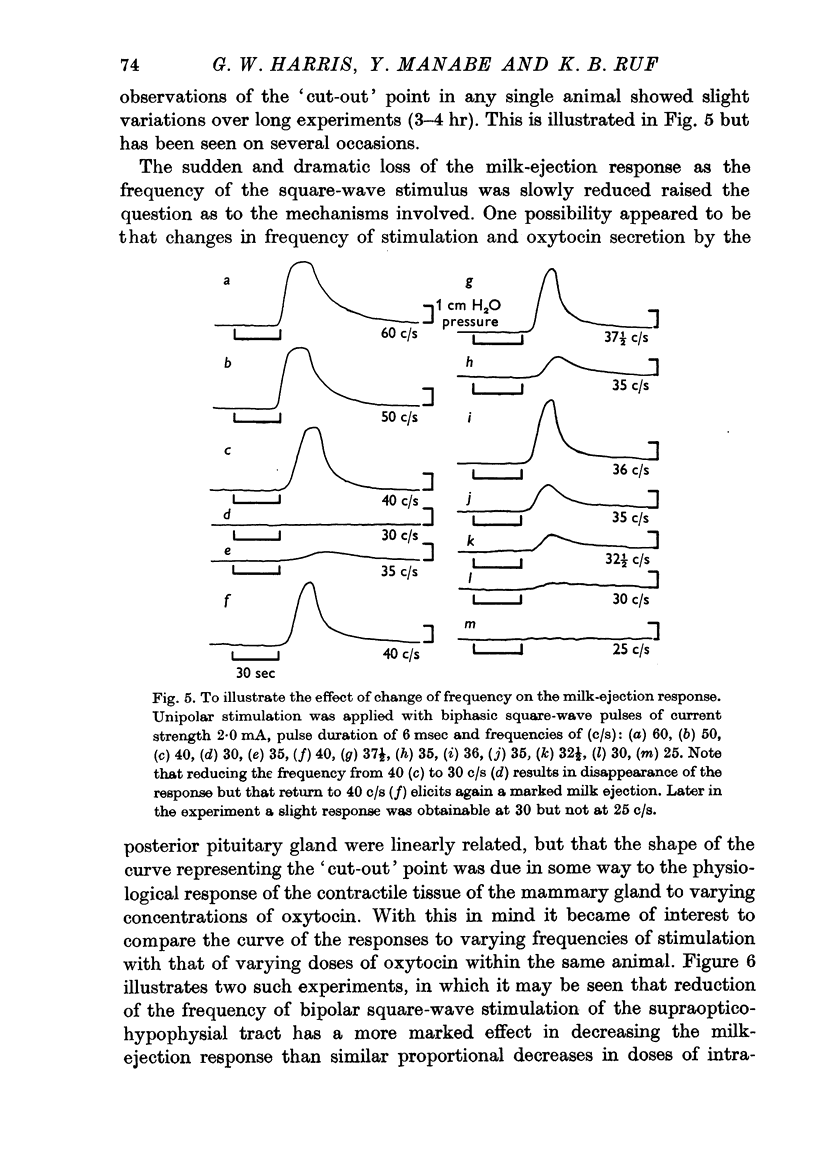
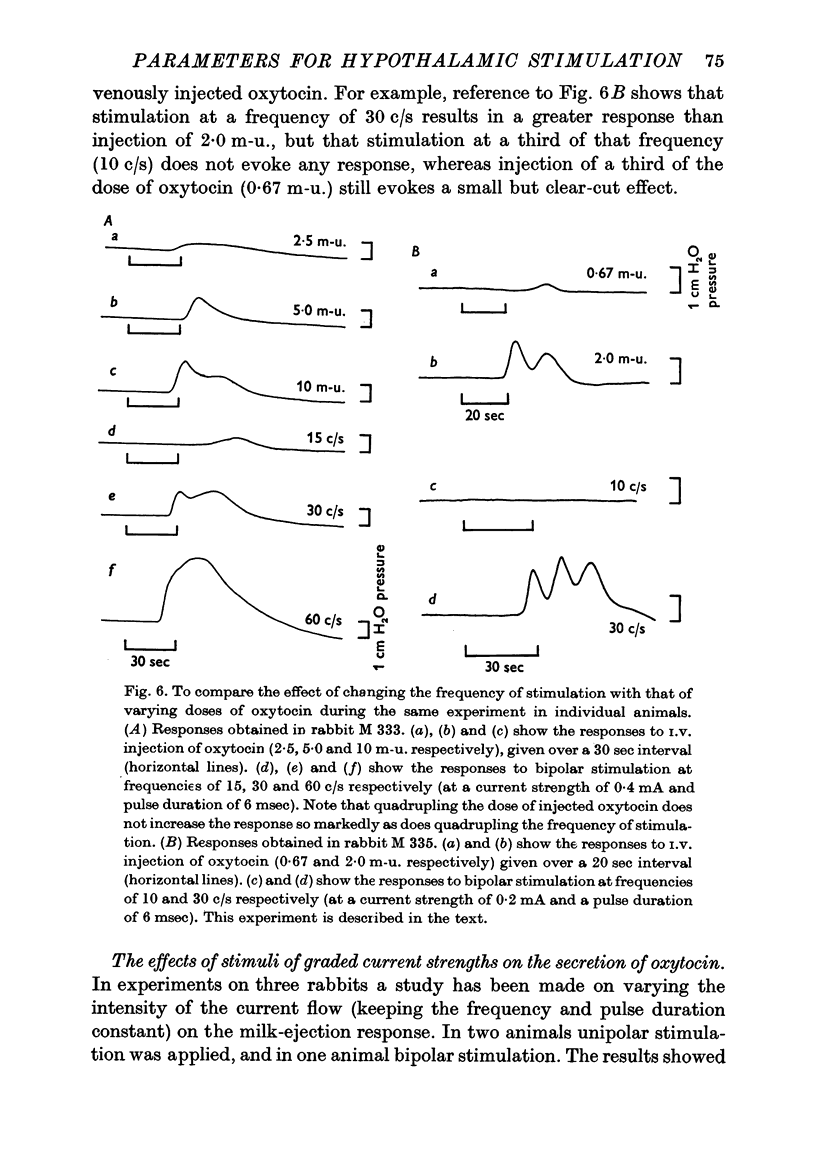
4. With constant and effective strength and duration of the pulse a slight diminution of the response was seen as the frequency was diminished from 100/sec to 50/sec. Further diminution in the frequency revealed that at some point between 50 and 10 c/s a sudden abrupt diminution in the magnitude of the responses occurred over a small range of frequency. This was a reversible phenomenon and it is suggested that it may be related to neurosecretory events occurring in the nerve terminals.
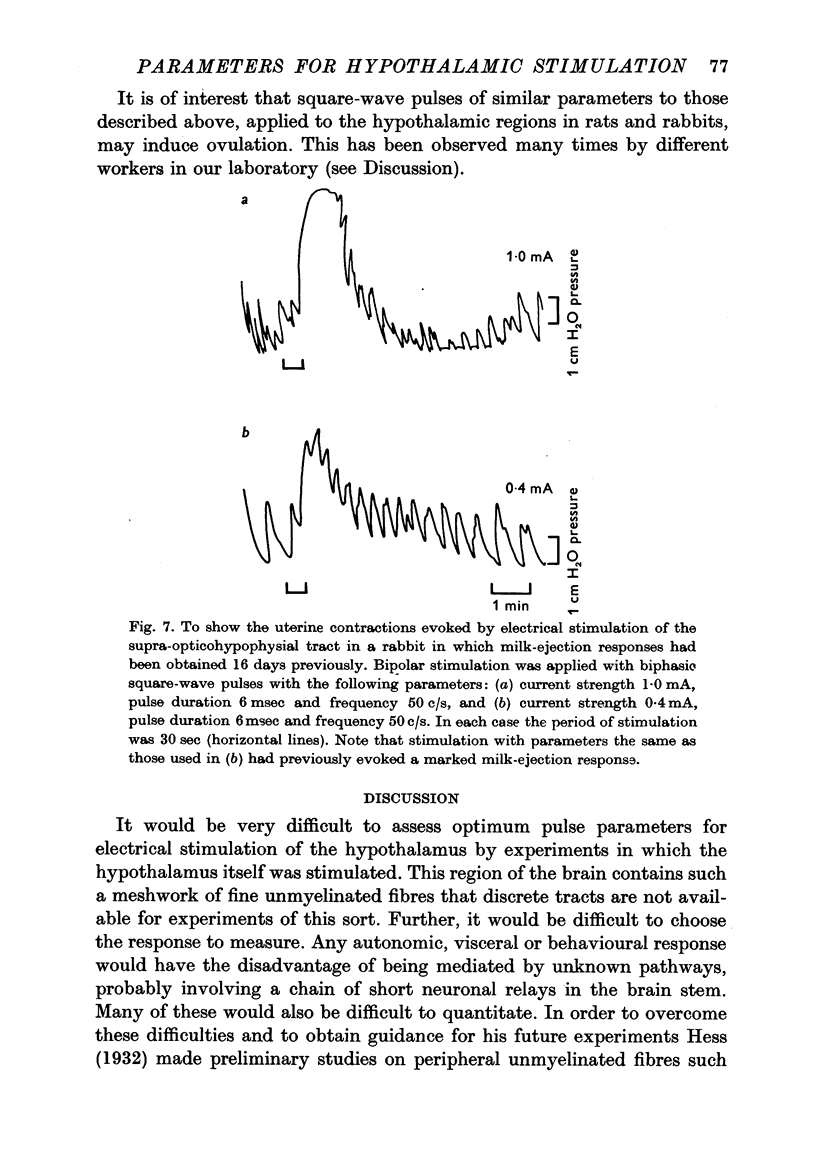
5. Stimuli with parameters within the ranges mentioned above are effective in eliciting an oxytocic response on the uterus in the rabbit and ovulation responses (when applied to the hypothalamus) in rats and rabbits. It appears likely that stimuli with such parameters are suitable for experiments concerned with stimulation of the hypothalamus.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRACLOUGH C. A., GORSKI R. A. Evidence that the hypothalamus is responsible for androgen-induced sterility in the female rat. Endocrinology. 1961 Jan;68:68–79. doi: 10.1210/endo-68-1-68. [DOI] [PubMed] [Google Scholar]
- CRITCHLOW V. Ovulation induced by hypothalamic stimulation in the anesthetized rat. Am J Physiol. 1958 Oct;195(1):171–174. doi: 10.1152/ajplegacy.1958.195.1.171. [DOI] [PubMed] [Google Scholar]
- CROSS B. A., HARRIS G. W. The role of the neurohypophysis in the milk-ejection reflex. J Endocrinol. 1952 Apr;8(2):148–161. doi: 10.1677/joe.0.0080148. [DOI] [PubMed] [Google Scholar]
- CROSS B. A., VAN DYKE H. B. The effects of highly purified posterior pituitary principles on the lactating mammary gland of the rabbit. J Endocrinol. 1953 Apr;9(2):232–235. doi: 10.1677/joe.0.0090232. [DOI] [PubMed] [Google Scholar]
- EVERETT J. W. OVULATION IN RATS FROM PREOPTIC STIMULATION THROUGH PLATINUM ELECTRODES. IMPORTANCE OF DURATION AND SPREAD OF STIMULUS. Endocrinology. 1965 Jun;76:1195–1201. doi: 10.1210/endo-76-6-1195. [DOI] [PubMed] [Google Scholar]
- Exley D., Gellert R. J., Harris G. W., Nadler R. D. The site of action of 'chlormadinone acetate' (6-chloro-delta 6-dehydro-17 alpha-acetoxyprogesterone) in blocking ovulation in the mated rabbit. J Physiol. 1968 Apr;195(3):697–714. doi: 10.1113/jphysiol.1968.sp008483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYWARD J. N., HILLIARD J., SAWYER C. H. TIME OF RELEASE OF PITUITARY GONADOTROPIN INDUCED BY ELECTRICAL STIMULATION OF THE RABBIT BRAIN. Endocrinology. 1964 Jan;74:108–113. doi: 10.1210/endo-74-1-108. [DOI] [PubMed] [Google Scholar]
- Harris G. W., Sherratt R. M. The action of chlormadinone acetate (6-chloro-delta6-dehydro-17alpha-acetoxyprogesterone) upon experimentally induced ovulation in the rabbit. J Physiol. 1969 Jul;203(1):59–66. doi: 10.1113/jphysiol.1969.sp008849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAKAMI M., SAWYER C. H. Induction of behavioral and electroencephalographic changes in the rabbit by hormone administration or brain stimulation. Endocrinology. 1959 Oct;65:631–643. doi: 10.1210/endo-65-4-631. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Seto K., Terasawa E., Yoshida K. Mechamisms in the limbic system controlling reproductive functions of the ovary with special reference to the positive feedback of progestin to the hippocampus. Prog Brain Res. 1967;27:69–102. doi: 10.1016/s0079-6123(08)63094-0. [DOI] [PubMed] [Google Scholar]
- Tindal J. S., Knaggs G. S., Turvey A. Preferential release of oxytocin from the neurohypophysis after electrical stimulation of the afferent path of the milk-ejection reflex in the brain of the guinea-pig. J Endocrinol. 1968 Feb;40(2):205–214. doi: 10.1677/joe.0.0400205. [DOI] [PubMed] [Google Scholar]
- WARD H. P. Stimulus factors in septal self-stimulation. Am J Physiol. 1959 Apr;196(4):779–782. doi: 10.1152/ajplegacy.1959.196.4.779. [DOI] [PubMed] [Google Scholar]


