Abstract
Experiments have been made to study the synaptic connexions between sensory cells in the C.N.S. of the leech. Each segmental ganglion contains six neurones that respond specifically to light touch applied to the skin; each of these `touch cells' innervates a discrete area on the surface of the body and has a characteristic set of properties by which it can be recognized. Using intracellular electrodes it has been shown that these sensory cells interact with one another through chemical and electrical synapses by way of a stereotyped set of pathways.
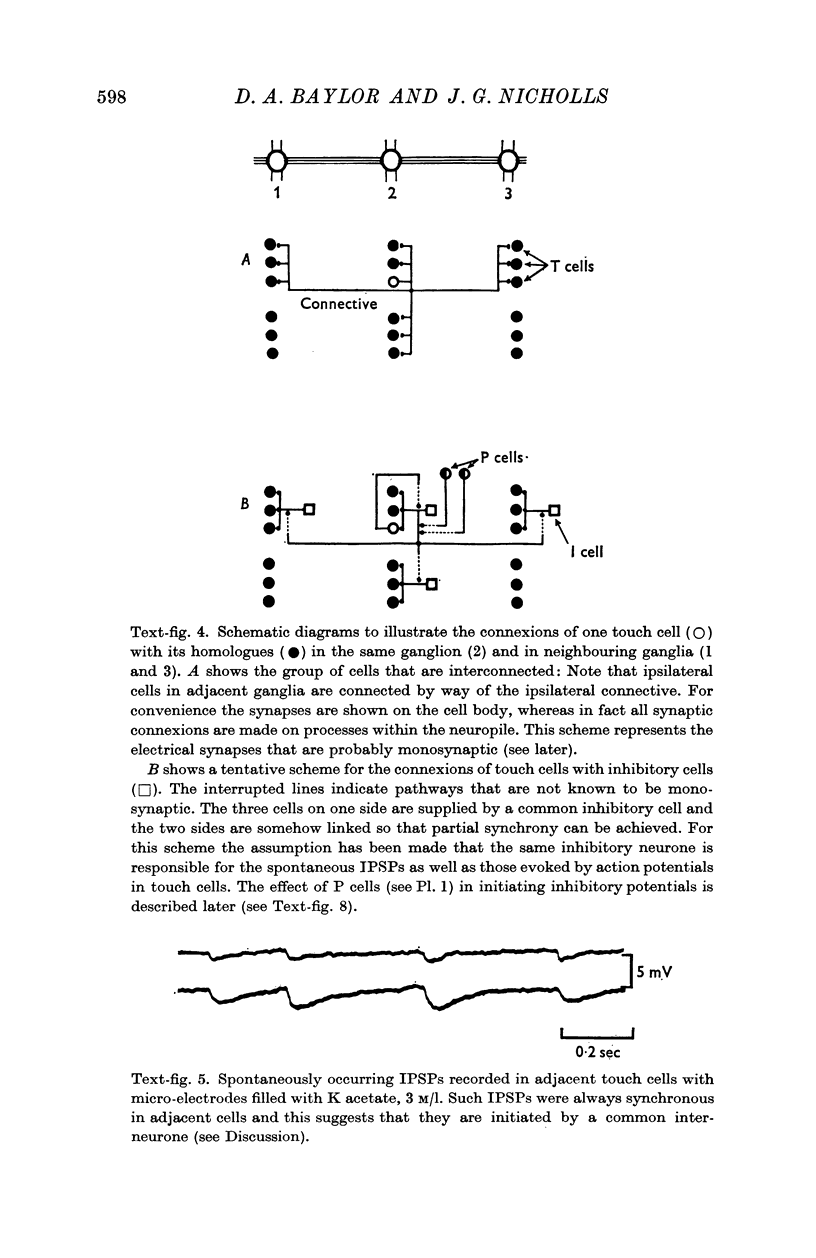
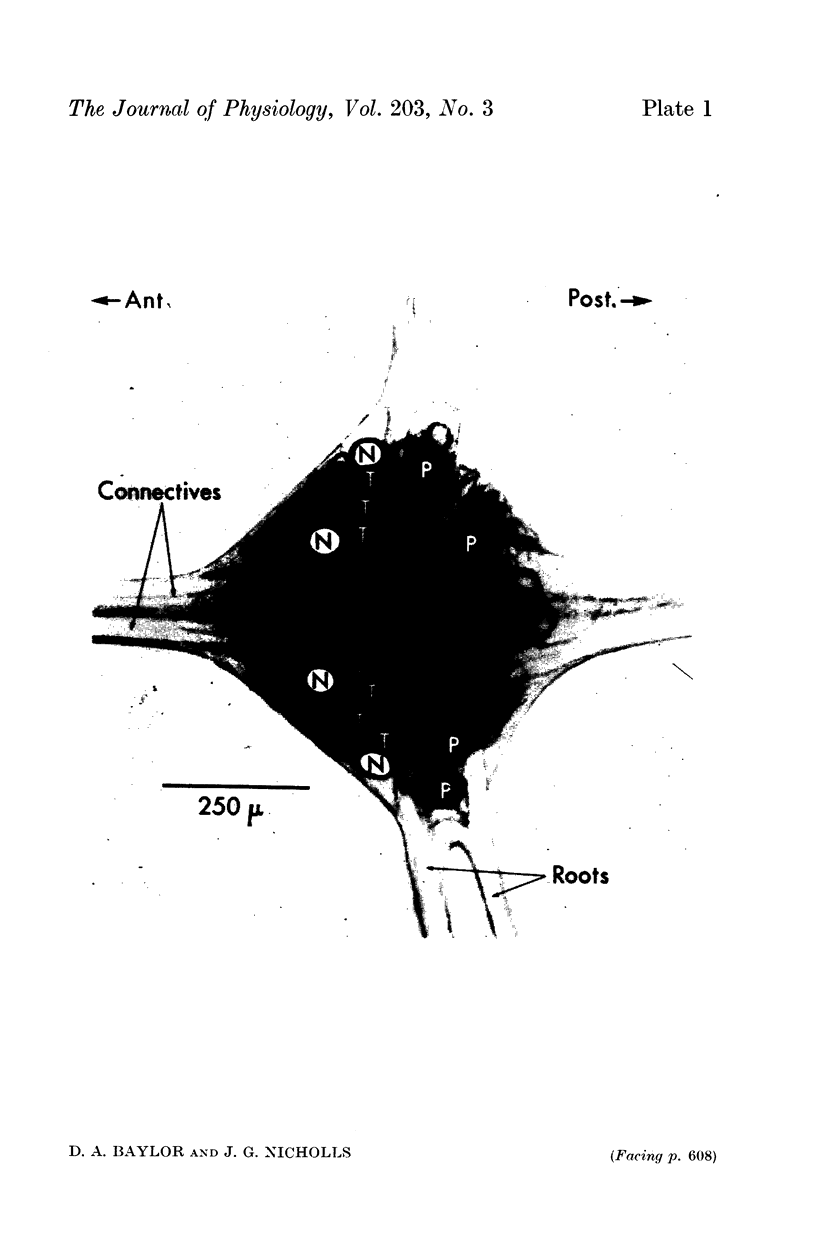
1. Action potentials occurring in one touch cell gave rise to synaptic potentials in the five other touch cells in the same ganglion and also in the three ipsilateral touch cells in the adjacent ganglia. Thus, synaptic interactions took place between sensory cells whose receptive fields lay within the same segment and on the same side of adjacent segments.
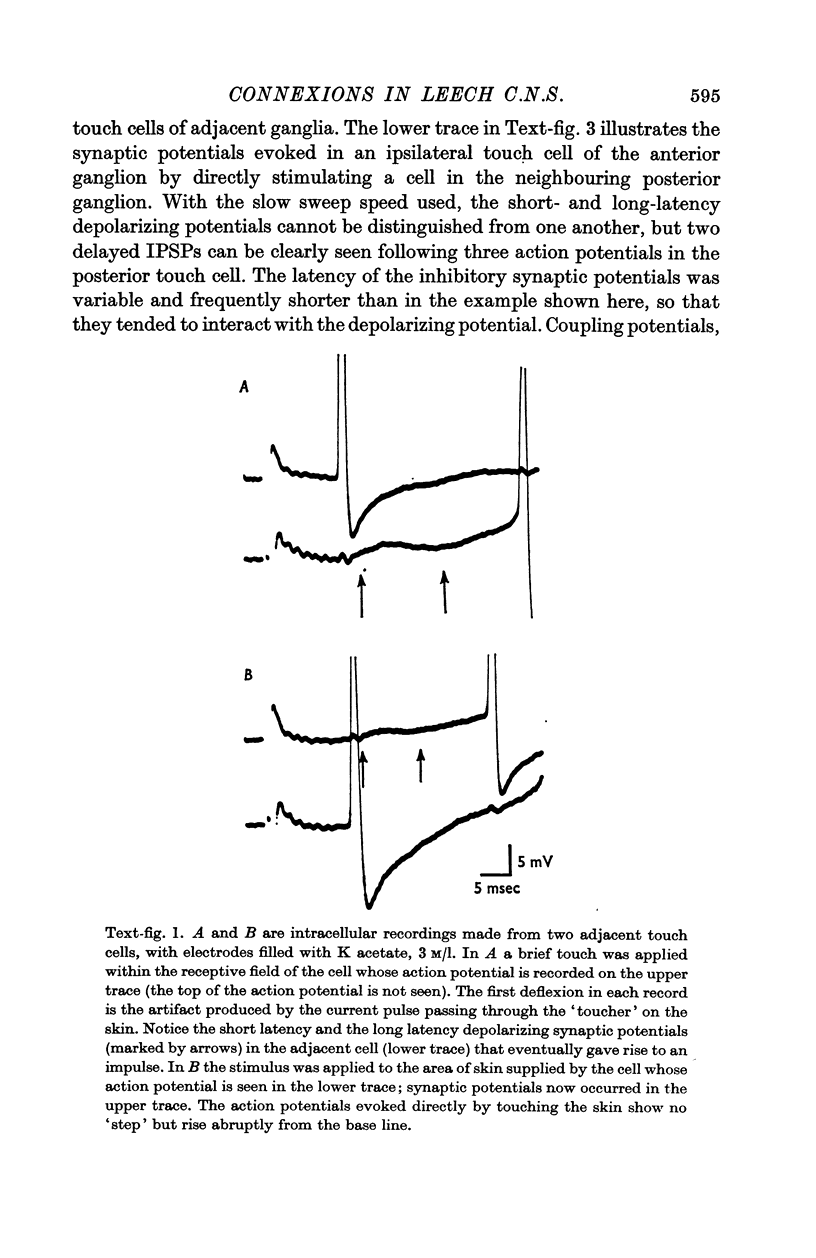
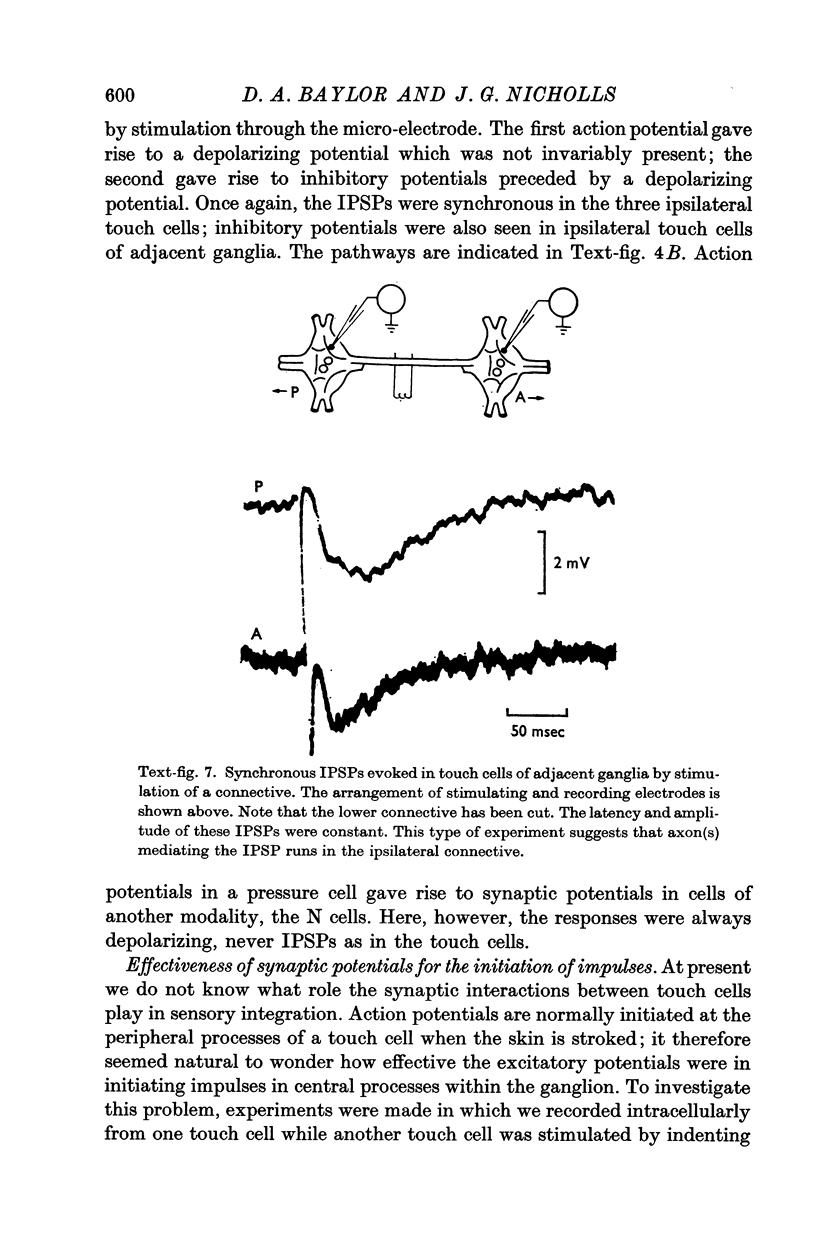
2. The post-synaptic potentials consisted of a short-latency coupling potential, followed by an excitatory potential and a number of inhibitory potentials. These delayed synaptic potentials occurred inconsistently and with a variable latency; they could also be recorded in the cell which had been stimulated. All of the touch cells appeared to be equally effective in initiating synaptic potentials.
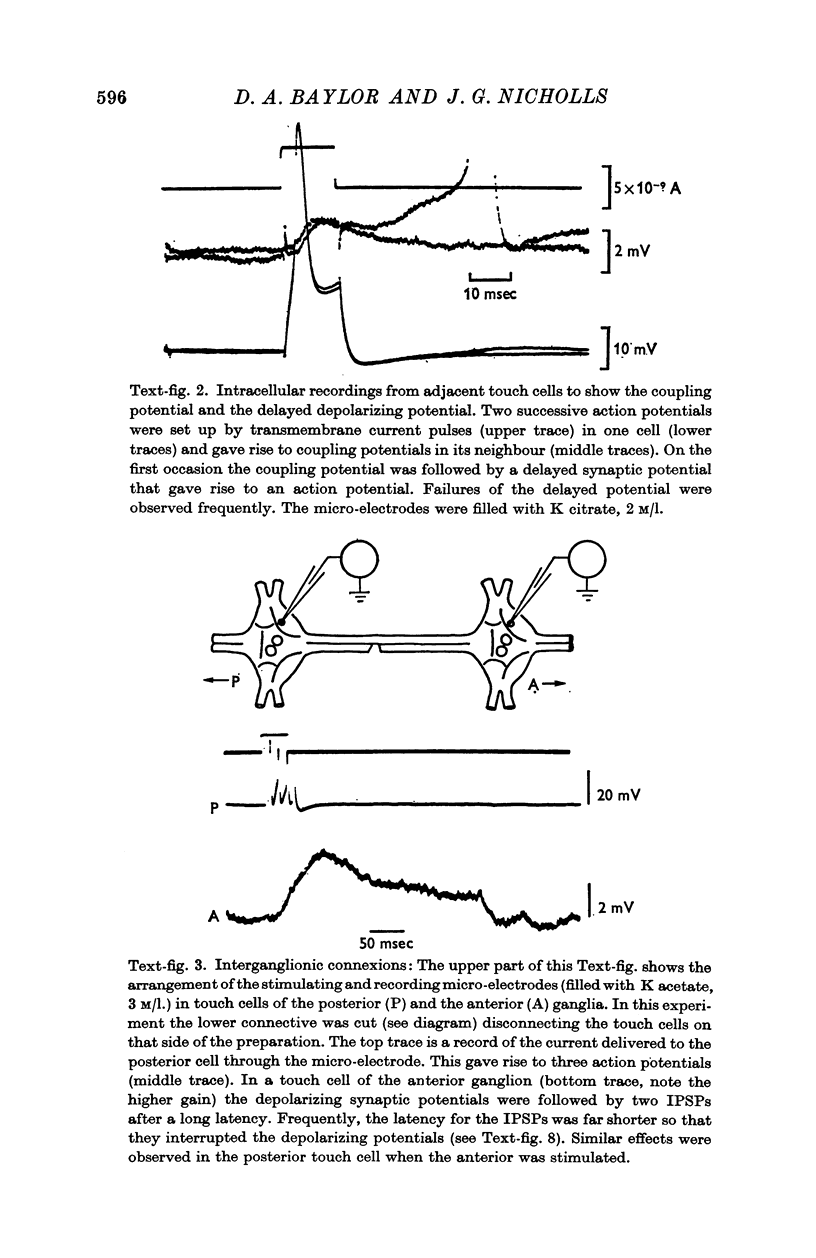
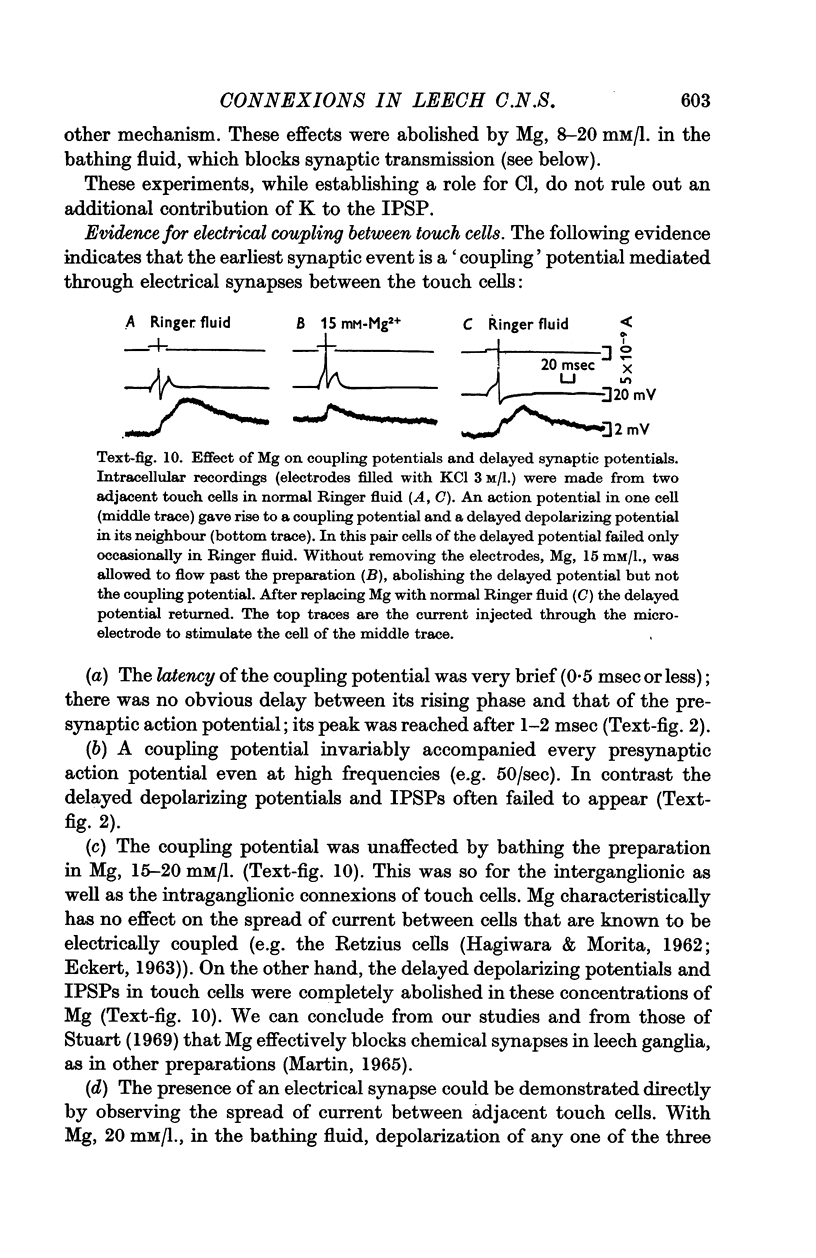
3. The short-latency coupling potential was shown to be mediated through an electrical synapse by observing a voltage change in one touch cell when current was injected into its neighbour. It was not abolished by high concentrations of Mg in the bathing fluid, which blocked chemical synapses in this ganglion. This electrical synapse displayed remarkable rectification; a depolarization could spread from cell to cell in both directions, while a hyperpolarization could spread in neither.
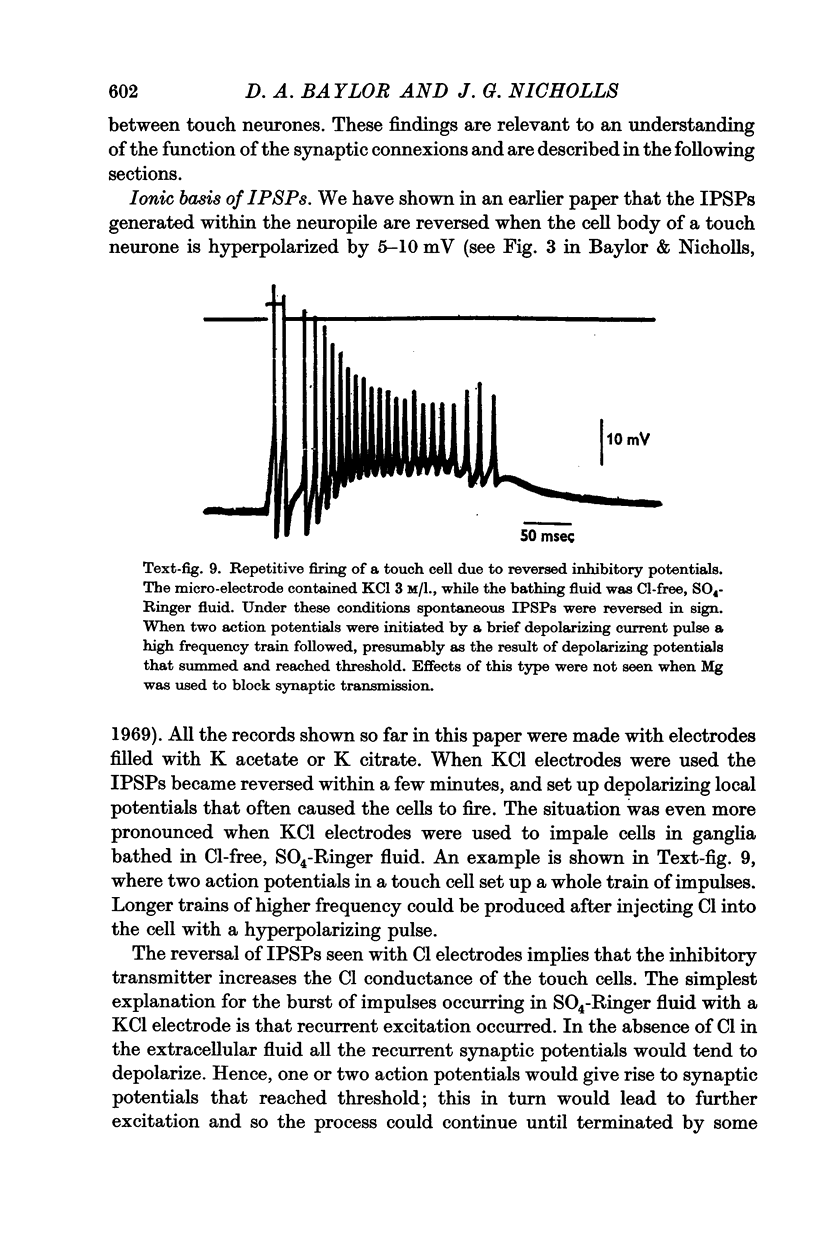
4. The inhibitory potentials were reversed by injecting Cl into the cell. In Cl-free Ringer solution this effect was so marked that the reversed IPSPs caused long trains of impulses in touch cells, which tended to excite each other by a process of positive feed-back.
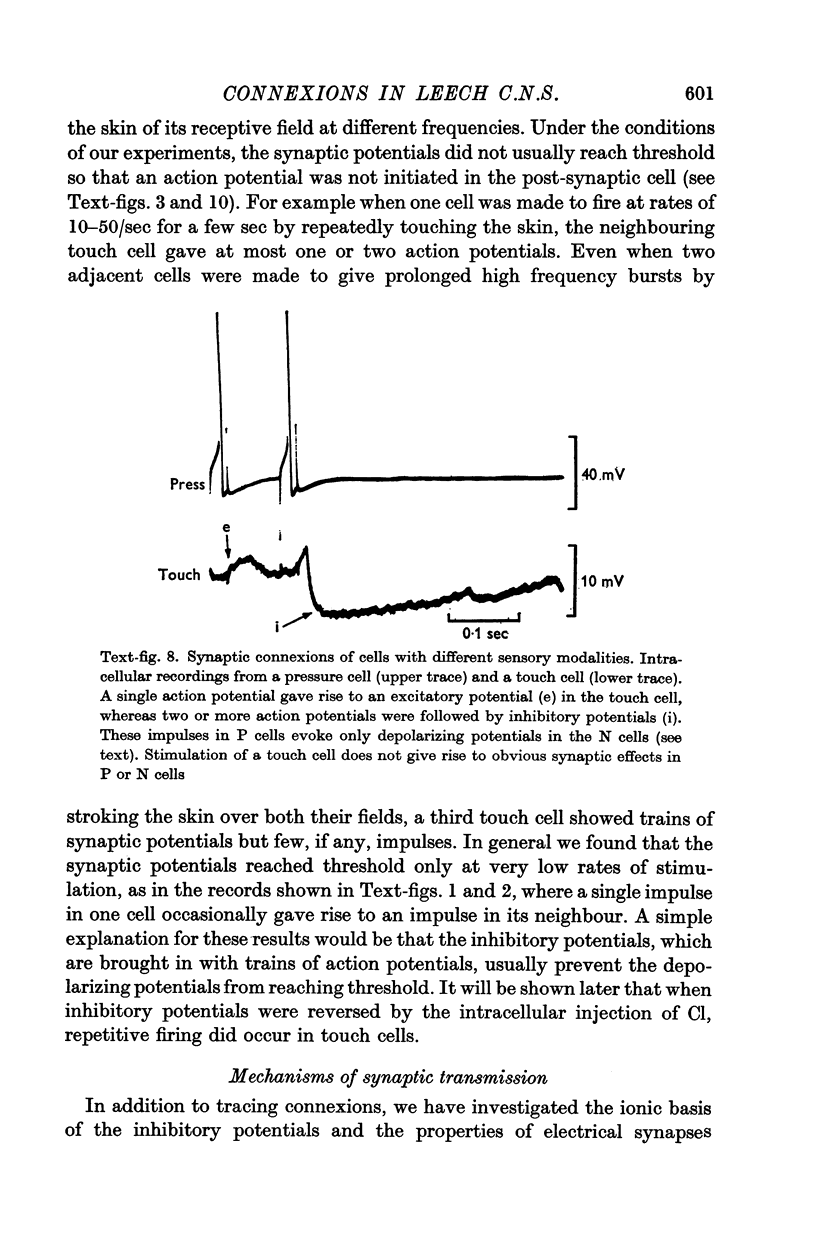
5. Synaptic potentials evoked by activation of a touch cell did not usually reach threshold since excitation and inhibition tended to cancel. The connexions between touch cells that mediated the delayed excitatory and inhibitory potentials are polysynaptic; the interneurones have not yet been found but some of their connexions could be inferred from electrical recordings.
6. Action potentials in sensory cells of a different modality (responding to pressure) also initiated synaptic potentials in the same family of touch cells.
7. The possible significance for integration of these synaptic interactions between sensory cells is discussed.
Full text
PDF





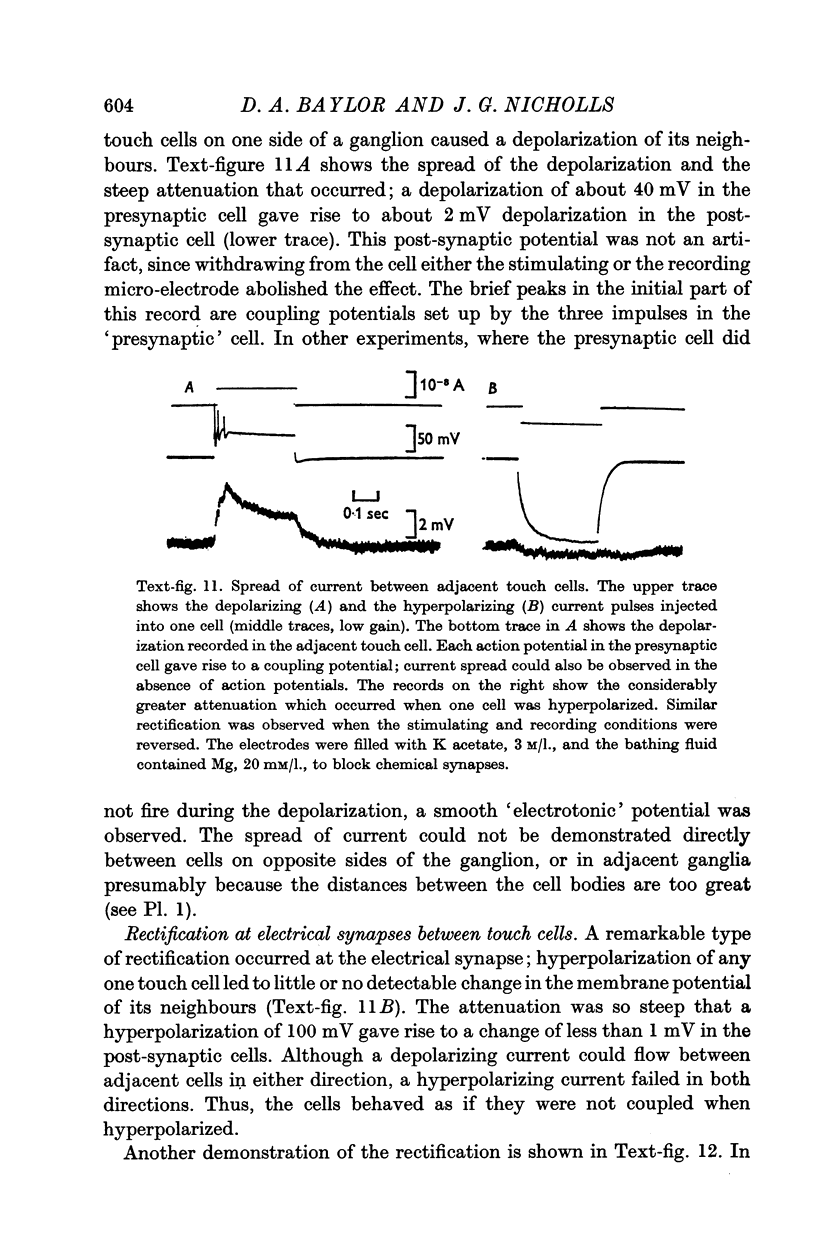





Images in this article
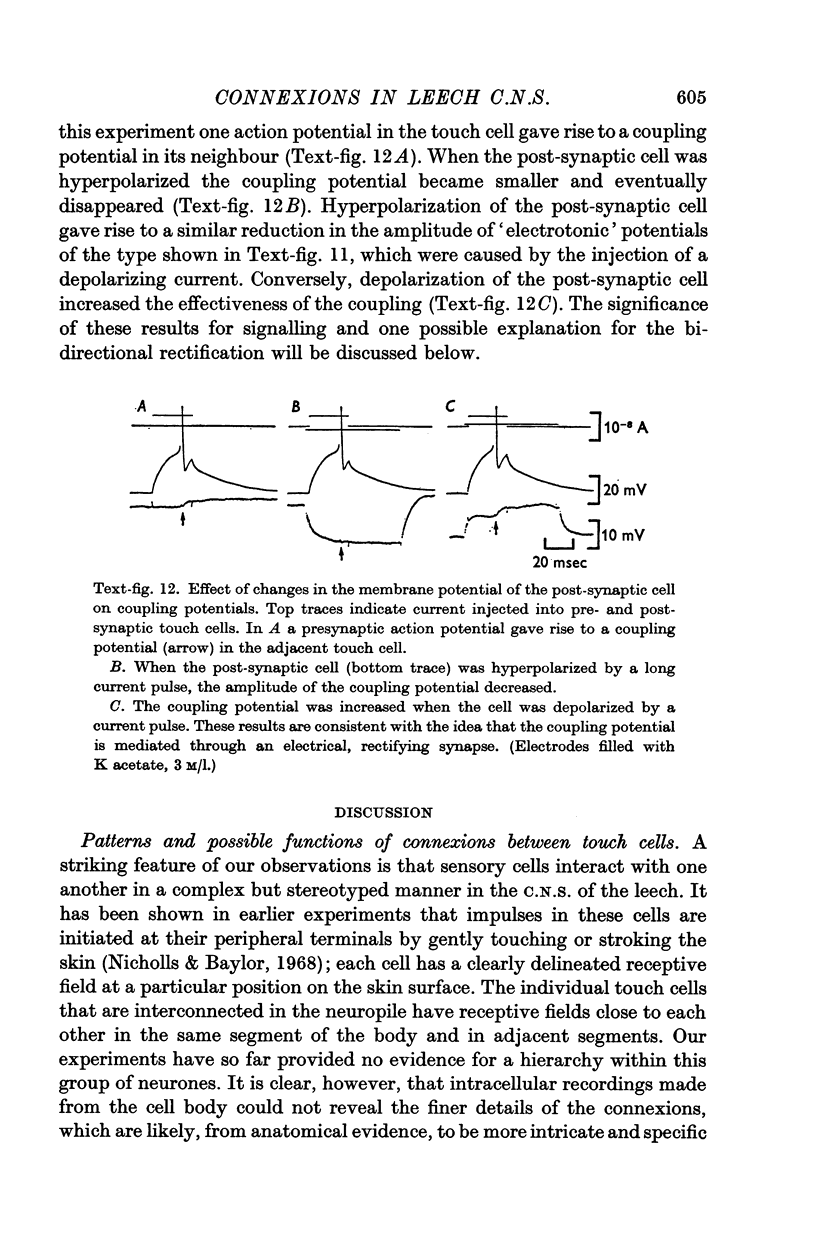
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Nicholls J. G. After-effects of nerve impulses on signalling in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):571–589. doi: 10.1113/jphysiol.1969.sp008880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsellino A., Fuortes M. G., Smith T. G. Visual responses in Limulus. Cold Spring Harb Symp Quant Biol. 1965;30:429–443. doi: 10.1101/sqb.1965.030.01.042. [DOI] [PubMed] [Google Scholar]
- COGGESHALL R. E., FAWCETT D. W. THE FINE STRUCTURE OF THE CENTRAL NERVOUS SYSTEM OF THE LEECH, HIRUDO MEDICINALIS. J Neurophysiol. 1964 Mar;27:229–289. doi: 10.1152/jn.1964.27.2.229. [DOI] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Presynaptic inhibition at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:543–562. doi: 10.1113/jphysiol.1961.sp006646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C. The mechanism of synaptic transmission. Ergeb Physiol. 1961;51:299–430. [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., MORITA H. Electrotonic transmission between two nerve cells in leech ganglion. J Neurophysiol. 1962 Nov;25:721–731. doi: 10.1152/jn.1962.25.6.721. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDELL L. M., WALL P. D. PRESYNAPTIC HYPERPOLARIZATION: A ROLE FOR FINE AFFERENT FIBRES. J Physiol. 1964 Aug;172:274–294. doi: 10.1113/jphysiol.1964.sp007417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLLS J. G., KUFFLER S. W. EXTRACELLULAR SPACE AS A PATHWAY FOR EXCHANGE BETWEEN BLOOD AND NEURONS IN THE CENTRAL NERVOUS SYSTEM OF THE LEECH: IONIC COMPOSITION OF GLIAL CELLS AND NEURONS. J Neurophysiol. 1964 Jul;27:645–671. doi: 10.1152/jn.1964.27.4.645. [DOI] [PubMed] [Google Scholar]
- Nicholls J. G., Baylor D. A. Specific modalities and receptive fields of sensory neurons in CNS of the leech. J Neurophysiol. 1968 Sep;31(5):740–756. doi: 10.1152/jn.1968.31.5.740. [DOI] [PubMed] [Google Scholar]
- Stretton A. O., Kravitz E. A. Neuronal geometry: determination with a technique of intracellular dye injection. Science. 1968 Oct 4;162(3849):132–134. doi: 10.1126/science.162.3849.132. [DOI] [PubMed] [Google Scholar]