Abstract
About 10% of the nalidixic acid-resistant (Nalr) mutants in a transposition-induced library exhibited a growth factor requirement as the result of cysH, icdA, metE, or purB mutation. Resistance in all of these mutants required a functional AcrAB-TolC efflux pump, but the EmrAB-TolC pump played no obvious role. Transcription of acrAB was increased in each type of Nalr mutant. In the icdA and purB mutants, each of the known signaling pathways appeared to be used in activating the AcrAB-TolC pump. The metabolites that accumulate upstream of the blocks caused by the mutations are hypothesized to increase the levels of the AcrAB-TolC pump, thereby removing nalidixic acid from the organism.
Quinolone antibiotics constitute one of the most widely used classes of antibacterial agent. Although the original quinolone, nalidixic acid (Nal) (Fig. 1), was effective primarily against gram-negative bacteria, fluoroquinolone derivatives are potent against a wide variety of gram-negative and gram-positive bacteria. The primary targets of the quinolones are DNA topoisomerase II (DNA gyrase) and topoisomerase IV (10, 19, 37).
FIG. 1.
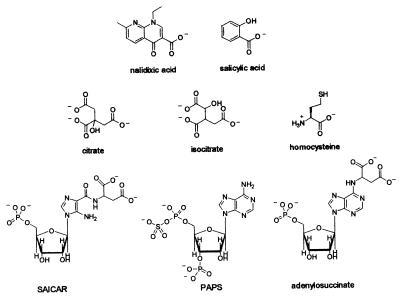
Structures of nalidixic acid and possible acrAB inducers.
Because of the therapeutic importance of these antibiotics, the appearance of bacterial mutants resistant to quinolones is of considerable concern. Many such mutants in a variety of bacteria have been characterized. These fall into two broad classes: those altered in the target topoisomerases and those failing to accumulate the drug to levels seen in the wild type. No mutant resistance stemming from the breakdown or modification of quinolones has been described.
Resistant mutants of Escherichia coli and of a wide variety of other gram-negative and gram-positive bacteria selected in the laboratory or isolated from clinical settings have been shown to be altered in one or more of the genes encoding components of the two topoisomerases (gyrA, gyrB, parC, and parE in E. coli) (34). These mutations produce resistance of various levels, and further mutations often increase the resistance (18).
Quinolone-resistant mutants containing mutations in genes other than those for the topoisomerases are also obtained frequently when only a low level of drug is used in selection or when a secondary mutation increases resistance beyond that of the primary mutation. Where the basis for such mutation has been determined, it has usually resulted from the activation of an efflux pump (31, 32) that removes the quinolone during or after entry so it does not reach a toxic level. In E. coli, activation of either the AcrAB-TolC pump (4, 9, 28) or the EmrAB-TolC pump (26) provides resistance. AcrAB-TolC appears to be the main pump providing intrinsic resistance to low levels of many toxic compounds in nature (38). Three signaling pathways controlling the expression of the acrAB genes and the formation of the AcrAB-TolC pump are known; these involve the MarAB, SoxRS, and RobA proteins (5, 11, 15, 33, 39). Thus, there are several potential ways of generating low-level resistance to quinolones through the activation of efflux pumps.
Screening mutants of E. coli and other bacteria selected on broth plates containing a low level of nalidixic acid revealed that resistance results from mutation at any one of many loci (16, 22). Unexpectedly, resistance mutations sometimes cause blocks in central biosynthetic pathways and corresponding growth factor requirements. The mutations include those in purB (adenylosuccinate lyase, adenine requirement) (16) and icdA (isocitrate dehydrogenase, glutamate requirement) (17) and others causing requirements for methionine or cysteine (17). In addition, Nalr mutants stemming from double mutations removing the dual aconitases (acnA acnB) (14) have been found (16, 22).
Why should a block in a biosynthetic pathway confer antibiotic resistance on the cell? Results from analysis of the icdA mutants provide a clue. These mutants accumulate a large quantity of citrate and isocitrate, the intermediate compounds before the block (Fig. 1). Double mutants lacking citrate synthase as well (gltA icdA) neither form these intermediates nor show the resistance to nalidixic acid of the icdA mutant (23, 24). Thus, one can hypothesize that the accumulation of the charged intermediate compounds triggers the resistance (23). purB mutants also accumulate intermediate compounds formed before the block (13); these compounds (SAICAR and adenylosuccinate; Fig. 1) resemble citrate and isocitrate in part. Thus, the resistance in purB mutants may also result from metabolite accumulation.
The association of metabolite accumulation and nalidixic acid resistance in icdA and purB mutants suggests a further hypothesis relating the two phenomena: the metabolites activate the formation of an efflux pump that removes nalidixic acid from the cell and thus prevents toxicity. In order to test this hypothesis, we isolated Nalr mutants of E. coli induced by transposon insertion, characterized the mutants, and tested the roles of the AcrAB-TolC and EmrAB-TolC pumps in the resistance.
MATERIALS AND METHODS
Strains, media, and culture conditions.
The bacterial strains used in this work are listed in Table 1. Nalr mutants were selected on HOPS medium, which contains 5 g of NaCl/liter, 16 g of agar/liter, 25 g of tryptone/liter, and 2.5 g of yeast extract/liter. After the basic medium was autoclaved, 2.5 ml of 1 M CaCl2, 1 ml of 20% glucose, and 5 ml of supplements (2 mg of adenine, 5 mg of glutamate, 5 mg of tryptophan, and antibiotics as appropriate) were added. Tryptone agar supplemented with adenine (purB) or antibiotics as necessary was used for subsequent work. When used, chloramphenicol was added to medium at 5 mg/liter (acrAB or tolC strains) or at 40 mg/liter (other strains). The same concentrations of kanamycin were used when selecting for kanamycin resistance. Correspondingly, tetracycline was used at 5 or 12 mg/liter.
TABLE 1.
Strains used
| Strain | Relevant characteristic | Source |
|---|---|---|
| RBH996 | marA::miniTn10kan | B159 from P. Miller |
| RBH1211 | Wild type, standard W3110 strain | J. R. Guest collection |
| RBH1852 | soxS3::Tn10 | BW831 from B. Weiss (40) |
| RBH1854 | As RBH1211, but from another source | E. Olson collection (25) |
| RBH1855 | As RBH1854, but ΔacrAB | B414 from E. Olson (25) |
| RBH1864 | rob::kan | RA4468 (5) from M. Bennik and B. Demple |
| RBH1868 | emrB::kan | OLS103 from J. Tomashek and K. Lewis |
| RBH1869 | tolC::Tn10 | J. Fralick (9) |
| RBH2086 | lacZ::Tn5-131 (Kns Tcs) | CE78 (6) from R. Bender |
| pNN602S | acrAB::lacZ fusion plasmid | H. Nikaido (27) |
Construction of a library of Nalr mutants.
Mutants of E. coli K-12 strain RBH1211 were induced by transposition from λNK1324 (λ Camr; obtained from U. Jacob) essentially as described previously (20). Selection was on HOPS plates containing 4 mg of Nal/liter and 40 mg of chloramphenicol/liter. After incubation overnight at 37°C, colonies were streaked onto similar medium but with 2 mg of Nal/liter, and plates were incubated overnight at 30°C. Single-colony isolates were made for further testing.
Characterization of mutants.
Mutants were streaked onto minimal glucose medium to identify growth factor requirements. Auxotrophic mutants were used as donors in transduction to RBH1854 with selection for chloramphenicol resistance. Transductants were tested for nalidixic acid resistance and growth factor requirements. Mutants in which those characters cotransduced with chloramphenicol resistance were assumed to be induced by the transposition. Transductants picked for further analysis were shown to be nonlysogenic for the viruses P1 and lambda.
The locations of the transposon insertions were determined by PCR with an rTth DNA polymerase XL kit (Applied Biosystems) according to the manufacturer's instructions. Twenty-five-nucleotide primers corresponded to the beginning and end of each open reading frame (ORF), and additional upstream primers corresponding to sequences approximately 100 nucleotides upstream of the ORF were used for insertions that did not map within the ORFs.
In order to determine the roles of the well-known AcrAB-TolC and EmrAB-TolC efflux pumps in the development of the Nal resistance, the pumps were knocked out in selected Nalr mutants. Transposon insertions were transduced from the RBH1854 derivatives into RBH1855 (acrAB, but otherwise isogenic with RBH1854) by using selection for chloramphenicol. The mutant genes tolC::Tn10 and emrB::kan were added to mutant derivatives of RBH1854 by transduction by using selection for tetracycline or kanamycin. In the same way, mutations of the signaling pathways (marA::miniTn10kan, rob::kan, and soxS3::Tn10) were transduced into derivatives of RBH1854 with selection for kanamycin or tetracycline.
Nalidixic acid resistance.
Strains were streaked onto tryptone agar with 0, 1, or 2.5 mg of nalidixic acid/liter and grown overnight at 37°C. The selected mutants grew on all three media, the wild type grew on media with 0 and 1 mg of Nal/liter, and acrAB and tolC strains grew only on medium lacking the antibiotic. For quantitative assays of resistance, diluted samples of strains grown overnight in tryptone broth at 37°C were spread on tryptone agar plates containing various amounts of Nal and plated at 37°C. Plates were counted after 24 to 40 h.
acrAB expression.
Relative transcription rates from the acrAB promoter were determined from a pacrAB-lacZ fusion in the low-copy-number plasmid pNN602S (27). This construct includes the acrAB regulator gene acrR. In order to prevent the appearance of LacZ (β-galactosidase) activity stemming from chromosomal lacZ transcription, the chromosomal lacZ+ gene was replaced with lacZ::Tn5-131 (Kns Tcr) (6) by using P1-mediated transduction with selection for tetracycline resistance.
In the expression experiments, 0.15 ml of an overnight culture in L broth (30°C) was subcultured in 20 ml of L broth at 30°C, and growth was continued with shaking at 30°C for approximately 2 h until harvest and LacZ assay essentially as described previously (27). Supplements (ethanol to 4%, lactose to 0.2%, nalidixic acid to 2 or 5 mg/liter) were added as appropriate 40 min after subculturing.
RESULTS
Isolation and characterization of Nalr mutants induced by transposon insertion.
From two experiments, a total of 260 Nalr mutants were recovered after plating organisms infected with λCamr on medium containing nalidixic acid (to select the mutants) plus chloramphenicol (to select organisms containing the Camr transposon) as described in Materials and Methods. Separate selection on plates containing chloramphenicol but not nalidixic acid showed that this corresponded to approximately 46,000 total organisms with transposon insertion, or a Nalr mutant per insertion frequency of 0.58%, assuming a single transposition per organism.
Single-colony isolates of each mutant were tested for growth on minimal agar medium containing glucose as the sole carbon and energy source. Of the total, 10 required glutamate for growth (icdA mutants), 13 required adenine (purB), 1 required cysteine, and 1 had an unidentified growth factor requirement satisfied by Casamino Acids. None required methionine, although methionine-requiring mutants were isolated in other screens. Thus, a total of 25 mutants exhibited a growth factor requirement (10%), consistent with a previous report of 2 to 40% auxotrophs among spontaneous mutants (16). None of the mutants showed high-level resistance to nalidixic acid (20 mg/liter).
In order to determine whether the nalidixic acid resistance and growth alterations were caused by a separate mutation rather than by the transposon insertion, we transduced Camr from some original isolates to a wild-type recipient by using selection on tryptone agar containing chloramphenicol. After single-colony isolation, we scored the transductants for Nalr and for growth properties.
In summary, 10 of 10 icdA mutations cotransduced with the Camr insertion and with Nalr, and 4 of 6 purB mutations cotransduced with Camr and with Nalr. (In the other two cases, neither purB nor Nalr cotransduced with Camr.) Nalr cotransduced with Camr in 10 of 26 original isolates that showed no growth factor requirement. Thus, most of the Nalr auxotrophs were caused by transposon insertion, but many of the original Nalr isolates showing normal growth were caused by mutation other than the insertion of the transposon. The locations of the transposon insertions were verified by PCR for two icdA and two purB mutants. For each type of mutant (icdA and purB), one insertion was within the ORF and one was immediately upstream.
The cysteine-requiring mutant responded to cysteine, sulfide, or sulfite, and so it was blocked in the reduction of sulfate to sulfite (21, 30). PCR showed that the transposon insertion was within the cysH gene encoding 3′-phosphoadenosine 5′-phosphosulfate (PAPS) sulfotransferase. A methionine-requiring mutant isolated earlier when testing the procedure responded to vitamin B12, as well, but not to homocysteine-thiolactone. This pinpointed the mutational defect to the last step in methionine synthesis, the formation of methionine by homocysteine methyltransferase, which is the product of the gene metE. PCR verified that the transposon insertion was within metE.
Characterizing the role of the AcrAB-TolC and EmrAB-TolC efflux pumps in resistance.
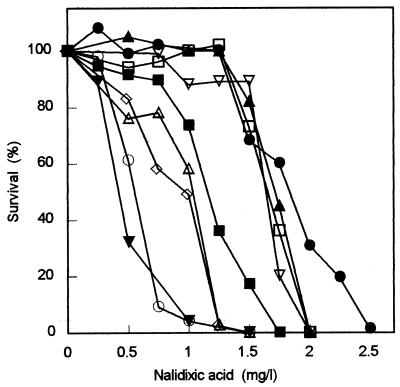
In order to see if the resistance of our mutants to Nal was associated with its efflux through one of these pumps, we modified the Nalr mutants so that they also contained acrAB, tolC, or emrB mutations (see Materials and Methods). Streaking onto tryptone agar containing nalidixic acid showed that emrB::kan had no obvious effect on the Nal resistance of icdA (five tested), purB (two tested), metE, or cysH mutants. However, each of the same mutants became Nal sensitive when the organism was deficient in the AcrAB-TolC pump because of defective AcrAB (ΔacrAB) or TolC (tolC::Tn10) (as shown for icdA derivatives by quantitative assay in Fig. 2). We conclude that in each of the mutants, the AcrAB-TolC pump must be functional in order to express Nal resistance but that EmrAB-TolC plays no obvious role.
FIG. 2.
Role of the AcrAB-TolC efflux pump and its activating pathways in the development of resistance of icdA mutants to nalidixic acid. •, icdA RH1854; ▪, wild-type (RH1854); ▾, ΔacrAB (RBH1855); ○, icdA ΔacrAB (icdA RBH1855). Other strains are derivatives of icdA RBH1854. Additional mutations are marA (▴), rob (▿), soxS (□), mar sox (⋄), and rob sox (▵).
Three signaling pathways for activating the AcrAB-TolC pump have been characterized (see the introduction). In order to determine which of the three, if any, were responsible for triggering drug resistance in our mutants, we inserted mutations inactivating each signal pathway (soxS3::Tn10, rob::kan, or marA::kan) separately into icdA and purB mutants. To our surprise, streak tests showed no diminution in resistance when any one signaling pathway was knocked out in these mutants. Quantitative assays supported the results from the streak tests (Fig. 2). These results suggested that either an unknown activation pathway was used or more than one of the known pathways triggered resistance in the mutants.
In order to determine which possibility was correct, we deleted pairs of the signaling pathways from the same icdA and purB mutants to see if resistance was lost. Streaking onto Nal-containing medium showed that for each Nalr mutant, the loss of either MarA and SoxS or Rob and SoxS prevented the development of resistance. Quantitative assays with an icdA derivative show the results clearly (Fig. 2). Thus, it appears that each signaling pathway (MarAB, Rob, and SoxRS) has a role in the development of resistance to nalidixic acid in the mutants tested. When a single path is inactivated, a small diminution in resistance may be seen via the quantitative assay, but it is too small to detect by streaking.
acrAB expression.
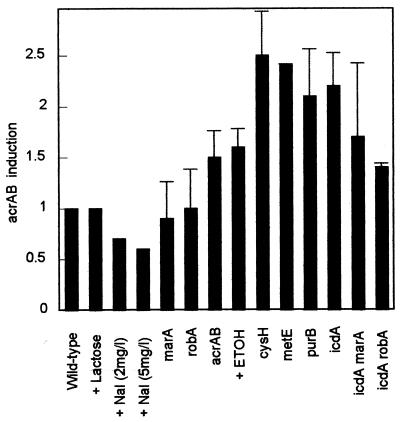
In order to see whether the activation of the acrAB-tolC pump implied by the above results stemmed from increased transcription, we measured relative transcription rates by using a pacrAB-lacZ fusion. The results (Fig. 3) show clearly that transcription from the acrAB promoter in each Nalr mutant was increased to over twice the level in the wild-type organism. Ethanol is known to induce the acrAB operon (27) and so served as a positive control. As expected, lactose did not affect transcription of the lacZ gene fused to the acrAB promoter. Expression was decreased by nalidixic acid. The lack of a functional chromosomal acrAB locus resulted in greater transcription from the acrAB promoter of the plasmid. The increased acrAB transcription in the icdA mutant was reduced by marA or robA mutation but was still greater than that in the wild type, consistent with the results shown in Fig. 2.
FIG. 3.
Increased acrAB transcription in Nalr mutants. β-Galactosidase activity from the acrAB-lacZ fusion in different mutants or after different treatments is expressed relative to that in the wild-type organism. Standard error bars are shown, except for the lactose or nalidixic acid additions, which show the results from single experiments.
DISCUSSION
These results show that in each of the mutants tested (cysH, icdA, metE, and purB), the expression of Nal resistance required the AcrAB-TolC efflux pump but not the EmrAB-TolC pump. In each type of mutant, expression from the acrAB promoter was increased. Each of the known signaling pathways for activating the expression of the acrAB genes played a role in developing the resistance in the two types of mutant examined in more detail (icdA and purB). When only a single signaling pathway was available, the resistance level was intermediate between that expressed by the wild type and that of the acrAB mutant. When any pair of pathways was available, the resistance level was slightly below that of an icdA mutant with all three pathways intact (Fig. 2), and acrAB transcription was correspondingly affected in the signaling mutants examined (marA and robA).
Comparison of the structures of the intermediate compounds formed before the metabolic block in metE (8, 36), icdA (23), and purB (13) mutants (Fig. 1) shows that each has at least one carboxylate. The well-studied AcrAB-TolC inducer, salicylate, also has a carboxylate (Fig. 1). Salicylate is known to induce the AcrAB-TolC efflux pump by at least two mechanisms, one of which involves Mar, the other(s) being unknown but not SoxRS (7). The repressor of marRAB, MarR, binds at the marRAB promoter; binding is antagonized in vitro by salicylate and some other inducers of the Mar pathway but not by inducers such as tetracycline (1, 2, 3, 29).
cysH mutants are thought to accumulate PAPS (21, 30); PAPS does not contain a carboxylate but is highly charged as a consequence of the sulfate and phosphate groups (Fig. 1). It seems likely that the anionic nature of the compounds is important to their inducing activity. However, structural studies with another multidrug-binding protein, QacR, show that a single regulatory protein can bind structurally diverse inducers in different ways (35).
As noted in the introduction, icdA mutants that also lack citrate synthase (gltA) are Nals. In contrast, mutants deficient in both aconitases and also in citrate synthase remain resistant to the drug (14), contrary to what is expected if citrate is not formed. However, recent work suggests that in these triple mutants, some citrate is formed by an alternative enzyme to citrate synthase, 2-methylcitrate synthase, and then trapped without further metabolism because of a lack of aconitase (12). Citrate is also formed in small amounts by an unknown pathway (presumably 2-methylcitrate synthase) in icdA gltA double mutants (23). However, in those strains the citrate does not form a pool large enough to induce AcrAB-TolC, probably because it is converted by aconitase to isocitrate, which then drains through the glyoxylate pathway (24). This route for further metabolism of citrate is not available in the acnA acnB gltA triple mutants.
Why should the internal metabolites induce an efflux pathway? Is it an accident or the product of long-term natural selection? We suggest that the AcrAB-TolC pump provides a means for damping infrequent short-term accumulations of charged metabolites that are inhibitory in high concentrations. By this reasoning, the charged intermediate compounds accumulating in the mutants are inducers of the pump that removes them from the organism if their concentrations become high as the result of temporary surges. The residual activity of the AcrAB-TolC pump would reflect the titration of the sum of the pools of the internal inducers. If true, the affinity or capacity of the pump for some inducers must be relatively low, because the complete blockage of the pathways in icdA and purB mutants results in large internal pools of the metabolites. Fortuitously, nalidixic acid is a substrate for the pump, and so activation by metabolite accumulation provides resistance against the antibiotic.
We envisage four roles for efflux pumps. Their best-known role is in the removal of toxins entering from outside. Other pumps remove end products of metabolism, for example, fermentation end products and toxins directed against other organisms. Although the pumps usually require an input of energy, some may conserve or capture potential energy. Finally, as suggested by these studies, pumps may serve to buffer the organism against infrequent surges in pools of potentially toxic normal metabolites. It will be interesting to learn which of these functions is primitive.
Acknowledgments
We thank those who donated strains and R. Bender, T. Goss, A. Bird, M. Sheehan, R. Liu, and C. Emal for their help.
The work was supported in part by NIH grant GM47156.
REFERENCES
- 1.Alekshun, M. N., and S. B. Levy. 1999. Alteration of the repressor activity of MarR, the negative regulator of the Escherichia coli marRAB locus, by multiple chemicals in vitro. J. Bacteriol. 181:4669-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alekshun, M. N., and S. B. Levy. 1999. The mar regulon: multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 7:410-413. [DOI] [PubMed] [Google Scholar]
- 3.Alekshun, M. N., S. B. Levy, T. R. Mealy, B. A. Seaton, and J. F. Head. 2001. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 A resolution. Nat. Struct. Biol. 8:710-714. [DOI] [PubMed] [Google Scholar]
- 4.Aono, R., N. Tsukagoshi, and M. Yamamoto. 1998. Involvement of outer membrane protein TolC, a possible member of the mar-sox regulon, in maintenance and improvement of organic solvent tolerance of Escherichia coli K-12. J. Bacteriol. 180:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ariza, R. R., Z. Li, N. Ringstad, and B. Demple. 1995. Activation of multiple antibiotic resistance and binding of stress-inducible promoters by Escherichia coli Rob protein. J. Bacteriol. 177:1655-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg, D. E., C. Egner, B. J. Hirschel, J. Howard, L. Johnsrud, R. A. Jorgensen, and T. D. Tlsty. 1980. Insertion, excision and inversion of Tn5. Cold Spring Harbor Symp. Quant. Biol. 45:115-123. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, S. P., S. B. Levy, J. Foulds, and J. L. Rosner. 1993. Salicylate induction of antibiotic resistance in Escherichia coli: activation of the mar operon and a mar-independent pathway. J. Bacteriol. 175:7856-7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, B. D., and E. S. Mingioli. 1950. Mutants of Escherichia coli requiring methionine or vitamin B12. J. Bacteriol. 60:17-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fralick, J. A. 1996. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J. Bacteriol. 178:5803-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gellert, M., K. Mizuuchi, M. H. O'Dea, T. Itoh, and J. I. Tomizawa. 1977. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc. Natl. Acad. Sci. USA 74:4772-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George, A. M., and S. B. Levy. 1983. Amplifiable resistance to tetracycline, chlorampenicol, and other antibiotics in Escherichia coli: involvement of a non-plasmid-encoded efflux of tetracycline. J. Bacteriol. 155:531-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerike, U., D. W. Hough, N. J. Russell, M. L. Dyall-Smith, and M. J. Danson. 1998. Citrate synthase and 2-methylcitrate synthase: structural, functional and evolutionary relationships. Microbiology 144:929-935. [DOI] [PubMed] [Google Scholar]
- 13.Gots, J. S., and E. G. Gollub. 1957. Sequential blockage in adenine biosynthesis by genetic loss of an apparent bifunctional deacylase. Proc. Natl. Acad. Sci. USA 43:826-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruer, M. J., A. J. Bradbury, and J. R. Guest. 1997. Construction and properties of aconitase mutants of Escherichia coli. Microbiology 143:1837-1846. [DOI] [PubMed] [Google Scholar]
- 15.Hachler, H., S. P. Cohen, and S. B. Levy. 1991. marA, a regulated locus which controls expression of chromosomal multiple antibiotic resistance in Escherichia coli. J. Bacteriol. 173:5532-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helling, R. B., and B. S. Adams. 1970. Nalidixic acid-resistant auxotrophs of Escherichia coli. J. Bacteriol. 104:1027-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helling, R. B., and J. S. Kukora. 1971. Nalidixic acid-resistant mutants of Escherichia coli deficient in isocitrate dehydrogenase. J. Bacteriol. 105:1224-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kern, W. V., M. Oethinger, A. S. Jellen-Ritter, and S. B. Levy. 2000. Non-target gene mutations in the development of fluoroquinolone resistance in Escherichia coli. Antimicrob. Agents Chemother. 44:814-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khodursky, A. B., E. L. Zechiedrich, and N. R. Cozzarelli. 1995. Topoisomerase IV is a target of quinolones in Escherichia coli. Proc. Natl. Acad. Sci. USA 92:11801-11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleckner, N., J. Bender, and S. Gottesman. 1991. Uses of transposons with emphasis on Tn10. Methods Enzymol. 204:139-180. [DOI] [PubMed] [Google Scholar]
- 21.Kredich, N. M. 1996. Biosynthesis of cysteine, p. 514-527. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, and B. Magasanik (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 22.Kumar, S. 1980. Types of spontaneous nalidixic acid resistant mutants of Escherichia coli. Indian J. Exp. Biol. 18:341-343. [PubMed] [Google Scholar]
- 23.Lakshmi, T. M., and R. B. Helling. 1976. Selection for citrate synthase deficiency in icd mutants of Escherichia coli. J. Bacteriol. 127:76-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakshmi, T. M., and R. B. Helling. 1978. Acetate metabolism in Escherichia coli. Can. J. Microbiol. 24:149-153. [DOI] [PubMed] [Google Scholar]
- 25.Liu, J. Y., P. F. Miller, M. Gosink, and E. R. Olson. 1999. The identification of a new family of sugar efflux pumps in Escherichia coli. Mol. Microbiol. 27:1845-1851. [DOI] [PubMed] [Google Scholar]
- 26.Lomovskaya, O., and K. Lewis. 1992. emr, an Escherichia coli locus for multidrug resistance. Proc. Natl. Acad. Sci. USA 89:8938-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma, D., M. Alberti, C. Lynch, H. Nikaido, and J. E. Hearst. 1996. The local repressor AcrR plays a modulating role in the regulation of acrAB genes of Escherichia coli by global stress signals. Mol. Microbiol. 19:101-112. [DOI] [PubMed] [Google Scholar]
- 28.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 16:45-55. [DOI] [PubMed] [Google Scholar]
- 29.Martin, R. G., and J. L. Rosner. 1995. Binding of purified multiple antibiotic-resistance repressor protein (MarR) to mar operator sequences. Proc. Natl. Acad. Sci. USA 92:5456-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuwald, A. F., B. R. Krishnan, I. Brikum, S. Kulakauskas, K. Suziedelis, T. Tomcsanyi, T. S. Leyh, and D. E. Berg. 1992. cysQ, a gene needed for cysteine synthesis in Escherichia coli K-12 only during aerobic growth. J. Bacteriol. 174:415-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikaido, H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 178:5853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikaido, H. 1999. Microdermatology: cell surface in the interaction of microbes with the external world. J. Bacteriol. 181:4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okusu, H., D. Ma, and H. Nikaido. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistant (Mar) mutants. J. Bacteriol. 178:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piddock, L. J. V. 1999. Mechanisms of fluoroquinolone resistance: an update, 1994-1998. Drugs 58(Suppl. 2):11-18. [DOI] [PubMed] [Google Scholar]
- 35.Schumacher, M. A., M. C. Miller, S. Grkovic, M. H. Brown, R. A. Skurray, and R. G. Brennan. 2001. Structural mechanisms of QacR induction and multidrug recognition. Science 294:2158-2163. [DOI] [PubMed] [Google Scholar]
- 36.Smith, D. A. 1961. Some aspects of the genetics of methionineless mutants of Salmonella typhimurium. J. Gen. Microbiol. 24:335-353. [Google Scholar]
- 37.Sugino, A., C. L. Peebles, K. N. Kreuzer, and N. R. Cozzarelli. 1977. Mechanisms of action of nalidixic acid. Purification of Escherichia coli nalA gene product and its relationship to DNA gyrase, a novel nicking-closing enzyme. Proc. Natl. Acad. Sci. USA 74:4767-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sulavik, M. C., C. Houseweart, C. Cramer, N. Jiwani, N. Murgolo, J. Greene, B. DiDomenico, K. J. Shaw, G. H. Miller, R. Hare, and G. Shimer. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob. Agents Chemother. 45:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White, D. G., J. D. Goldman, B. Demple, and S. B. Levy. 1997. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli. J. Bacteriol. 179:6122-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu, J., and B. Weiss. 1992. Two-stage induction of the soxRS (superoxide response) regulon of Escherichia coli. J. Bacteriol. 174:3915-3920. [DOI] [PMC free article] [PubMed] [Google Scholar]