Abstract
Ralstonia eutropha JMP134 can grow on several chlorinated aromatic pollutants, including 2,4-dichlorophenoxyacetate and 2,4,6-trichlorophenol (2,4,6-TCP). Although a 2,4,6-TCP degradation pathway in JMP134 has been proposed, the enzymes and genes responsible for 2,4,6-TCP degradation have not been characterized. In this study, we found that 2,4,6-TCP degradation by JMP134 was inducible by 2,4,6-TCP and subject to catabolic repression by glutamate. We detected 2,4,6-TCP-degrading activities in JMP134 cell extracts. Our partial purification and initial characterization of the enzyme indicated that a reduced flavin adenine dinucleotide (FADH2)-utilizing monooxygenase converted 2,4,6-TCP to 6-chlorohydroxyquinol (6-CHQ). The finding directed us to PCR amplify a 3.2-kb fragment containing a gene cluster (tcpABC) from JMP134 by using primers designed from conserved regions of FADH2-utilizing monooxygenases and hydroxyquinol 1,2-dioxygenases. Sequence analysis indicated that tcpA, tcpB, and tcpC encoded an FADH2-utilizing monooxygenase, a probable flavin reductase, and a 6-CHQ 1,2-dioxygenase, respectively. The three genes were individually inactivated in JMP134. The tcpA mutant failed to degrade 2,4,6-TCP, while both tcpB and tcpC mutants degraded 2,4,6-TCP to an oxidized product of 6-CHQ. Insertional inactivation of tcpB may have led to a polar effect on downstream tcpC, and this probably resulted in the accumulation of the oxidized form of 6-CHQ. For further characterization, TcpA was produced, purified, and shown to transform 2,4,6-TCP to 6-CHQ when FADH2 was supplied by an Escherichia coli flavin reductase. TcpC produced in E. coli oxidized 6-CHQ to 2-chloromaleylacetate. Thus, our data suggest that JMP134 transforms 2,4,6-TCP to 2-chloromaleylacetate by TcpA and TcpC. Sequence analysis suggests that tcpB may function as an FAD reductase, but experimental data did not support this hypothesis. The function of TcpB remains unknown.
2,4,6-Trichlorophenol (2,4,6-TCP) and other polychlorophenols have been used as wood preservatives for decades around the world, resulting in severe groundwater contamination around sawmills (24, 32). In addition, polychlorophenol derivatives are often used as herbicides and fungicides (5, 15). The corresponding polychlorophenols are often metabolic intermediates during the biodegradation of these derivatives. For example, 2,4,5-TCP is the first metabolic intermediate of 2,4,5-trichlorophenoxyacetate degradation by Burkholderia cepacia AC1100 (23, 50), and 2,4,6-TCP is produced during prochloraz degradation by Aureobacterium sp. strain C964 (6). Several 2,4,6-TCP-degrading bacteria have been reported (1, 2, 6, 9, 10, 25, 30, 42, 45). Recently, Clement et al. (10) have shown that Ralstonia eutropha JMP134 and JMP222 (which does not harbor the 2,4-dichlorophenoxyacetic acid-degrading plasmid pJP4) grow on 2,4,6-TCP as the sole carbon source. The reported cell yields for JMP134 and JMP222 growing on only 2,4,6-TCP are extremely low, and the culture's turbidity at 660 nm reaches a maximum of 0.043 after 5 days of incubation (10). The detection of 2,6-dichloro-p-hydroquinone (2,6-DiCH) during 2,4,6-TCP degradation by JMP134 whole cells and the identification of 6-chlorohydroxyquinol (6-CHQ) 1,2-dioxygenase activities in JMP134 cell extracts have led to a hypothesis that 2,4,6-TCP is degraded via 2,6-DiCH to 6-CHQ, which is subject to ring cleavage to 2-chloromaleylacetate (2-CMA) (37). However, the enzymes that convert 2,4,6-TCP to 2,6-DiCH and then to 6-CHQ are not detectable in the cell extracts (37). Thus, the enzymes and genes responsible for the pathway have not been characterized. We report here the optimization of growth conditions for JMP134 to degrade 2,4,6-TCP, the identification of 2,4,6-TCP monooxygenase activities in JMP134 cell extracts, the purification and characterization of a reduced flavin adenine dinucleotide (FADH2)-utilizing TCP monooxygenase, and the cloning and functional characterization of a gene cluster (tcpABC) responsible for converting 2,4,6-TCP to 2-CMA in JMP134.
MATERIALS AND METHODS
Materials.
All reagents used were of the highest purity available and were purchased from Sigma Chemical Co. (St. Louis, Mo.), Aldrich Chemical Co. (Milwaukee, Wis.), or Fisher Scientific Co. (Pittsburgh, Pa.). 2,6-DiCH was freshly prepared by reducing 2,6-dichloro-p-benzoquinone with 1 mM ascorbic acid. 6-CHQ was a gift from R. Crawford (University of Idaho). All PCRs were performed with Taq DNA polymerase and primers purchased from Gibco BRL (Gaithersburg, Md.). Restriction endonucleases and DNA-modifying enzymes were purchased from Gibco BRL and New England Biolabs (Beverly, Mass.).
Bacterial strains, culture conditions, and plasmids.
R. eutropha JMP134 was grown at 30°C in a mineral salt medium consisting of (per liter of deionized water) 0.58 g of K2HPO4, 0.19 g of KH2PO4, 0.25 g of NaNO3, 0.1 g of MgSO4 · 7H2O, and 1 ml of a trace element solution. The trace element solution contained 10 ml of concentrated HCl per liter and consisted of the following: MgSO4, 10 g/liter; CaCO3, 2 g/liter; FeSO4 · 7H2O, 4.5 g/liter; ZnSO4 · 7H2O, 1.44 g/liter; MnSO4 · 4H2O, 1.12 g/liter; CuSO4 · 5H2O, 0.25 g/liter; CoSO4 · 7H2O, 0.25 g/liter; and H3BO3, 0.06 g/liter. The carbon source was 0.2 to 0.5% (wt/vol) monosodium glutamate with various amounts of 2,4,6-TCP. Escherichia coli strains Nova Blue and BL21(DE3) were used as the hosts for pET30 LIC clones (Novagen, Madison, Wis.), and strain TOPO10 was used to host plasmids constructed from the TA cloning vectors pCR2.1 and pCR2.1-TOPO (Invitrogen, Carlsbad, Calif.), which were also used as suicidal plasmids for JMP134. All E. coli strains were routinely grown at 37°C in Luria-Bertani (LB) medium or on LB agar (40), except BL21(DE3) was cultured at 24°C when used to produce functional enzymes. Kanamycin was used at 30 μg · ml−1 in culture media.
Partial purification of 2,4,6-TCP monooxygenase from R. eutropha JMP134.
All purification steps were performed at 4°C. The cells were harvested from 3 liters of culture and suspended in 20 ml of 20 mM potassium phosphate (KPi) buffer (pH 7.0) containing 1 mM EDTA. The protease inhibitor phenylmethylsulfonyl fluoride freshly prepared in absolute ethanol was added to a final concentration of 0.5 mM. The cells were disrupted by passing through a French pressure cell model FA-030 (Aminco, Urbana, Ill.) three times at 260 MPa. The lysate was centrifuged at 17,000 × g for 10 min to remove cell debris and unbroken cells. Ammonium sulfate was added to the supernatant to 30% saturation with constant stirring. The mixture was centrifuged at 17,000 × g for 10 min, and the pellet was discarded. Additional ammonium sulfate was added to the supernatant to 70% saturation with constant stirring. The mixture was then centrifuged at 17,000 × g for 10 min, and the pellet was saved. The pellet was dissolved in 5 ml of 20 mM KPi buffer (pH 7.0) with 25% saturation of ammonium sulfate and loaded onto a phenyl agarose (Sigma) column (1.5 by 12.5 cm) equilibrated with the same buffer. The proteins were eluted with a linear gradient of ammonium sulfate (25 to 0%, 100 ml) in the KPi buffer with 1 mM dithiothreitol (DTT) at a flow rate of 1 ml · min−1. Individual fractions were collected, and solid ammonium sulfate was added to each fraction to 70% saturation. The samples were centrifuged, and the pellets were resuspended in 20 mM KPi (pH 7.0) buffer containing 0.5 mM DTT and dialyzed against the same buffer for 2 h. 2,4,6-TCP-degrading activities in each fraction were analyzed.
Cloning of a tcp gene cluster.
The sequences of four hydroxyquinol (HQ) 1,2-dioxygenases are available from GenBank: TftH from B. cepacia AC1100 involved in 2,4,5-trichlorophenoxyacetate degradation (12), HadC from Ralstonia (formerly Burkholderia) pickettii DTP0602 involved in 2,4,6-TCP degradation (42), DxnF from Sphingomonas sp. strain RW1 involved in dibenzo-p-dioxin degradation (3), and an HQ 1,2-dioxygenase from Arthrobacter sp. strain BA-5-17 (35). The amino acid sequences of the enzymes were aligned by using PILEUP, a GCG software program (Genetics Computer Group, Madison, Wis.) provided by the VADMS Center at our university. Using the conserved regions from the N termini (Q84EFILLS) and the C termini (D264AVFGVR), primers HQF and HQR (Table 1) were designed and synthesized. The primers and JMP134 DNA were used in a PCR with a thermal profile of 30 s at 94°C, 30 s at 55°C, and 1 min at 72°C for 30 cycles. A single PCR product of 541 bp matching the predicted size was produced. The PCR product was cloned into pCR2.1 (Invitrogen), forming plasmid pKOC, and the insert was sequenced. Sequence analysis of FADH2-utilizing monooxygenases has suggested that two previously reported chlorophenol 4-monooxygenases (TftD and HadA) are FADH2-utilizing monooxygenases (13, 18, 49). The two chlorophenol 4-monooxygenases are 61.4% identical in amino acid sequences. Using the conserved regions from the N termini (Q7YLESLND) and C termini (F441ENFNGT), degenerate primers TftDF1 and TftDR1 (Table 1) were designed and synthesized. The primers and JMP134 DNA were used in PCR as described above. A single PCR product of 1.3 kb matching the predicted size was produced and directly sequenced with the two primers. Primers TftDRcr and HQOFcr (Table 1) were used in PCR amplification of the DNA sequence between the FADH2-utilizing monooxygenase gene and HQ 1,2-dioxygenase gene. A single PCR product of 1.8 kb was produced. The product was directly sequenced with the two primers.
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequencea | Positions of primers (GenBank accession no. AF498371) |
|---|---|---|
| TftDF1b | 5′-AGTACCTGGAGTC(G/C)CT(G/C)AACGAC-3′ | 213-235 |
| TftDR1b | 5′-CGG(C/G)GT(C/G)CCGTTGAATTCTCGAA-3′ | 1543-1520 |
| HQFb | 5′-AGGAGTTCATCCTGCT(G/C)(A/T)G-3′ | 2623-2641 |
| HQRb | 5′-CGCAC(G/C)CCGAACAC(A/T)GCGTC-3′ | 3163-3144 |
| TftDRcr | 5′-ATCGGCACCAACAAGATC-3′ | 1136-1153 |
| HQOFcr | 5′-AGGAATCGGATAGGCCTG-3′ | 2999-2982 |
| TftDRf | 5′-CTTGTTGGTGCCGATATG-3′ | 1150-1133 |
| TftDFcr | 5′-TGTTCCTTGTCGTAGTGG-3′ | 429-412 |
| TcpABC5 | 5′-GGAGTCCAGTGATGCAAG-3′ | 2407-2424 |
| TcpF6 | 5′-ATGACGTGCAGTACGAAG-3′ | 2887-2904 |
| TcpAiF | 5′-CCAAGAAGCACGACCTGA-3′ | 621-638 |
| TcpABC4 | 5′-CAGCCCGGCTGGTGTTCGT-3′ | 1952-1969 |
| TcpABC1 | 5′-AAGATAGCCGCCTTGCAG-3′ | 2205-2188 |
| TcpAF4-NdeI | 5′-AGACCTGCATATGATTCGCACTGG-3′ | 184-207 |
| TcpAR5-BamHI | 5′-GAGGCGGATCCACAGTCATCG-3′ | 1754-1734 |
| TcpF16-NdeI | 5′-GGAGTCCACATATGCAAGAGTATG-3′ | 2407-2430 |
| TcpR15 | 5′-CTTTCCGGTGCTAGAGGGTTGG-3′ | 3258-3237 |
| TcpF2 | 5′-TCCGCATGCTGACTATGC-3′ | 1554-1571 |
Boldface indicates changes from template DNA for introduction of restriction sites (underlined).
Degenerate primer.
Thermal asymmetric interlaced PCR (TAIL-PCR) (33) was used to amplify the 5′-end sequence of tcpA and the 3′-end sequence of tcpC. Two nested tcpA-specific primers, TftDRf and TftDFcr (Table 1), and three random primers were used to amplify the 5′ upstream region of tcpA. Two nested tcpC-specific primers, TcpABC5 and TcpF6 (Table 1), and three random primers were used to amplify the 3′ downstream region of the tcpC. After two successive rounds of PCR, the products of the first and second PCRs were separated on an agarose gel and compared. The products from the second PCR that showed the expected decrease in size were gel purified and sequenced. The nucleotide sequence of the complete tcp gene cluster was confirmed by direct sequencing of a single PCR product of the gene cluster.
Chromosomal disruption of tcpA, tcpB, and tcpC in R. eutropha JMP134.
A 530-bp internal fragment of tcpA and a 252-bp internal fragment of tcpB were PCR amplified from JMP134 DNA by using primer pair TcpAiF-TftDRf and primer pair TcpABC4-TcpABC1, respectively (Table 1). The PCR products were individually cloned into pCR2.1-TOPO (Invitrogen), forming plasmids pKOA and pKOB, respectively. Plasmid DNA (5 to 10 μg) of pKOA, pKOB, or pKOC (also used for initial sequencing) was independently electroporated into electrocompetent JMP134 cells prepared as follows. JMP134 cells (100 ml) grown in the mineral salt medium were harvested by centrifugation at 6,000 × g for 15 min at 4°C when the turbidity of the culture at 600 nm was between 0.5 and 0.6. The cell pellet was washed with 50 ml of ice-cold 1 M sorbitol solution, and the cells were collected by centrifugation at 6,000 × g. The washing was repeated two more times. The washed cell pellet was finally suspended in 400 μl of 1 M sorbitol, and 40 μl of cells was used in each electroporation. Recombinant strains were selected on the mineral salt agar containing kanamycin.
Expression of tcpA in E. coli and purification of recombinant TcpA.
For ease of protein purification, tcpA was cloned into the pET-30-LIC vector to yield plasmid pTcpA. The forward primer TcpAF4-NdeI and the reverse primer TcpAR5-BamHI (Table 1) were designed so that tcpA was cloned as a C-terminal His-tag fusion gene. The primers were used for PCR amplification of tcpA from JMP134 DNA. The PCR product was cut with NdeI and BamHI and then ligated into the plasmid pET30-LIC previously digested with NdeI and BamHI, producing plasmid pTcpA. DNA sequencing of pTcpA was performed to confirm the cloned tcpA gene did not have any point mutation resulted from PCR amplification. Electrocompetent E. coli BL21(DE3) cells (Novagen) were transformed by pTcpA. BL21(DE3)(pTcpA) cells (200 ml) were grown in LB medium with kanamycin at 37°C. When the turbidity of the culture at 600 nm reached 0.6, 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the culture to induce protein production at 24°C for 4 h. The cells were harvested and suspended in 6 ml of 20 mM KPi buffer (pH 7.0) containing 1 mM DTT plus 0.5 mM phenylmethylsulfonyl fluoride. The cells were disrupted by passing through a French pressure cell (model FA-030; Aminco, Urbana, Ill.) three times at 260 MPa. The lysate was centrifuged at 17,000 × g for 10 min, and the supernatant that contained 7.9 mg of protein · ml−1 was saved as cell extracts.
Recombinant TcpA in the cell extract was purified with an Ni2+-nitrilotriacetic acid (NTA)-agarose matrix according to the manufacturer's instructions (Qiagen, Valencia, Calif). Briefly, 3 ml of the cell extracts containing ca. 24 mg of protein was mixed with 1 ml of Ni2+-NTA-agarose matrix and 20 mM imidazole for 1 h at 4°C. The mixture was packed into a small column, and the column was washed with 5 ml of 20 mM KPi buffer (pH 7.0) containing 20 mM imidazole. TcpA was then eluted from the column with 5 ml of the same buffer containing 200 mM imidazole, with recovery of 7.7 mg of protein. Two milliliters of TcpA eluted from the Ni2+-NTA column (ca. 3 mg of protein) was loaded onto a Superdex 200 size exclusion column (1 by 30 cm) (Pharmacia, Alameda, Calif.). The column was eluted with an isocratic flow (0.5 ml · min−1) of 20 mM KPi buffer (pH 7.0) containing 1 mM DTT and 150 mM NaCl. Active fractions were pooled and saved.
Expression of tcpC in E. coli.
TcpC was produced in E. coli to examine its role as a functional 6-CHQ 1,2-dioxygenase. The forward primer TcpF16-NdeI and the reverse primer TcpR15 (Table 1) were used for PCR amplification of tcpC from JMP134 DNA. The PCR product was cloned into pCR2.1-TOPO (Invitrogen), forming plasmid pTA-TcpC. Plasmid pTA-TcpC was cut with NdeI and EcoRI, and the tcpC insert was ligated into the plasmid pET30-LIC previously digested with NdeI and EcoRI, producing plasmid pTcpC. DNA sequencing of pTcpC was performed to confirm cloning of tcpC. Electrocompetent E. coli BL21(DE3) cells were transformed with pTcpC. TcpC was produced in 200 ml of BL21(DE3)(pTcpC) cells under conditions identical to those for producing recombinant TcpA. The BL21(DE3)(pTcpC) cells were harvested and suspended in 10 ml of 20 mM KPi buffer (pH 7.0) containing 0.5 mM phenylmethylsulfonyl fluoride. The cells were broken by passing through the French pressure cell three times at 260 MPa. The lysate was centrifuged at 17,000 × g for 10 min, and the supernatant that contained 3.2 mg of protein · ml−1 was saved as cell extracts. Expression of TcpC was confirmed by running 32 μg of the cell extracts on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, showing ca. 25% of soluble protein was TcpC.
Enzyme assay.
The 2,4,6-TCP monooxygenase activity was assayed at 33°C by measuring the consumption of 2,4,6-TCP. A standard 40-μl assay mixture contained 20 mM KPi buffer (pH 7.0), 100 μM 2,4,6-TCP, 10 μM flavin adenine dinucleotide (FAD), 5 mM NADH, 0.4% Tween 20, 1 mM ascorbic acid, 1 U of catalase (Sigma), 9 U of a general E. coli flavin reductase (Fre) (49), and various amounts of proteins. Stock solutions (100 mM) of 2,4,6-TCP, 2,6-DiCH, and 6-CHQ were prepared in absolute ethanol. The reaction was initiated by adding NADH to the reaction mixture and terminated by adding 40 μl of acetonitrile-acetic acid mixture (9/1 [vol/vol]). The samples were centrifuged at 13,000 × g for 2 min, and the supernatants were analyzed by high-performance liquid chromatography (HPLC) (47). 6-CHQ 1,2-dioxygenase activity in cell extracts was assayed spectrophotometrically by measuring the production of 2-CMA at 253 nm (44, 52) at 30°C. The 1-ml reaction mixture contained 120 μg of protein, 100 mM KPi buffer (pH 7.0), 200 μM 6-CHQ, 1 mM freshly prepared sodium borohydride, and 10 μM FeSO4 · 7H2O. FAD reductase activities in cell extracts were determined spectrophotometrically by monitoring the oxidation of NADH (ɛ340 = 6220 M−1 · cm−1) in 20 mM KPi buffer (pH 7.0) containing 400 μM NADH and 10 μM FAD at 30°C. One unit of FAD reductase activity was defined as the oxidation of 1 nmol of NADH per min.
pH and temperature optima.
TcpA activity was measured at various pH values within the range of 5.8 to 7.8 by using 20 mM KPi buffer in a total volume of 40 μl at 33°C. The reaction mixture was the same as that described above for the enzyme assay. The temperature optimum for the enzyme activity was determined at pH 7.0 at different temperatures.
Analytical methods.
Gas chromatography-mass spectrometry (GC-MS) analysis of 2,4,6-TCP monooxygenase reaction end products was performed on a QP5050A GC-MS system (Shimadzu, Columbia, Md.) equipped with a DB-5 (30 m by 0.25 mm) capillary column (J&W Scientific, Folsom, Calif.). Samples were extracted and derivatized by a previously described method (26). Briefly, the reaction solutions were acidified to pH 4 (10 μl of concentrated HCl per ml of reaction mixture), and aromatic compounds were extracted into ethyl acetate (1:1 [vol/vol]; total volume, 2 ml). The organic phase was dried, and the remaining solid was dissolved in a 200-μl mixture of pyridine and acetic anhydride (1:3 [vol/vol]). The solution was heated at 45°C for 20 min and then analyzed directly by GC-MS at a flow rate of 0.8 ml · min−1 of helium. The oven parameters were 50°C for 3.5 min, with a 30°C · min−1 increase to final temperature of 300°C for 3 min. The injector and detector were at 250 and 300°C, respectively. The sample was analyzed with a scan interval of 0.34 s and an m/z range of 40 to 400. HPLC was used for quantification of the concentrations of 2,4,6-TCP, 2,6-DiCH, and 6-CHQ (47). Protein concentrations were determined with a protein dye reagent (8) with bovine serum albumin as a standard. SDS-PAGE was done by the method of Laemmli (27), and gels were stained for proteins with GelCode Blue (Pierce, Rockford, Ill.). Glutamate concentrations in the media were determined by a fluorometric method with o-phthaldialdehyde (16).
Nucleotide sequence accession number.
The DNA sequence obtained in this study has been added to the GenBank database (accession no. AF498371).
RESULTS
2,4,6-TCP degradation by R. eutropha JMP134 was inducible and subject to catabolic repression.
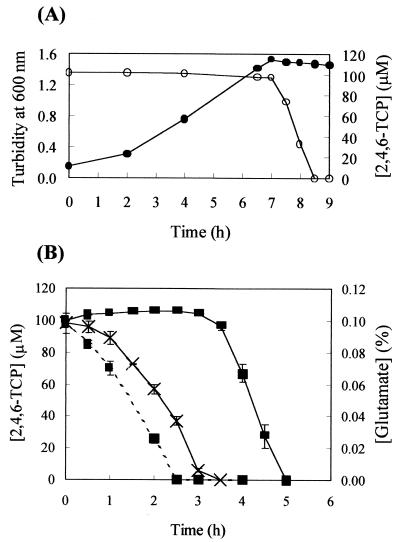
In order to study the enzymes responsible for 2,4,6-TCP degradation, we have tried to optimize the cell yield. The cell yields for JMP134 grown on 0.2% glutamate in a mineral medium with 100 μM 2,4,6-TCP were much higher than for cells grown on 2,4,6-TCP; however, 2,4,6-TCP was not degraded until the culture reached stationary phase (Fig. 1A). When growing with 0.5% glutamate, JMP134 did not degrade 2,4,6-TCP, even when it reached stationary phase. With high concentrations of glutamate, the cells did not use up glutamate, but stopped growing due to the accumulation of NH4+ and increased pH in the medium. When early stationary phase was reached, JMP134 cells grown in the mineral medium containing glutamate were harvested by centrifugation and suspended in glutamate-free mineral salt medium containing 100 μM 2,4,6-TCP. 2,4,6-TCP degradation started within 1 h (Fig. 1B). Similar cell suspensions containing 100 μM 2,4,6-TCP and 0.1% (wt/vol) glutamate immediately degraded glutamate. However, 2,4,6-TCP was not degraded until glutamate was completely consumed (Fig. 1B). The start of 2,4,6-TCP degradation lagged behind the completion of glutamate consumption by about 1 h. These results indicate 2,4,6-TCP degradation was inducible by 2,4,6-TCP and was subject to catabolic repression by glutamate.
FIG. 1.
(A) Growth (•) of JMP134 and its degradation of 2,4,6-TCP (○) in mineral salt medium with 0.2% (wt/vol) glutamate and 100 μM 2,4,6-TCP. A 10% inoculum of an overnight culture was used to start growth. (B) Effect of glutamate on induction of 2,4,6-TCP degradation in JMP134. JMP134 cultures grown on the mineral salt medium containing 0.4% (wt/vol) glutamate were harvested at early stationary phase by centrifugation. The cells were suspended to a turbidity of 1.0 at 600 nm in mineral salt medium containing either no glutamate (×) or 0.1% glutamate (▪). 2,4,6-TCP (100 μM) was added to the cell suspensions. Concentrations of 2,4,6-TCP (solid line) and glutamate (dashed line) remaining in the medium supernatant were assayed over time. The turbidity of the cell suspensions with no glutamate remained at 1.0 over the course of the experiment. The turbidity of the cell suspensions with glutamate increased from 1.0 to 1.4 and remained at 1.4 after glutamate was completely consumed. Data are means of duplicates with ranges.
Detection of 2,4,6-TCP monooxygenase activities in JMP134 cell extracts and partial purification of the enzyme.
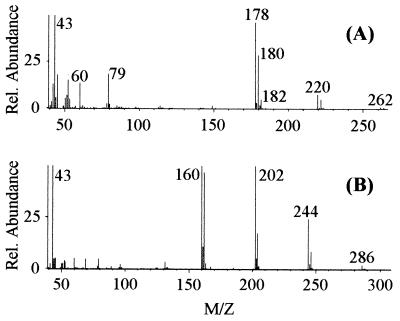
Cells growing on 0.2% glutamate to early stationary phase were induced with 250 μM 2,4,6-TCP. When 2,4,6-TCP was completely consumed, the cells were harvested and cell extracts were obtained. The extracts consumed 2,4,6-TCP in the presence of NADH at 0.64 nmol · min−1 · mg−1. On the other hand, cell extracts from glutamate-grown cells did not transform 2,4,6-TCP, further validating that the 2,4,6-TCP-degrading activity is inducible by 2,4,6-TCP. After ammonium sulfate precipitation and dialysis in 20 mM KPi buffer (pH 7.0), the dialyzed sample lost the 2,4,6-TCP-degrading activity. Addition of FAD restored the enzymatic activity. After the sample was fractionated with a phenyl agarose column, no active fractions were recovered. When Fre, an E. coli flavin reductase (17), was included in the reaction mixture together with NADH and FAD, the 2,4,6-TCP-degrading activity was detected in the fractions at around 10% saturation of ammonium sulfate. HPLC analysis showed the complete consumption of 6 nmol of 2,4,6-TCP in 10 min by 0.3 mg of partially purified cell extracts. Two compounds that had the same retention times and absorption spectra as 2,6-DiCH (0.3 nmol) and 6-CHQ (4.8 nmol) standards were produced. We included 1 mM ascorbic acid in the reaction mixture to minimize the autooxidation of 2,6-DiCH and 6-CHQ. GC-MS analysis of the acetylated reaction products detected two compounds. The retention times of the minor and major products were 10.03 and 10.65 min, respectively. The mass spectrum of the 10.03-min peak (Fig. 2A) was typical for a molecule containing two chlorines and was identified as acetylated 2,6-DiCH, with the molecular ion peak at m/z 262, and its fragments at m/z 220 (loss of —COCH3) and at m/z 178 (loss of two —COCH3). The peaks at m/z 182 (M + 4), 180 (M + 2), and 178 (M+) and their relative intensities are characteristic of a molecule containing two chlorine atoms. The relative intensities are consistent with the natural abundance of 76% for 35Cl and 24% for 37Cl. The mass spectrum of the 10.65-min peak (Fig. 2B) was typical for a molecule with one chlorine and identified as acetylated 6-CHQ with the molecular ion peak at m/z 286 and its fragments at m/z 244 (loss of —COCH3), at m/z 202 (loss of two —COCH3), and at m/z 160 (loss of all three —COCH3). The assignment of the two products as 2,6-DiCH and 6-CHQ was confirmed by GC-MS analysis of the acetylated authentic compounds. On the basis of this initial characterization, we hypothesized that R. eutropha JMP134 uses a single FADH2-utilizing monooxygenase to oxidize 2,4,6-TCP to 6-CHQ.
FIG. 2.
Mass spectra of the 2,4,6-TCP degradation products produced by partially purified 2,4,6-TCP monooxygenase from JMP134 with Fre to supply FADH2. (A) Mass spectrum of the GC peak at 10.03 min, identical to that of acetylated 2,6-DiCH. (B) Mass spectrum of the GC peak at 10.65 min, identical to that of acetylated 6-CHQ.
Cloning and sequencing of a tcp gene cluster from JMP134.
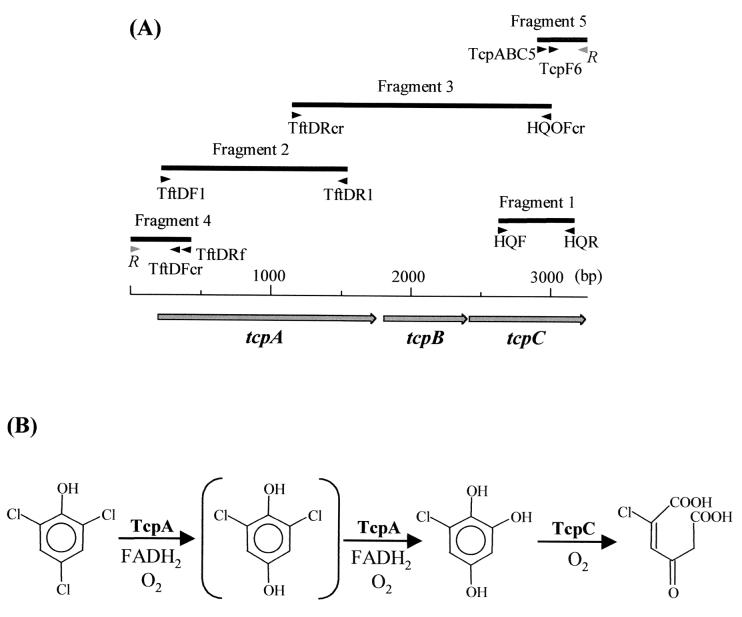
An HQ 1,2-dioxygenase activity that oxidizes 6-CHQ is detected in JMP134 cell extracts (37). We designed two degenerate primers from two highly conserved regions of four HQ 1,2-dioxygenases and amplified a single PCR product from JMP134 DNA (Fig. 3A, fragment 1). This PCR product contained a partial open reading frame (ORF) that showed 77% identity to HadC of R. pickettii (42). A similar approach amplified a 1.3-kb internal fragment of a potential TCP monooxygenase gene from JMP134 (Fig. 3A, fragment 2). The deduced amino acid sequence of this fragment was most similar to HadA of R. pickettii with 89% identity. A 1.8-kb PCR product (Fig. 3A, fragment 3) was PCR amplified from JMP134 DNA with primers located within fragments 1 and 2. This PCR product contained the 3′ end of the TCP monooxygenase gene, a complete ORF (tcpB) with deduced amino acid sequence that showed 76% identity to HadB of R. pickettii, and the 5′ end of the potential HQ 1,2-dioxygenase gene. Using TAIL-PCR, we successfully amplified the 5′ end of the TCP monooxygenase gene and the 3′ end of the HQ 1,2-diooxygenase gene (Fig. 3A, fragments 4 and 5, respectively). After compiling the DNA sequences of these five PCR fragments, we obtained 3,259 bp of DNA sequence that contained three complete ORFs, designated as tcpABC (Fig. 3A).
FIG. 3.
Organization of the tcpABC gene cluster of R. eutropha JMP134 and the roles of gene products in the proposed 2,4,6-TCP degradation pathway. (A) The DNA sequence of the gene cluster was assembled by compiling the DNA sequences of five PCR fragments (represented by black boxes) amplified from JMP134 DNA. The black arrowheads below each fragment represent the primers (Table 1) used to generate the fragment. The gray arrowheads (R) represent the random primers used in TAIL-PCR to amplify fragments 4 and 5. (B) The functions of TcpA and TcpC in 2,4,6-TCP degradation were studied in this report.
Genetic and functional analyses of the tcp genes for 2,4,6-TCP metabolism in JMP134.
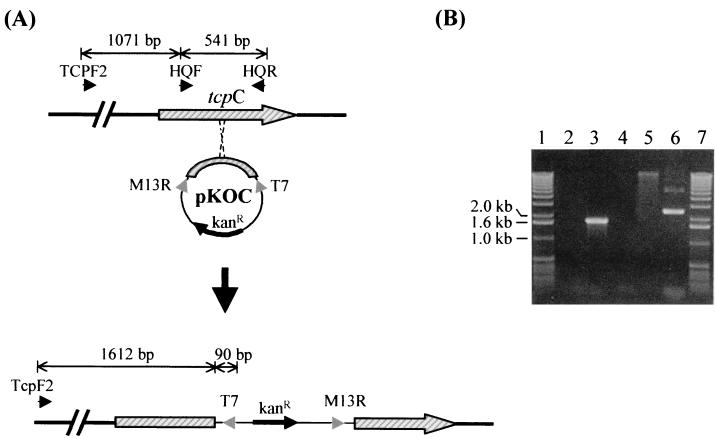
Each of the tcp genes was disrupted via homologous integration of a suicidal plasmid that carried an internal fragment of the respective tcp gene in JMP134, and the integration was confirmed by PCR. For example, pKOC, which carried a 541-bp internal fragment of tcpC amplified with primers HQF and HQR (Table 1), was electroporated into JMP134. The integration of the whole pKOC at tcpC resulted in a kanamycin-resistant recombinant clone that contained two truncated copies of tcpC on the chromosome (Fig. 4A). Integration of the whole pKOC was confirmed by PCR with primer T7, which specifically annealed to the plasmid, and primer TcpF2, which annealed 5′ to tcpC, resulting in a 1.7-kb product (Fig. 4B, lane 3). The same approaches were used to inactivate tcpA and tcpB and to confirm the insertions.
FIG. 4.
(A) Schematic representation of the homologous crossover between the tcpC internal fragment on pKOC and the tcpC on the genome. The integration resulted in two truncated copies of tcpC: one without the N-terminal region and the other without the C-terminal region. (B) Confirmation of the integration of pKOC by PCR with different primers and genomic DNA of the tcpC mutant. A PCR with primer pair T7 plus TcpF2 and DNA isolated from the tcpC mutant amplified the correct 1.7-kb product (lane 3). Similar PCRs with primer pairs T7 plus M13R (lane 2) and M13R plus TcpF2 (lane 4) produced no product, as expected. Plasmid preparation from the tcpC mutant (lane 5) did not recover any plasmid. Lanes 1 and 7 contained molecular mass standards in kilobases (Gibco BRL), and lane 6 contained plasmid pKOC.
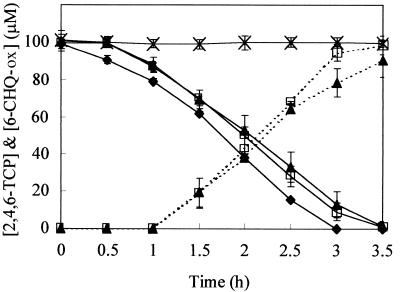
The tcpC mutant degraded 2,4,6-TCP at rates comparable to those in wild-type JMP134 cells (Fig. 5). However, the tcpC mutant accumulated a reddish-orange metabolite in its culture medium. HPLC analysis identified the reddish-orange metabolite as an oxidized product of 6-CHQ (6-CHQ-ox), because it had the same retention time and absorption spectrum (λmax = 290 nm) as an oxidized 6-CHQ standard prepared by incubating 6-CHQ in the mineral salt medium at 30°C for 20 min. The activity of TcpC was further tested. When 200 μM 6-CHQ was incubated with cell extracts of E. coli BL21(DE3)(pTcpC) in which TcpC was overproduced (ca. 25% of soluble protein), a peak at 253 nm appeared, consistent with previous reports that indicated 2-CMA production (44, 52). The 253-nm peak did not appear when E. coli BL21(DE3) cell extracts containing no TcpC were used. These results confirm that tcpC encodes a functional 6-CHQ 1,2-dioxygenase that oxidizes 6-CHQ to 2-CMA.
FIG. 5.
Degradation of 2,4,6-TCP (solid line) and accumulation of 6-CHQ-ox (dashed line) by cell suspensions of JMP134 and its tcp mutants. Cells were grown to early stationary phase in the mineral salt medium containing 0.4% (wt/vol) glutamate, harvested, and suspended to a turbidity of 1.0 at 600 nm in glutamate-free mineral salt medium containing 100 μM 2,4,6-TCP. ♦, JMP134 wild type; ×, tcpA mutant; □, tcpB mutant; and ▴, tcpC mutant. Data are means of duplicates with ranges.
The tcpB gene is not required for 2,4,6-TCP degradation, since the tcpB mutant degraded 2,4,6-TCP as fast as the tcpC mutant (Fig. 5). Insertional inactivation of tcpB could have a polar effect on the expression of tcpC. Thus, the tcpB mutant also accumulated 6-CHQ-ox (Fig. 5). Although the deduced amino acid sequence of tcpB showed some homology to flavin reductases, which supply reduced flavin mononucleotide (FMNH2) to bacterial luciferases in Vibrio harveyi and V. fischeri (21, 29), specific FAD reductase activities in the cell extracts prepared from the 2,4,6-TCP-induced tcpB mutant (152 ± 49 U/mg protein)(average ± standard deviation of three measurements) was not significantly lower than those of 2,4,6-TCP-induced JMP134 (235 ± 78 U/mg of protein) or uninduced JMP134 (238 ± 72 U/mg of protein).
The tcpA mutant did not degrade 2,4,6-TCP (Fig. 5). Thus, tcpA probably encodes the 2,4,6-TCP-degrading FADH2-utilizing monooxygenase detected in JMP134 cell extracts.
Production, purification, and characterization of recombinant TcpA.
Recombinant TcpA was produced in E. coli BL21(DE3)(pTcpA) as a C-terminal His-tagged fusion protein. After nickel affinity chromatography and size exclusion chromatography, a single band with an apparent molecular weight of 60,000, which agreed with the calculated molecular weight of 60,791.78 for the recombinant TcpA, was detected by SDS-PAGE. The native molecular mass of recombinant TcpA was determined to be about 60 kDa by size-exclusion chromatography, suggesting that the protein is a monomer. Pure recombinant TcpA alone did not degrade 2,4,6-TCP in a reaction mixture containing 2,4,6-TCP, NADH, and FAD, but it metabolized 2,4,6-TCP when Fre was added. In controls, Fre plus NADH and FAD did not lead to any consumption of 2,4,6-TCP. When riboflavin and flavin mononucleotide (FMN) replaced FAD in the reaction mixture, the enzymes did not transform 2,4,6-TCP. The reaction was oxygen dependent, since identical reactions performed in an anaerobic glove box showed no 2,4,6-TCP degradation. In a 2.5-min incubation, 40 μg of recombinant TcpA completely transformed 6.5 nmol of 2,4,6-TCP with the formation of 6.0 ± 1.4 nmol of 6-CHQ and 0.3 ± 0.1 nmol of 2,6-DiCH (average of three experiments ± standard deviation). The limited accumulation of 2,6-DiCH was consistent with the results when partially purified JMP134 cell extracts were incubated with 2,4,6-TCP. Pure recombinant TcpA converted 2,6-DiCH to 6-CHQ at a very slow rate of 3.5 ± 0.2 nmol · min−1 · mg−1; 2,6-DiCH degradation by JMP134 cell extracts was undetectable, perhaps due to marginal activity. The effects of temperature and pH on recombinant TcpA activity were determined. The apparent optimum temperature for TcpA activity was 33°C, with 66, 88, 85, 86, and 42% of the optimal activity retained at 22, 27, 30, 37, and 45°C, respectively. The 20 mM KPi buffer with pH values ranging from 6.0 to 7.8 did not appear to have any effect on TcpA activity. There was no significant change in TcpA activity when 2 to 100 μM 2,4,6-TCP was used in the reaction mixture. Thus, TcpA's Km for 2,4,6-TCP is estimated to be less than 1 μM, and the apparent specific activity of TcpA for 2,4,6-TCP was determined to be 327 ± 54 nmol · min−1 · mg−1, which is almost 100 times higher than that for 2,6-DiCH.
DISCUSSION
Our data showed that the expression of the 2,4,6-TCP degradation genes in JMP134 was inducible by 2,4,6-TCP and subject to catabolic repression by glutamate (Fig. 1). Catabolic repression on the expression of degradation pathways of aromatic compounds by small organic acid molecules has been reported (34, 41, 53). The mechanism of catabolic repression of the 2,4,6-TCP degradation pathway in JMP134 by glutamate is unknown, but can occur at the transcriptional level, as reported in Pseudomonas spp. (19, 31).
A 2,4,6-TCP degradation pathway (Fig. 3B) in JMP134 has been proposed by Padilla et al. (37) on the basis of whole-cell studies and cell extract experiments. In the present study, we identified and characterized genes and enzymes of the pathway (Fig. 3B). TcpA degraded 2,4,6-TCP to 6-CHQ, and the tcpA mutant lost the capability to degrade 2,4,6-TCP completely (Fig. 5). However, it is unclear whether 2,6-DiCH is a metabolic intermediate during 2,4,6-TCP degradation by TcpA. There was a small amount of 2,6-DiCH produced from 2,4,6-TCP degradation by partially purified JMP134 cell extracts and recombinant TcpA, but pure recombinant TcpA converted 2,6-DiCH to 6-CHQ at an extremely slow rate, and the conversion of 2,6-DiCH to 6-CHQ by JMP134 cell extracts was not detectable. One possible explanation is that TcpA oxidizes 2,4,6-TCP to 2,6-DiCH and then to 6-CHQ without releasing 2,6-DiCH. Once 2,6-DiCH is released, it is not a good substrate for TcpA. Thus, further study is necessary to determine whether 2,6-DiCH is the first metabolic intermediate during 2,4,6-TCP degradation in JMP134.
This 2,4,6-TCP degradation pathway in JMP134 is different from the characterized 2,4,5-TCP and pentachlorophenol degradation pathways. B. cepacia AC1100 degrades 2,4,5-TCP by using TftD, which has recently been reclassified as an FADH2-utilizing monooxygenase by sequence analysis (13, 18, 49). TftC, formerly classified as the small component of the two-component TCP 4-monooxygenase, was shown to be TftD's partner FAD reductase by both sequence comparison and experimental data (T. M. Louie and L. Xun, unpublished data). TftD oxidizes both 2,4,5-TCP and 2,5-DiCH, but at a faster rate for 2,5-DiCH than for 2,4,5-TCP (47). Consequently, the proteins oxidize 2,4,5-TCP to 5-CHQ with transient accumulation of 2,5-DiCH, which is not detectable at the end of the reaction. 5-CHQ does not undergo ring cleavage directly like 6-CHQ in the 2,4,6-TCP degradation pathway of JMP134. Rather, 5-CHQ is further dechlorinated to HQ before HQ 1,2-dioxygenase (TftH) oxidizes it to maleylacetate (12, 51). Sphingomonas chlorophenolica ATCC 39723 degrades pentachlorophenol and 2,4,6-TCP to 2,6-DiCH that is directly oxidized to 2-chloromaleylacetate by 2,6-DiCH 1,2-dioxygenase (36, 48). Therefore, S. chlorophenolica ATCC 39723 and R. eutropha JMP134 degrade 2,4,6-TCP through two different pathways.
This study demonstrates that TcpA is an FADH2-utilizing monooxygenase because recombinant TcpA expressed in E. coli oxidized 2,4,6-TCP to 6-CHQ only in the presence of oxygen and FADH2. Without Fre to supply FADH2, the purified enzyme was not active. Additionally, the deduced protein sequence of tcpA displays significant identity to a group of aromatic compound-hydroxylating FADH2-utilizing monooxygenases (Table 2) (13, 18, 49). Phylogenetic analysis of these FADH2-utilizing monooxygenases showed that TcpA clustered with TftD of B. cepacia AC1100 and HadA of R. pickettii DTP0602 (data not shown). This cluster of FADH2-utilizing monooxygenase is distinctive from another cluster consisting of HpaB of E. coli W and PheA1 of Geobacillus thermoglucosidasius A7, which oxidize phenol or nonchlorinated phenol derivatives. The clustering of TcpA with TftD and HadA is consistent with their shared capability to use 2,4,6-TCP as a substrate, but TftD and HadA have been reported to oxidize 2,4,6-TCP only to 2,6-DiCH (42, 47).
TABLE 2.
Proteins that displayed significant identity to JMP134 tcp gene products
| JMP134 protein | Representative homologs | Organism | GenBank accession no. | % Identitya |
|---|---|---|---|---|
| TcpA | HadA, chlorophenol-4-hydroxylase | Ralstonia pickettii DTP0602 | BAA13105 | 87 |
| TftD, chlorophenol 4-monooxygenase | Burkholderia cepacia AC1100 | AAC23548 | 61 | |
| PheA1, phenol 2-hydroxylase component A | Geobacillus thermoglucosidasius A7 | AAF66546 | 24 | |
| PhzO, phenazine hydroxylase | Pseudomonas chlororaphis | AAG17551 | 22 | |
| HpaB 4-hydroxyphenylacetate 3-monooxygenase | Escherichia coli W | CAA86048 | 20 | |
| TcpB | HadB, unknown function | Ralstonia pickettii DTP0602 | BAA13106 | 74 |
| Putative enzyme | Agrobacterium tumefaciens C58 | AAK88228 | 57 | |
| Putative enzyme | E. coli O157:H7 | BAB34677 | 56 | |
| Putative enzyme | E. coli K12 | AAC74093 | 55 | |
| Putative nitroreductase | Caulobacter crescentus | AAK22048 | 50 | |
| TcpC | HadC, hydroxyquinol 1,2-dioxygenase | Ralstonia pickettii DTP0602 | BAA13107 | 64 |
| TftH, hydroxyquinol 1,2-dioxygenase | Burkholderia cepacia AC1100 | AAC43338 | 53 | |
| Hydroxyquinol 1,2-dioxygenase | Arthrobacter sp. strain BA-5-17 | BAA82713 | 48 | |
| DxnF, hydroxyquinol 1,2-dioxygenase | Sphingomonas sp. strain RW1 | CAA51371 | 46 | |
| Putative hydroxyquinol 1,2-dioxygenase | Agrobacterium tumefaciens C58 | AAK88258 | 45 |
Percentage of amino acid identity between two protein sequences was determined by CLUSTAL W (43).
Genetic and biochemical characterizations of tcpC suggest that TcpC is a 6-CHQ 1,2-dioxygenase that oxidizes 6-CHQ to 2-CMA. The tcpC mutant degraded 2,4,6-TCP only to 6-CHQ with accumulation of 6-CHQ-ox in the culture medium (Fig. 5). Additionally, E. coli cell extracts containing TcpC oxidized 6-CHQ to 2-CMA. TcpC shows an extremely high degree of sequence identity to several HQ 1,2-dioxygenases (Table 2). Two other HQ 1,2-dioxygenases have been shown to use 6-CHQ as a substrate and produce 2-chloromaleylacetate (28, 52). The assignment of TcpC as a 6-CHQ 1,2-dioxygenase is also in agreement with reported results that 6-CHQ 1,2-dioxygenase activities are present in 2,4,6-TCP-induced JMP134 cell extracts (37).
Many FADH2- and FMNH2-utilizing monooxygenases have partner flavin reductases, and the genes encoding the partners are usually located in the same operon or are physically linked (7, 14, 20, 22, 38, 46). tcpB is located directly 3′ to tcpA (Fig. 3), and a conserved domain search of TcpB showed that it belongs to the nitroreductase family (pfam00881) (4), which includes the V. harveyi NADPH:FMN oxidoreductase (Frp) and the V. fischeri NAD(P)H:FMN oxidoreductase (FRase I), which provide bacterial luciferases with FMNH2 (21, 29). Thus, TcpB may function as the partner flavin reductase for TcpA. However, tcpB is not required for the function of TcpA in vivo (Fig. 5). This finding is not surprising, because the background FAD reductase activities in JMP134 (ca. 240 U/mg of protein) were relatively high when compared to that of E. coli (ca. 60 U/mg protein) (11). The high background flavin reductases may supplant the role of tcpB and provide FADH2 to TcpA in the tcpB mutant. Prieto and García (39) have observed that HpaB expressed alone in E. coli is capable of obtaining FADH2 from housekeeping flavin reductases to support low HpaB activity. We also observed that IPTG-induced E. coli BL21(DE3)(pTcpA) cells slowly accumulated 6-CHQ-ox from 2,4,6-TCP (data not shown), suggesting low-level TcpA activity is supported by E. coli housekeeping flavin reductases in vivo. Although sequence analysis suggested that TcpB is a flavin reductase for TcpA, experimental data did not support the hypothesis. The function of TcpB remains unknown.
In summary, this study delineates the complete enzymatic pathway for the degradation of 2,4,6-TCP to 2-CMA in R. eutropha JMP134. This research expands our knowledge of the diversity of reaction mechanisms and pathways of polychlorophenol degradation by bacteria. This information is ultimately useful for designing effective approaches for bioremediating environmental pollutants in the natural environment.
Acknowledgments
This research was funded in part by NSF grant MCB-9722970 and by grant DE-FG02-00ER62891 from the NABIR program, Office of Biological and Environmental Research of U.S. Department of Energy.
REFERENCES
- 1.Adriaens, P., Q. Fu, and D. Grbic-Galic. 1995. Bioavailability and transformation of highly chlorinated dibenzo-p-dioxins and dibenzofurans in anaerobic soils and sediments. Environ. Sci. Technol. 29:2252-2260. [DOI] [PubMed] [Google Scholar]
- 2.Aranda, C., F. Godoy, B. Gonzalez, J. Homo, and M. Martinez. 1999. Effects of glucose and phenylalanine upon 2,4,6-trichlorophenol degradation by Pseudomonas paucimobilis S37 cells in a no-growth state. Microbios 100:73-82. [PubMed] [Google Scholar]
- 3.Armengaud, J., K. N. Timmis, and R.-M. Wittich. 1999. A functional 4-hydroxysalicylate/hydroxyquinol degradative pathway gene cluster is linked to the initial dibenzo-p-dioxin pathway genes in Sphingomonas sp. strain RW1. J. Bacteriol. 181:3452-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman, A., E. Birney, L. Cerruti, R. Durbin, L. Etwiller, S. R. Eddy, S. Griffiths-Jones, K. L. Howe, M. Marshall, and E. L. Sonnhammer. 2002. The Pfam protein families database. Nucleic Acids Res. 30:276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birchmore, R. J., and J. C. Meneley. 1979. Prochloraz—a new foliar fungicide with potential in a wide range of crops. Phytopathology 69:1022-1026. [Google Scholar]
- 6.Bock, C., R. M. Kroppenstedt, U. Schmidt, and H. Diekmann. 1996. Degradation of prochloraz and 2,4,6-trichlorophenol by environmental bacterial strains. Appl. Microbiol. Biotechnol. 45:257-262. [DOI] [PubMed] [Google Scholar]
- 7.Bohuslavek, J., J. W. Payne, Y. Liu, H. Bolton, Jr., and L. Xun. 2001. Cloning, sequencing, and characterization of a gene cluster involved in EDTA degradation from the bacterium BNC1. Appl. Environ. Microbiol. 67:688-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 9.Briglia, M., F. A. Rainey, E. Stackebrandt, G. Schraa, and M. S. Salkinoja-Salonen. 1996. Rhodococcus percolatus sp. nov., a bacterium degrading 2,4,6-trichlorophenol. Int. J. Syst. Bacteriol. 46:23-30. [DOI] [PubMed] [Google Scholar]
- 10.Clement, P., V. Matus, L. Cardenas, and B. Gonzalez. 1995. Degradation of trichlorophenols by Alcaligenes eutrophus JMP134. FEMS Microbiol. Lett. 127:51-55. [DOI] [PubMed] [Google Scholar]
- 11.Covès, J., V. Nivière, M. Eschenbrenner, and M. Fontecave. 1993. NADPH-sulfite reductase from Escherichia coli. A flavin reductase participating in the generation of the free radical of the ribonucleotide reductase. J. Biol. Chem. 268:18604-18609. [PubMed] [Google Scholar]
- 12.Daubaras, D. L., K. Saido, and A. M. Chakrabarty. 1996. Purification of hydroxyquinol 1,2-dioxygenase and maleylacetate reductase: the lower pathway of 2,4,5-trichlorophenoxyacetic acid metabolism by Burkholderia cepacia AC1100. Appl. Environ. Microbiol. 62:4276-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delaney, S. M., D. V. Mavrodi, R. F. Bonsall, and L. S. Thomashow. 2001. phzO, a gene for biosynthesis of 2-hydroxylated phenazine compounds in Pseudomonas aureofaciens 30-84. J. Bacteriol. 183:318-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichhorn, E., J. R. van der Ploeg, and T. Leisinger. 1999. Characterization of a two-component alkanesulfonate monooxygenase from Escherichia coli. J. Biol. Chem. 274:26639-26646. [DOI] [PubMed] [Google Scholar]
- 15.Firestone, D. 1978. The 2,3,7,8-tetrachlorodibenzo-para-dioxin problem: a review. Ecol. Bull. 27:39-52. [Google Scholar]
- 16.Fisher, G. H., I. Arias, I. Quesada, S. D'Aniello, F. Errico, M. M. Di Fiore, and A. D'Aniello. 2001. A fast and sensitive method for measuring picomole levels of total free amino acids in very small amounts of biological tissues. Amino Acids 20:163-173. [DOI] [PubMed] [Google Scholar]
- 17.Fontecave, M., R. Eliasson, and P. Reichard. 1987. NAD(P)H:flavin oxidoreductase of Escherichia coli. A ferric iron reductase participating in the generation of the free radical of ribonucleotide reductase. J. Biol. Chem. 262:12325-12331. [PubMed] [Google Scholar]
- 18.Galán, B., E. Díaz, M. A. Prieto, and J. L. García. 2000. Functional analysis of the small component of the 4-hydroxyphenylacetate 3-monooxygenase of Escherichia coli W: a prototype of a new flavin:NAD(P)H reductase subfamily. J. Bacteriol. 182:627-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hester, K. L., J. Lehman, F. Najar, L. Song, B. A. Roe, C. H. MacGregor, P. W. Hager, P. V. Phibbs, Jr., and J. R. Sokatch. 2000. Crc is involved in catabolite repression control of the bkd operons of Pseudomonas putida and Pseudomonas aeruginosa. J. Bacteriol. 182:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hubner, A., C. E. Danganan, L. Xun, A. M. Chakrabarty, and W. Hendrickson. 1998. Genes for 2,4,5-trichlorophenoxyacetic acid metabolism in Burkholderia cepacia AC1100: characterization of the tftC and tftD genes and locations of the tft operons on multiple replicons. Appl. Environ. Microbiol. 64:2086-2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inouye, S. 1994. NAD(P)H-flavin oxidoreductase from the bioluminescent bacterium Vibrio fischeri ATCC 7744 is a flavoprotein. FEBS Lett. 347:163-168. [DOI] [PubMed] [Google Scholar]
- 22.Kahnert, A., P. Vermeij, C. Wietek, P. James, T. Leisinger, and M. A. Kertesz. 2000. The ssu locus plays a key role in organosulfur metabolism in Pseudomonas putida S-313. J. Bacteriol. 182:2869-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karns, J. S., S. Duttagupta, and A. M. Chakrabarty. 1983. Regulation of 2,4,5-trichlorophenoxyacetic acid and chlorophenol metabolism in Pseudomonas cepacia AC1100. Appl. Environ. Microbiol. 46:1182-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitunen, V. H., R. Valo, and M. S. Salkinoja-Salonen. 1987. Contamination of soil around wood-preserving facilities by polychlorinated aromatic compounds. Environ. Sci. Technol. 21:96-101. [Google Scholar]
- 25.Kiyohara, H., T. Hatta, Y. Ogawa, T. Kakuda, H. Yokoyama, and N. Takizawa. 1992. Isolation of Pseudomonas pickettii strains that degrade 2,4,6-trichlorophenol and their dechlorination of chlorophenols. Appl. Environ. Microbiol. 58:1276-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knapp, D. R. 1979. Handbook of analytical derivatization reactions. John Wiley & Sons, New York, N.Y.
- 27.Laemmli, U. K. 1970.Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 28.Latus, M., H. Seitz, J. Eberspächer, and F. Lingens. 1995. Purification and characterization of hydroxyquinol 1,2-dioxygenase from Azotobacter sp. strain GP1. Appl. Environ. Microbiol. 61:2453-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei, B., and S. C. Tu. 1998. Mechanism of reduced flavin transfer from Vibrio harveyi NADPH-FMN oxidoreductase to luciferase. Biochemistry 37:14623-14629. [DOI] [PubMed] [Google Scholar]
- 30.Li, D.-Y., J. Eberspächer, B. Wagner, J. Kuntzer, and F. Lingens. 1991. Degradation of 2,4,6-trichlorophenol by Azotobacter sp. strain GP1. Appl. Environ. Microbiol. 57:1920-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McFall, S. M., B. Abraham, C. G. Narsolis, and A. M. Chakrabarty. 1997. A tricarboxylic acid cycle intermediate regulating transcription of a chloroaromatic biodegradative pathway: fumarate-mediated repression of the clcABD operon. J. Bacteriol. 179:6729-6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Middaugh, D. P., R. L. Thomas, S. E. Lantz, C. S. Heard, and J. G. Mueller. 1994. Field-scale testing of a hyperfiltration unit for removal of creosote and pentachlorophenol from ground water: chemical and biological assessment. Arch. Environ. Contam. Toxicol. 26:309-319. [Google Scholar]
- 33.Motomura, M., N. Chihaya, T. Shinozawa, T. Hamasaki, and K. Yabe. 1999. Cloning and characterization of the O-methyltransferase I gene (dmtA) from Aspergillus parasiticus associated with the conversions of demethylsterigmatocystin to sterigmatocystin and dihydrodemethylsterigmatocystin to dihydrosterigmatocystin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 65:4987-4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller, C., L. Petruschka, H. Cuypers, G. Burchhardt, and H. Herrmann. 1996. Carbon catabolite repression of phenol degradation in Pseudomonas putida is mediated by the inhibition of the activator protein PhlR. J. Bacteriol. 178:2030-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murakami, S., T. Okuno, E. Matsumura, S. Takenaka, R. Shinke, and K. Aoki. 1999. Cloning of a gene encoding hydroxyquinol 1,2-dioxygenase that catalyzes both intradiol and extradiol ring cleavage of catechol. Biosci. Biotechnol. Biochem. 63:859-865. [DOI] [PubMed] [Google Scholar]
- 36.Ohtsubo, Y., K. Miyauchi, K. Kanda, T. Hatta, H. Kiyohara, T. Senda, Y. Nagata, Y. Mitsui, and M. Takagi. 1999. PcpA, which is involved in the degradation of pentachlorophenol in Sphingomonas chlorophenolica ATCC39723, is a novel type of ring-cleavage dioxygenase. FEBS Lett. 459:395-398. [DOI] [PubMed] [Google Scholar]
- 37.Padilla, L., V. Matus, P. Zenteno, and B. Gonzalez. 2000. Degradation of 2,4,6-trichlorophenol via chlorohydroxyquinol in Ralstonia eutropha JMP134 and JMP222. J. Basic Microbiol. 40:243-249. [DOI] [PubMed] [Google Scholar]
- 38.Prieto, M. A., E. Díaz, and J. L. García. 1996. Molecular characterization of the 4-hydroxyphenylacetate catabolic pathway of Escherichia coli W: engineering a mobile aromatic degradative cluster. J. Bacteriol. 178:111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prieto, M. A., and J. L. García. 1994. Molecular characterization of 4-hydroxyphenylacetate 3-hydroxylase of Escherichia coli. A two-protein component enzyme. J. Biol. Chem. 269:22823-22829. [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Santos, P. M., J. M. Blatny, I. Di Bartolo, S. Valla, and E. Zennaro. 2000. Physiological analysis of the expression of the styrene degradation gene cluster in Pseudomonas fluorescens ST. Appl. Environ. Microbiol. 66:1305-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takizawa, N., H. Yokoyama, K. Yanagihara, T. Hatta, and H. Kiyohara. 1995. A locus of Pseudomonas pickettii DTP0602, had, that encodes 2,4,6-trichlorophenol-4-dechlorinase with hydroxylase activity, and hydroxylation of various chlorophenols by the enzyme. J. Ferment. Bioeng. 80:318-326. [Google Scholar]
- 43.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tiedje, J. M., J. M. Duxbury, M. Alexander, and J. E. Dawson. 1969. 2,4-D metabolism: pathway of degradation of chlorophenols by Arthrobacter sp. J. Agric. Food Chem. 17:1021-1026. [DOI] [PubMed] [Google Scholar]
- 45.Wang, C. C., C. M. Lee, C. J. Lu, M. S. Chuang, and C. Z. Huang. 2000. Biodegradation of 2,4,6-trichlorophenol in the presence of primary substrate by immobilized pure culture bacteria. Chemosphere 41:1873-1879. [DOI] [PubMed] [Google Scholar]
- 46.Xu, Y., M. W. Mortimer, T. S. Fisher, M. L. Kahn, F. J. Brockman, and L. Xun. 1997. Cloning, sequencing and analysis of a gene cluster from Chelatobacter heintzii ATCC 29600 encoding nitrilotriacetate monooxygenase and NADH:flavin mononucleotide oxidoreductase. J. Bacteriol. 179:1112-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xun, L. 1996. Purification and characterization of chlorophenol 4-monooxygenase from Burkholderia cepacia AC1100. J. Bacteriol. 178:2645-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xun, L., J. Bohuslavek, and M. Cai. 1999. Characterization of 2,6-dichloro-p-hydroquinone 1,2-dioxygenase (PcpA) of Sphingomonas chlorophenolica ATCC 39723. Biochem. Biophys. Res. Commun. 266:322-325. [DOI] [PubMed] [Google Scholar]
- 49.Xun, L., and E. R. Sandvik. 2000. Characterization of 4-hydroxyphenylacetate 3-hydroxylase (HpaB) of Escherichia coli as a reduced flavin adenine dinucleotide-utilizing monooxygenase. Appl. Environ. Microbiol. 66:481-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xun, L., and K. B. Wagnon. 1995. Purification and properties of 2,4,5-trichlorophenoxyacetate oxygenase from Pseudomonas cepacia AC1100. Appl. Environ. Microbiol. 61:3499-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaborina, O., D. L. Daubaras, A. Zago, L. Xun, K. Saido, T. Klem, D. Nikolic, and A. M. Chakrabarty. 1998. Novel pathway for conversion of chlorohydroxyquinol to maleylacetate in Burkholderia cepacia AC1100. J. Bacteriol. 180:4667-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaborina, O., M. Latus, J. Eberspächer, L. A. Golovleva, and F. Lingens. 1995. Purification and characterization of 6-chlorohydroxyquinol 1,2-dioxygenase from Streptomyces rochei 303: comparison with an analogous enzyme from Azotobacter sp. strain GP1. J. Bacteriol. 177:229-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zylstra, G. J., R. H. Olsen, and D. P. Ballou. 1989. Genetic organization and sequence of the Pseudomonas cepacia genes for the alpha and beta subunits of protocatechuate 3,4-dioxygenase. J. Bacteriol. 171:5915-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]