Abstract
1. Experiments were made on single neurones and glial cells in the central nervous system of the leech to study the accumulation of K that occurs in the extracellular spaces around neurones as a result of impulse activity.
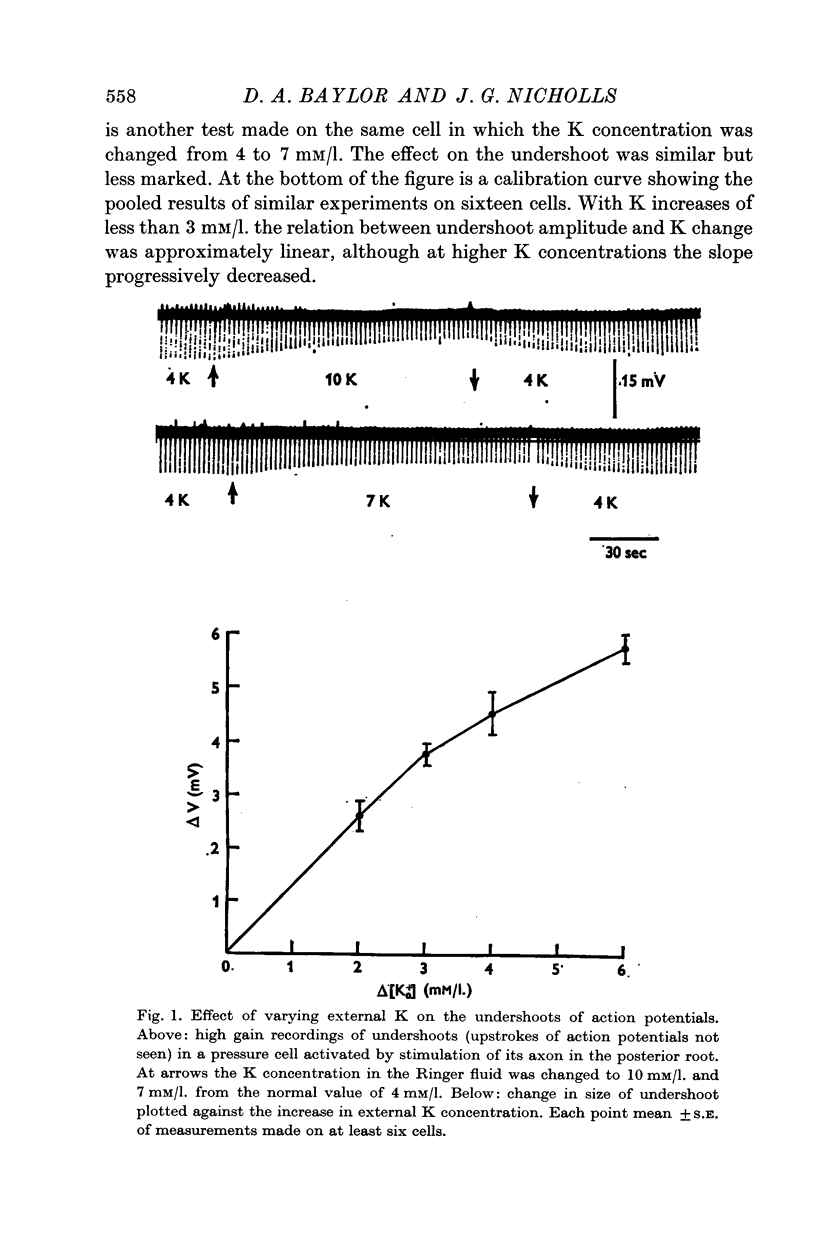
2. The resting potential of a neurone is too insensitive to be used for the estimation of small changes in K concentration. The undershoot of the action potential, however, provided a reliable indicator of the K accumulation that occurs around a neurone during activity.
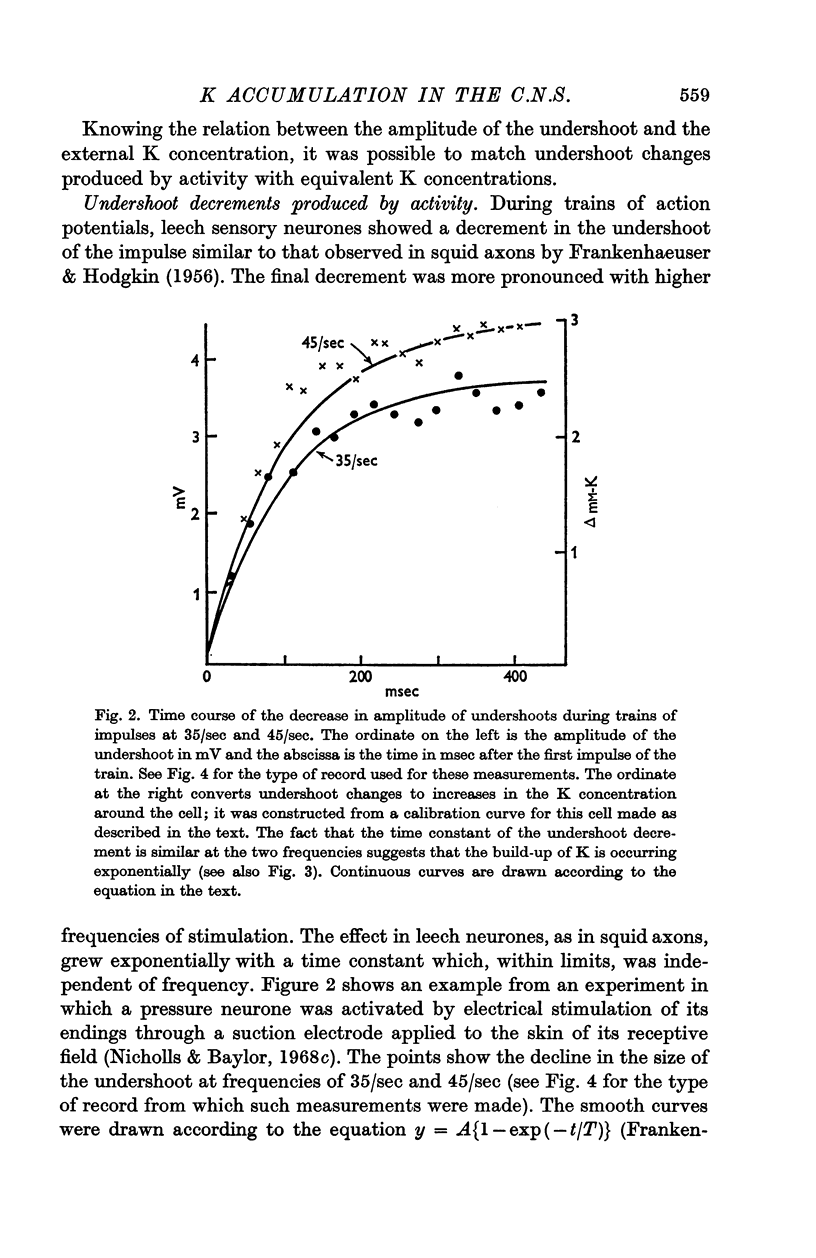
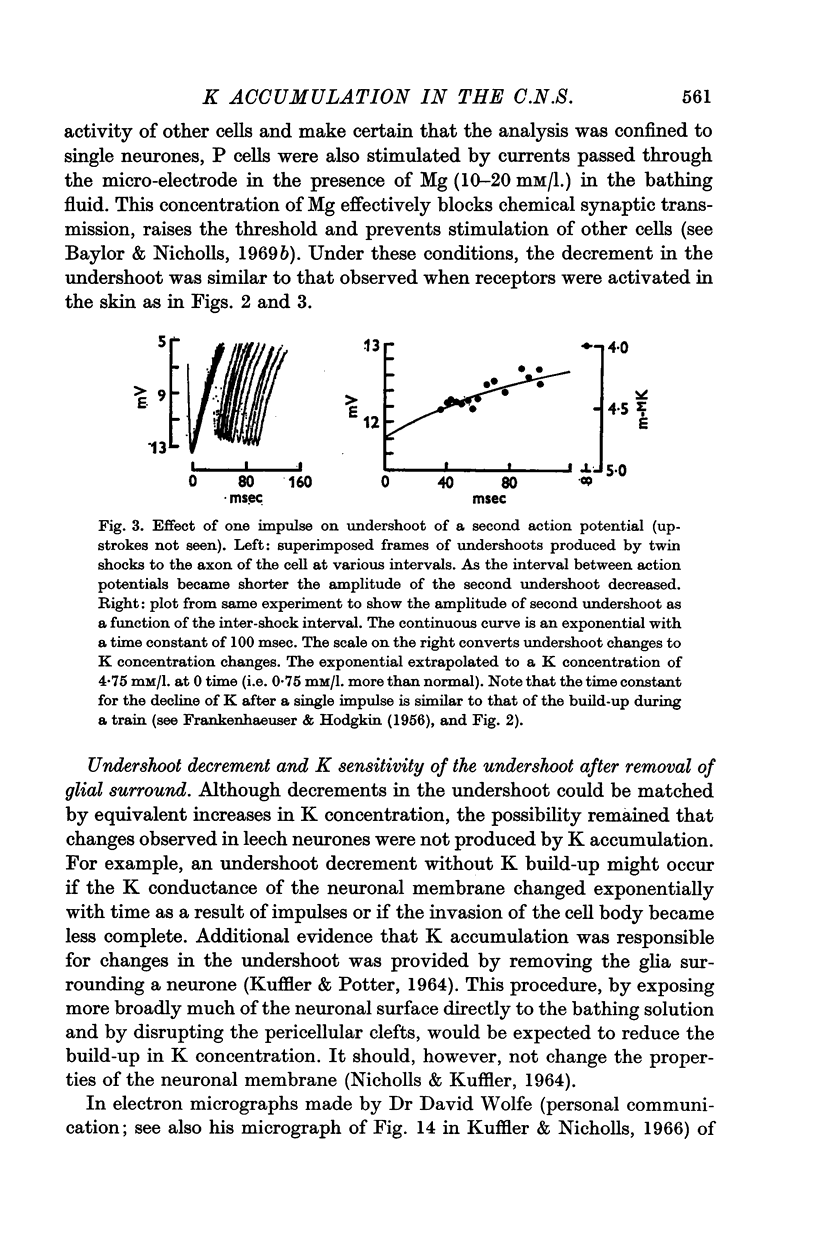
3. After a single impluse the amplitude of the undershoot of a second action potential was decreased; the effect corresponded to a peak increase in K concentration of about 0·8 mM/l. immediately after the spike and declined exponentially with a time constant of about 100 msec. With trains of impulses the K concentration increased exponentially, again with a time constant of about 100 msec. The final value of K depended on the frequency and could build up to about double the normal concentration of 4 mM/l.
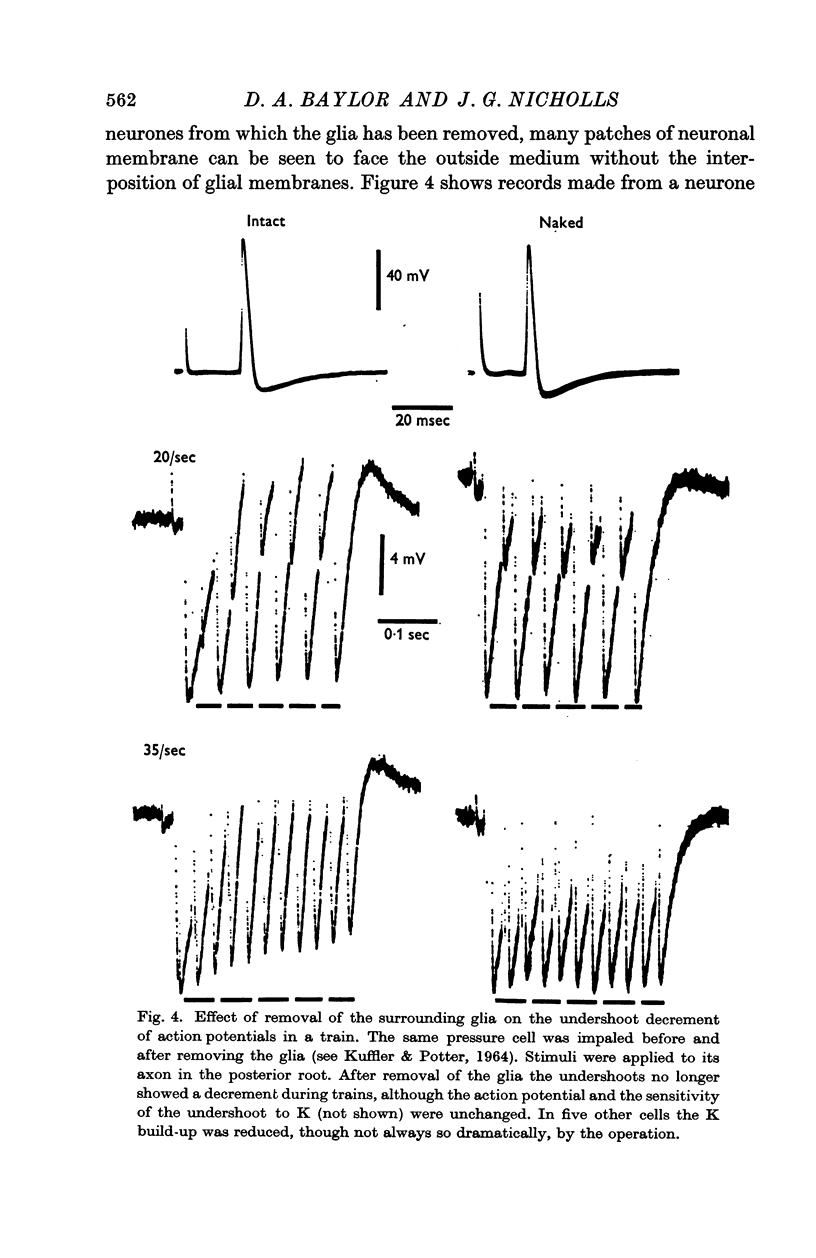
4. The build-up of K was markedly reduced when the extracellular space surrounding a neurone was enlarged by removing its glial investment.
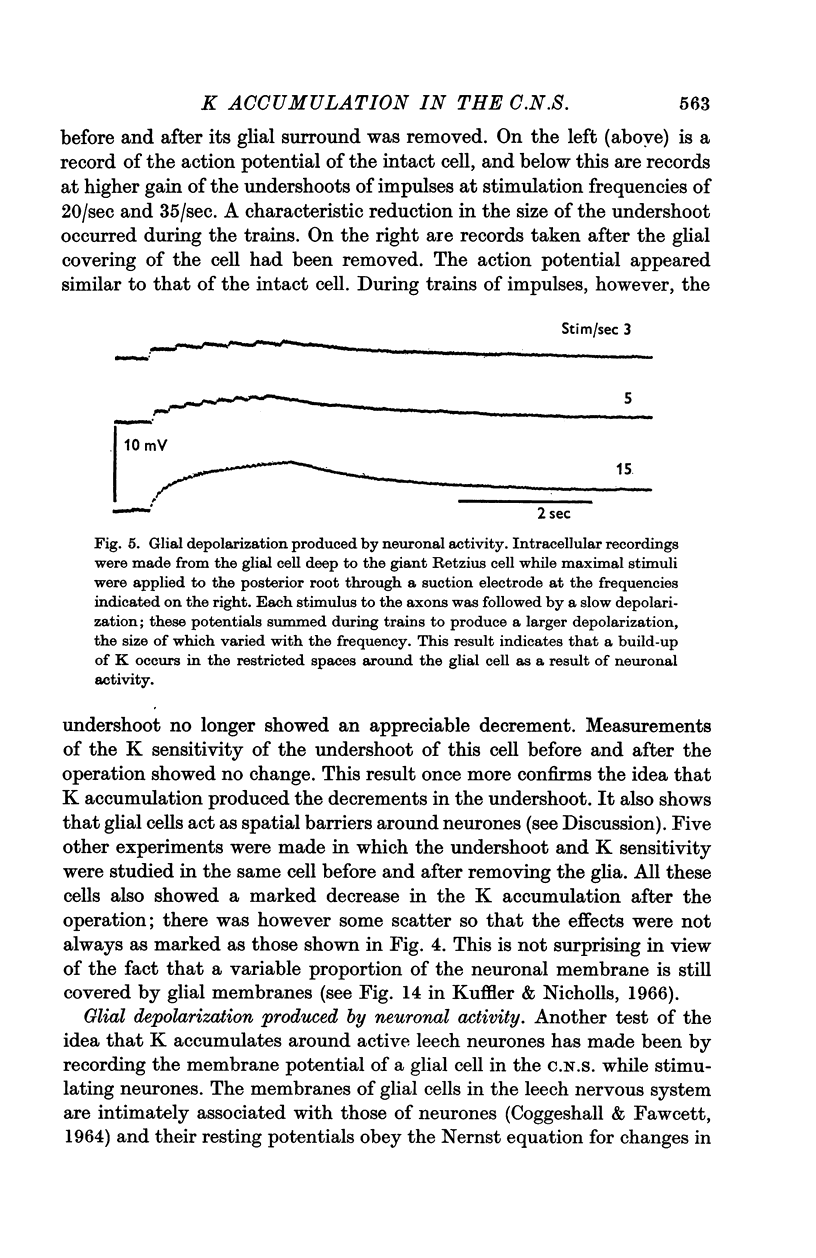
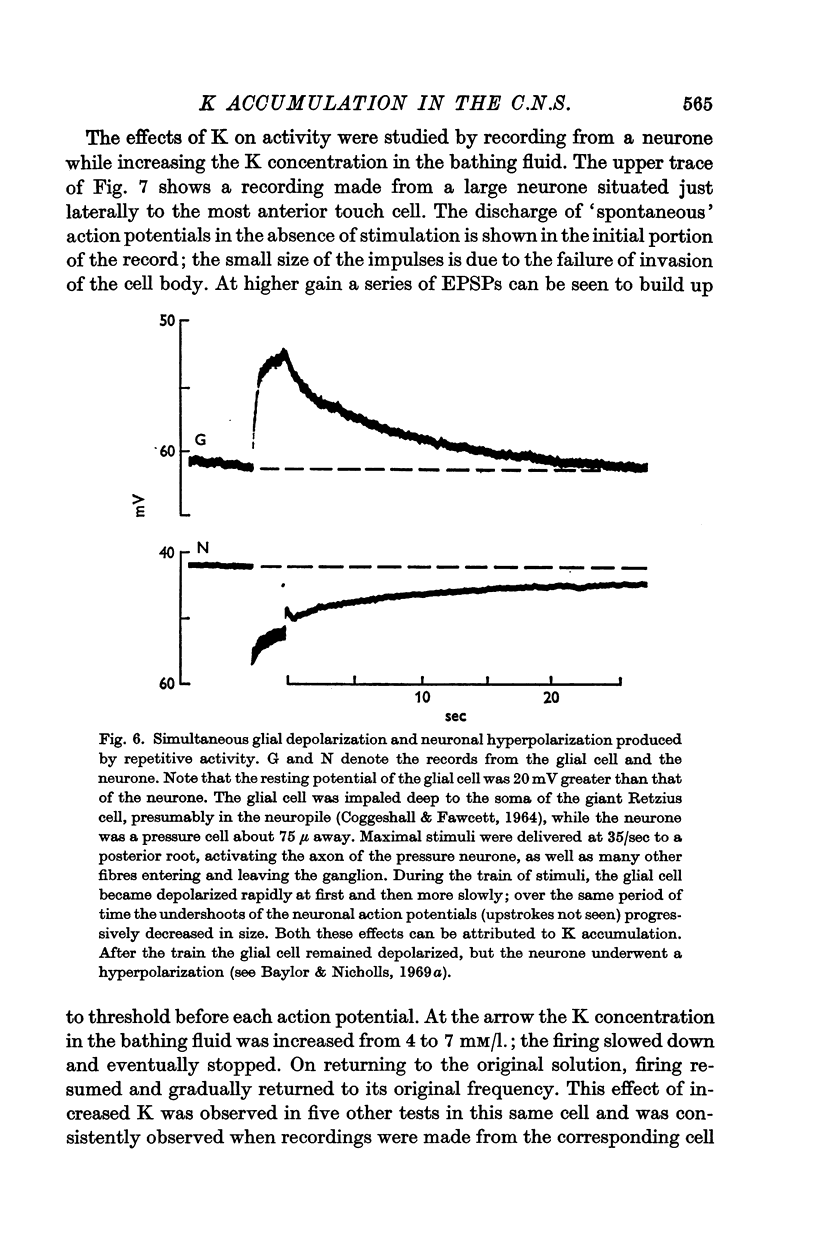
5. Synchronous, repetitive activation of groups of neurones caused a slow depolarization of neighbouring glial cells in the C.N.S. of the leech, similar to that observed in amphibia and mammals. The change in glial membrane potential was also used to estimate the changes in K concentration and these values agreed with measurements derived from the undershoot.
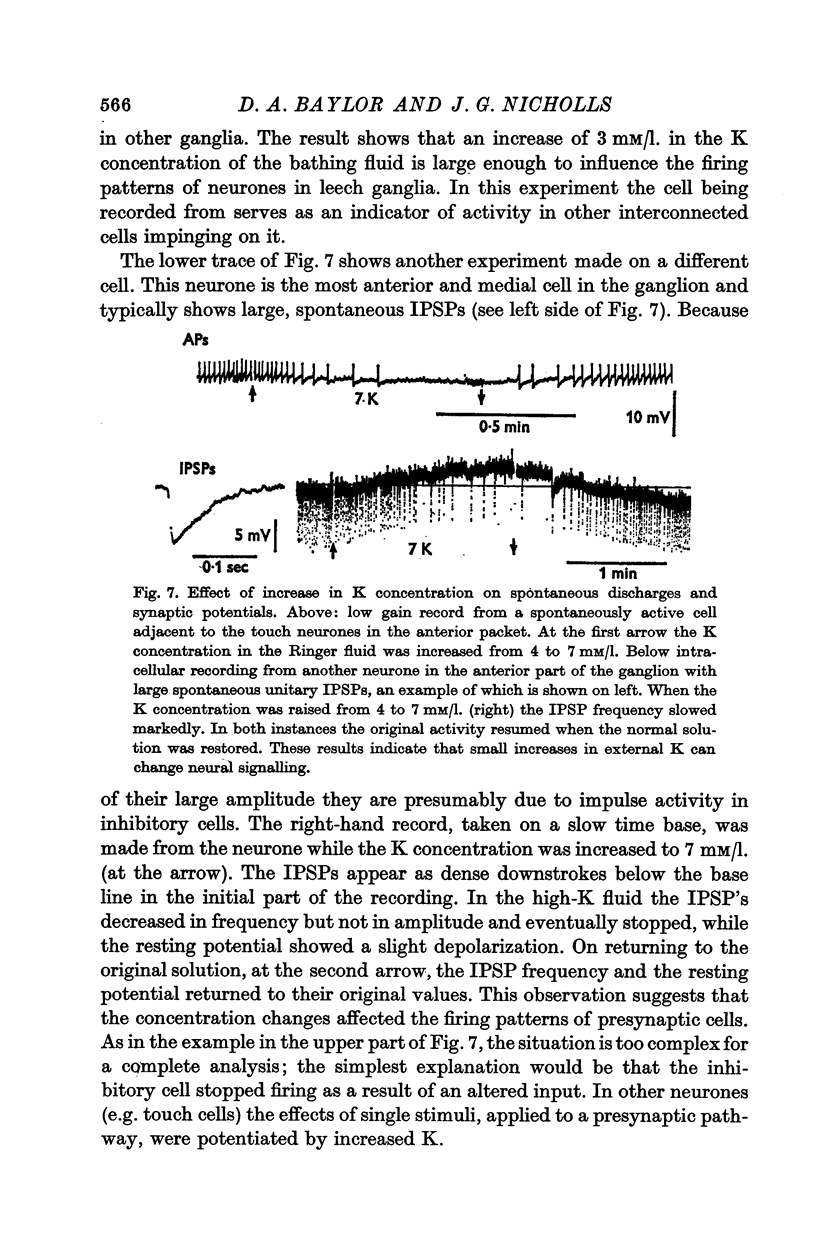
6. Increases of K concentration in the bathing fluid of the same order as those caused by neural firing markedly affected the frequency of `spontaneous' neuronal discharges and synaptic potentials occurring within certain neurones in the C.N.S.
7. The possible effects of physiologically occurring increases of K concentration on integration are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Nicholls J. G. After-effects of nerve impulses on signalling in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):571–589. doi: 10.1113/jphysiol.1969.sp008880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nicholls J. G. Chemical and electrical synaptic connexions between cutaneous mechanoreceptor neurones in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):591–609. doi: 10.1113/jphysiol.1969.sp008881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COGGESHALL R. E., FAWCETT D. W. THE FINE STRUCTURE OF THE CENTRAL NERVOUS SYSTEM OF THE LEECH, HIRUDO MEDICINALIS. J Neurophysiol. 1964 Mar;27:229–289. doi: 10.1152/jn.1964.27.2.229. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURUKAWA T., FURSHPAN E. J. Two inhibitory mechanisms in the Mauthner neurons of goldfish. J Neurophysiol. 1963 Jan;26:140–176. doi: 10.1152/jn.1963.26.1.140. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The sodium and potassium content of cephalopod nerve fibers. J Physiol. 1951 Jun;114(1-2):151–182. doi: 10.1113/jphysiol.1951.sp004609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The leakage of radioactive potassium from stimulated nerve. J Physiol. 1951 Mar;113(1):99–114. doi: 10.1113/jphysiol.1951.sp004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., POTTER D. D. GLIA IN THE LEECH CENTRAL NERVOUS SYSTEM: PHYSIOLOGICAL PROPERTIES AND NEURON-GLIA RELATIONSHIP. J Neurophysiol. 1964 Mar;27:290–320. doi: 10.1152/jn.1964.27.2.290. [DOI] [PubMed] [Google Scholar]
- Karahashi Y., Goldring S. Intracellular potentials from "idle" cells in cerebral cortex of cat. Electroencephalogr Clin Neurophysiol. 1966 Jun;20(6):600–607. doi: 10.1016/0013-4694(66)90024-1. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- NICHOLLS J. G., KUFFLER S. W. EXTRACELLULAR SPACE AS A PATHWAY FOR EXCHANGE BETWEEN BLOOD AND NEURONS IN THE CENTRAL NERVOUS SYSTEM OF THE LEECH: IONIC COMPOSITION OF GLIAL CELLS AND NEURONS. J Neurophysiol. 1964 Jul;27:645–671. doi: 10.1152/jn.1964.27.4.645. [DOI] [PubMed] [Google Scholar]
- Nicholls J. G., Baylor D. A. Specific modalities and receptive fields of sensory neurons in CNS of the leech. J Neurophysiol. 1968 Sep;31(5):740–756. doi: 10.1152/jn.1968.31.5.740. [DOI] [PubMed] [Google Scholar]
- Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- SHANES A. M. Effect of temperature on potassium liberation during nerve activity. Am J Physiol. 1954 Jun;177(3):377–382. doi: 10.1152/ajplegacy.1954.177.3.377. [DOI] [PubMed] [Google Scholar]


