Abstract
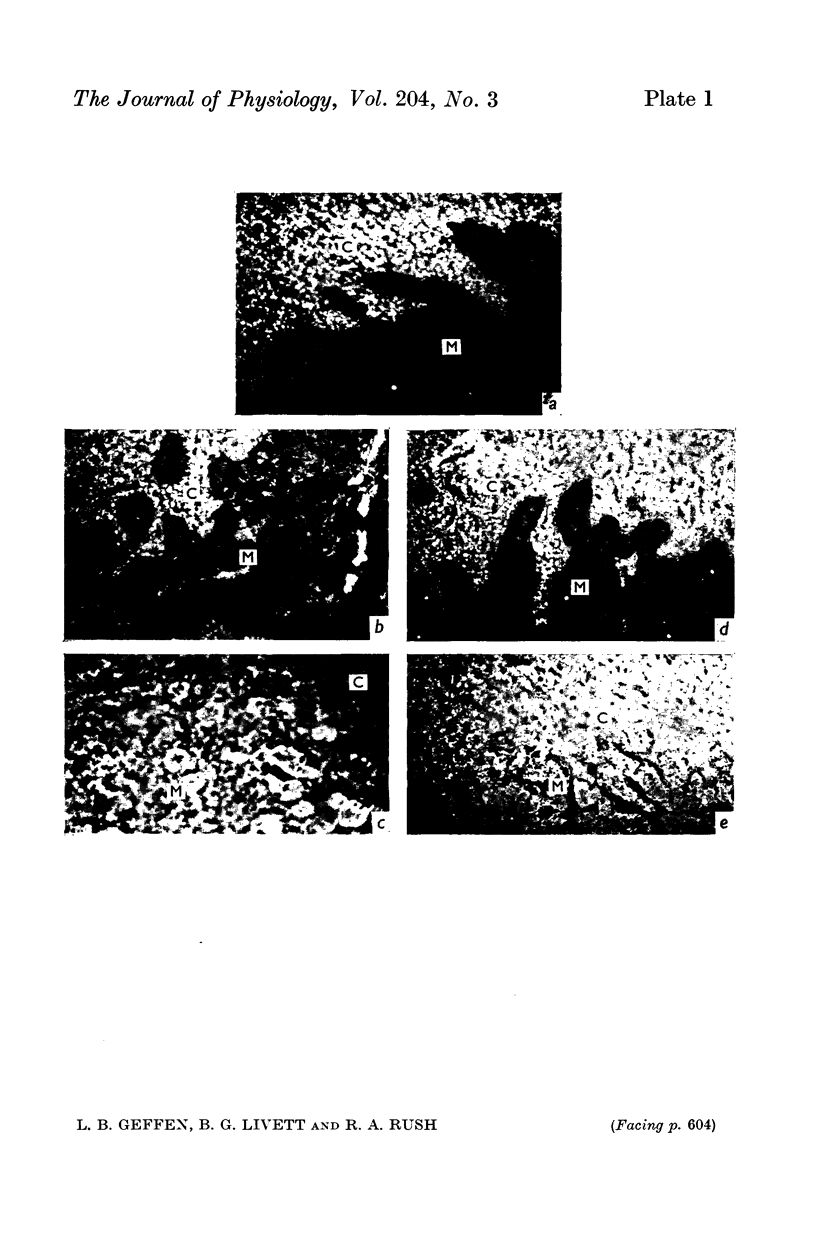
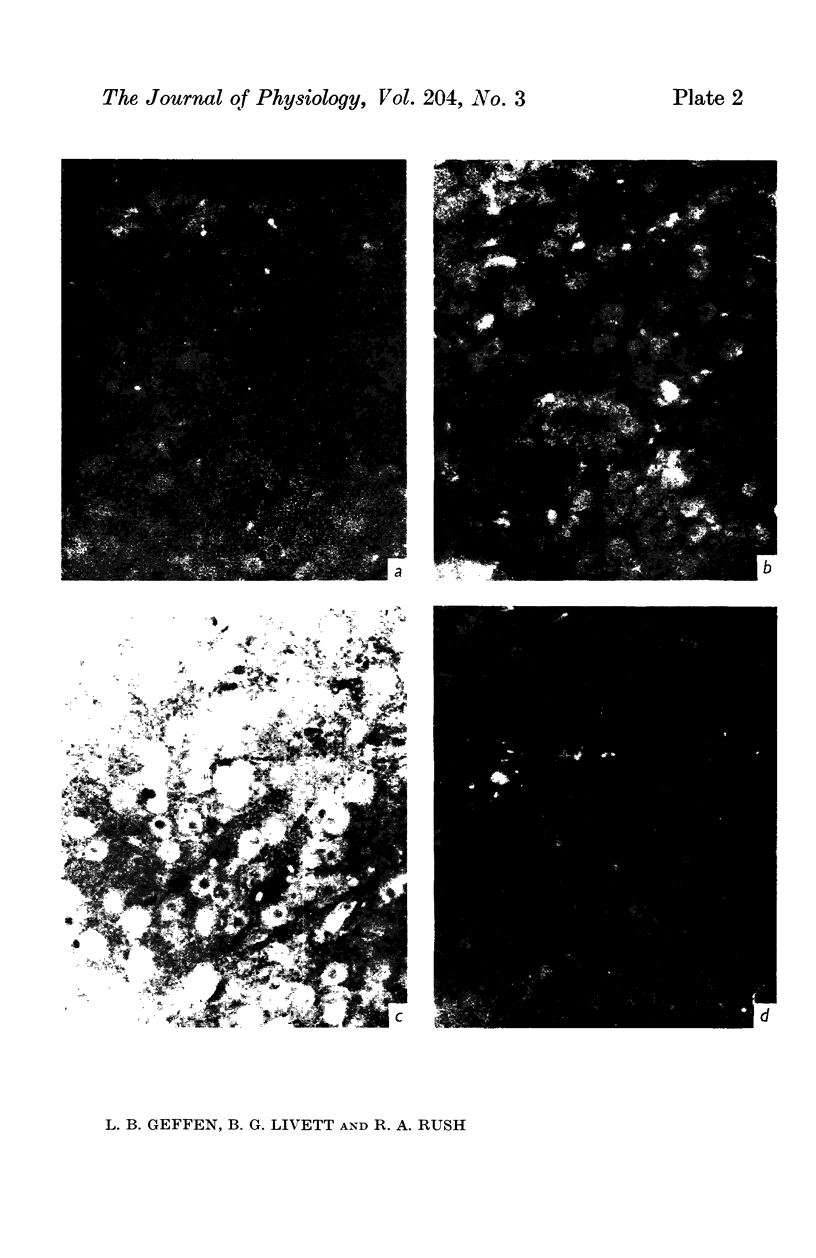
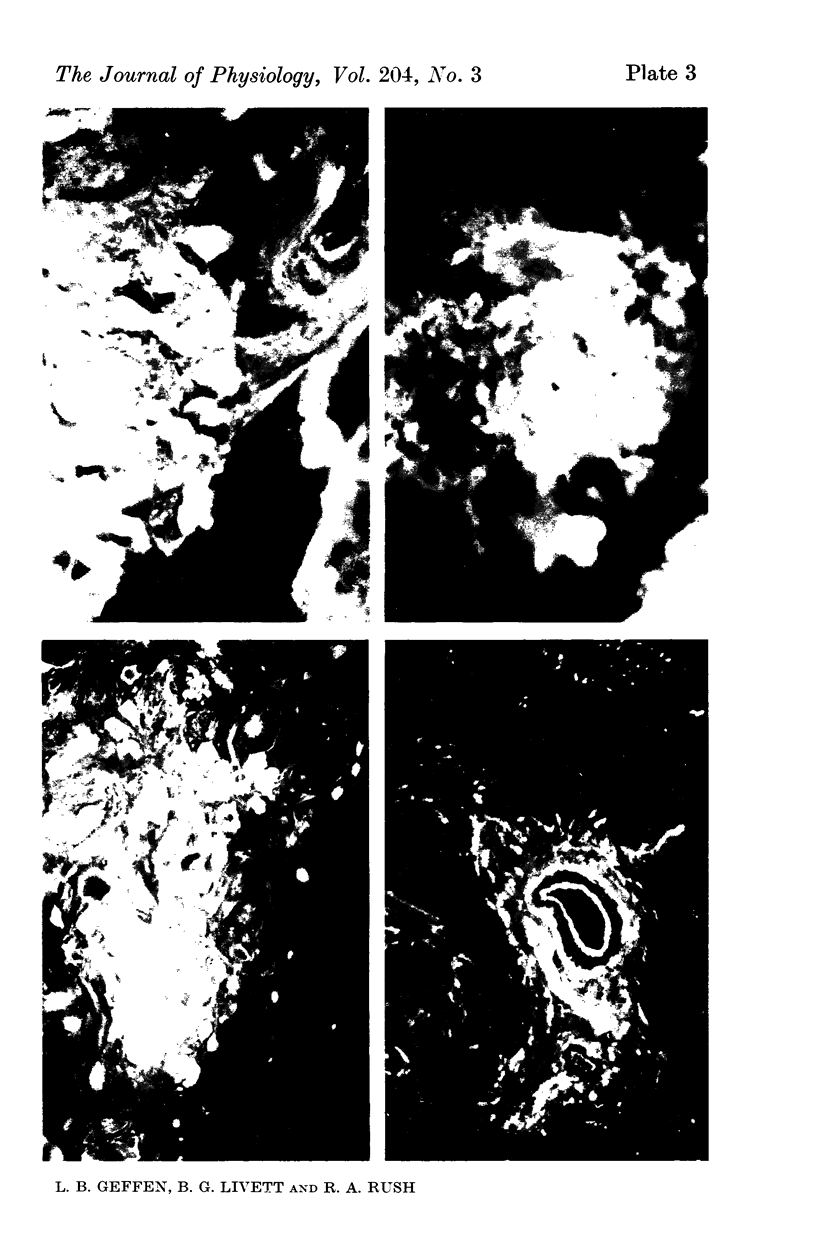
1. The distribution of specific proteins in sympathetic neurones has been examined by immunofluorescent histology using antibodies prepared against soluble protein components of the catecholamine storage vesicles of the adrenal medulla.
2. Two antigen preparations were separated by ion exchange chromatography of the soluble proteins released on osmotic lysis of catecholamine storage vesicles which had been isolated by centrifugation from homogenates of sheep adrenal medulla. One fraction (AgDH) had high dopamine-β-hydroxylase activity, while another (AgCB), consisting of the bulk of the protein, had some capacity to bind catecholamines. On disk gel electrophoresis the antigens ran as single bands with very different mobilities.
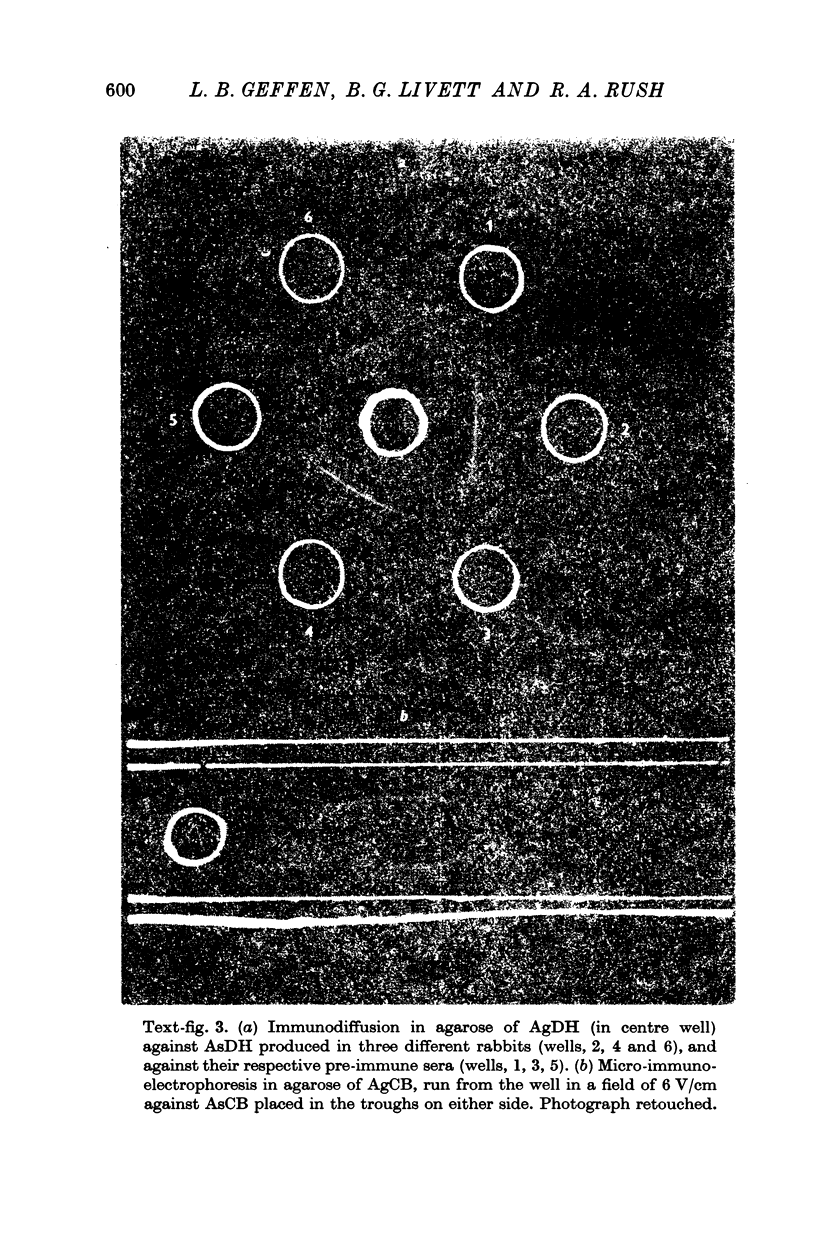
3. Antisera (AsDH) and (AsCB) produced in rabbits to the two antigens were shown to react specifically with their antigens by immunodiffusion and electrophoresis in agarose.
4. Indirect immunofluorescent staining of tissue sections was achieved by layering first the rabbit anti-sera, followed by goat anti-rabbit globulin serum which had been conjugated with fluorescein isothiocyanate.
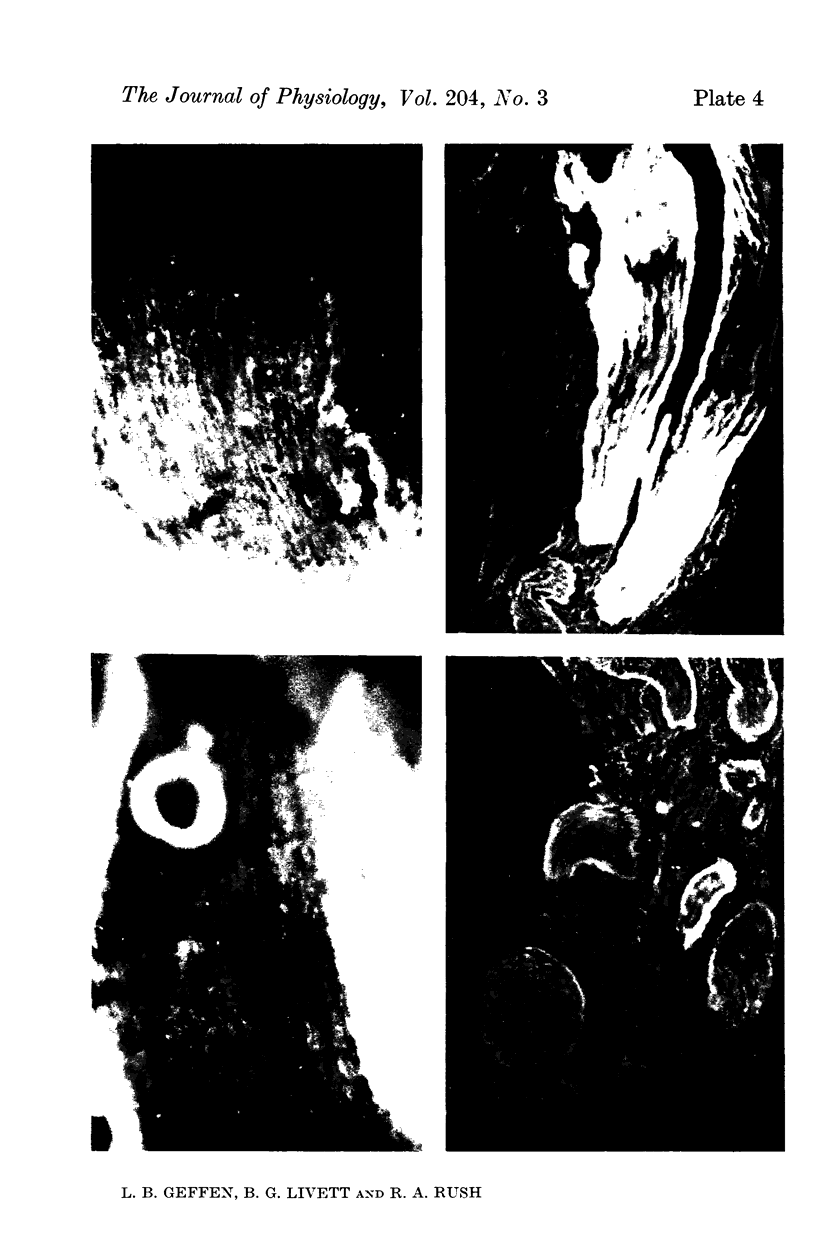
5. The adrenal medulla and the cell bodies of sympathetic ganglia showed the most intense green fluorescence with the immune rabbit sera, and hardly stained when pre-immune serum from the same animal was used. The reactivity of the antisera could be abolished by incubation with the corresponding antigen.
6. The preterminal and terminal axons of sympathetic nerves also stained specifically but less intensely with both antisera. When the nerves were ligated for up to 24 hr, the portion immediately proximal to the constriction showed an enhanced reaction to the antisera.
7. The results provide evidence that sympathetic neurones contain proteins immunologically identical to those involved in the synthesis and storage of noradrenaline in the adrenal medulla, and support the concept that granular vesicles are synthesized in the perikaryon of the neurone and are transported somatofugally in the axon.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belpaire F., Laduron P. Tissue fractionation and catecholamines. I. Latency and activation properties of dopamine-beta-hydroxylase in adrenal medulla. Biochem Pharmacol. 1968 Mar;17(3):411–421. doi: 10.1016/0006-2952(68)90251-7. [DOI] [PubMed] [Google Scholar]
- Blaschko H., Comline R. S., Schneider F. H., Silver M., Smith A. D. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature. 1967 Jul 1;215(5096):58–59. doi: 10.1038/215058a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DAVIS G. A., SANTEN R. J., AGRANOFF B. W. THE PRODUCTION OF A LINEAR DENSITY GRADIENT BY MEANS OF A PROPORTIONING PUMP. Anal Biochem. 1965 Apr;11:153–154. doi: 10.1016/0003-2697(65)90056-4. [DOI] [PubMed] [Google Scholar]
- Dahlström A. Observations on the accumulation of noradrenaline in the proximal and distal parts of peripheral adrenergic nerves after compression. J Anat. 1965 Oct;99(Pt 4):677–689. [PMC free article] [PubMed] [Google Scholar]
- Dahlström A. The intraneuronal distribution of noradrenaline and the transport and life-span of amine storage granules in the sympathetic adrenergic neuron. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1967;257(1):93–115. doi: 10.1007/BF00537460. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. The mechanism of release of catecholamines from the adrenal medulla. Pharmacol Rev. 1966 Mar;18(1):471–480. [PubMed] [Google Scholar]
- Friedman S., Kaufman S. 3,4-dihydroxyphenylethylamine beta-hydroxylase. Physical properties, copper content, and role of copper in the catalytic acttivity. J Biol Chem. 1965 Dec;240(12):4763–4773. [PubMed] [Google Scholar]
- Geffen L. B., Ostberg A. Distribution of granular vesicles in normal and constricted sympathetic neurones. J Physiol. 1969 Oct;204(3):583–592. doi: 10.1113/jphysiol.1969.sp008933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffen L. B., Rush R. A. Transport of noradrenaline in sympathetic nerves and the effect of nerve impulses on its contribution to transmitter stores. J Neurochem. 1968 Sep;15(9):925–930. doi: 10.1111/j.1471-4159.1968.tb11634.x. [DOI] [PubMed] [Google Scholar]
- Gibb J. W., Spector S., Udenfriend S. Production of antibodies to dopamine-beta-hydroxylase of bovine adrenal medulla. Mol Pharmacol. 1967 Sep;3(5):473–478. [PubMed] [Google Scholar]
- Kapeller K., Mayor D. The accumulation of noradrenaline in constricted sympathetic nerves as studied by fluorescence and electron microscopy. Proc R Soc Lond B Biol Sci. 1967 Mar 28;167(1008):282–292. doi: 10.1098/rspb.1967.0027. [DOI] [PubMed] [Google Scholar]
- Kirshner N., Sage H. J., Smith W. J., Kirshner A. G. Release of catecholamines and specific protein from adrenal glands. Science. 1966 Oct 28;154(3748):529–531. doi: 10.1126/science.154.3748.529. [DOI] [PubMed] [Google Scholar]
- LEVIN E. Y., LEVENBERG B., KAUFMAN S. The enzymatic conversion of 3,4-dihydroxyphenylethylamine to norepinephrine. J Biol Chem. 1960 Jul;235:2080–2086. [PubMed] [Google Scholar]
- Laduron P., Belpaire F. Transport of noradrenaline and dopamine-beta-hydroxylase in sympathetic nerves. Life Sci. 1968 Jan 1;7(1):1–7. doi: 10.1016/0024-3205(68)90355-x. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Geffen L. B., Austin L. Proximo-distal transport of [14C]noradrenaline and protein in sympathetic nerves. J Neurochem. 1968 Sep;15(9):931–939. doi: 10.1111/j.1471-4159.1968.tb11635.x. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Geffen L. B., Rush R. A. Immunohistochemical evidence for the transport of dopamine-B-hydroxylase and a catecholamine binding protein in sympathetic nerves. Biochem Pharmacol. 1969 Apr;18(4):923–924. doi: 10.1016/0006-2952(69)90063-x. [DOI] [PubMed] [Google Scholar]
- Mueller R. A., Shideman F. E. The prolongation of reserpine-induced cardiac norepinephrine depletion by metabolic inhibitors. Biochem Pharmacol. 1968 Mar;17(3):451–467. doi: 10.1016/0006-2952(68)90255-4. [DOI] [PubMed] [Google Scholar]
- Nairn R. C. Standardization in immunofluorescence. Clin Exp Immunol. 1968 Jul;3(6):465–476. [PMC free article] [PubMed] [Google Scholar]
- Pellegrino de Iraldi A., De Robertis E. The neurotubular system of the axon and the origin of granulated and non-granulated vesicles in regenerating nerves. Z Zellforsch Mikrosk Anat. 1968;87(3):330–344. doi: 10.1007/BF00333684. [DOI] [PubMed] [Google Scholar]
- Potter L. T. Role of intraneuronal vesicles in the synthesis, storage, and release of catecholamines. Circ Res. 1967 Dec;21(6 Suppl):13–24. [PubMed] [Google Scholar]
- SCHUEMANN H. J., SCHNELL K., PHILIPPU A. SUBCELLULAERE VERTEILUNG VON NORADRENALIN UND ADRENALIN IM MEERSCHWEINCHENHERZEN. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964 Oct 23;249:251–266. [PubMed] [Google Scholar]
- Sage H. J., Smith W. J., Kirshner N. Mechanism of secretion from the adrenal medulla. I. A microquantitative immunologic assay for bovine adrenal catecholamine storage vesicle protein and its application to studies of the secretory process. Mol Pharmacol. 1967 Jan;3(1):81–89. [PubMed] [Google Scholar]
- Schneider F. H., Smith A. D., Winkler H. Secretion from the adrenal medulla: biochemical evidence for exocytosis. Br J Pharmacol Chemother. 1967 Sep;31(1):94–104. doi: 10.1111/j.1476-5381.1967.tb01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. Purification and properties of an acidic protein from chromaffin granules of bovine adrenal medulla. Biochem J. 1967 May;103(2):483–492. doi: 10.1042/bj1030483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. J., Kirshner N. A specific soluble protein from the catecholamine storage vesicles of bovine adrenal medulla. I. Purification and chemical characterization. Mol Pharmacol. 1967 Jan;3(1):52–62. [PubMed] [Google Scholar]
- Trifaró J. M., Poisner A. M., Douglas W. W. The fate of the chromaffin granule during catecholamine release from the adrenal medulla. I. Unchanged efflux of phospholipid and cholesterol. Biochem Pharmacol. 1967 Nov;16(11):2095–2100. doi: 10.1016/0006-2952(67)90006-8. [DOI] [PubMed] [Google Scholar]