Abstract
1. Bio-assay techniques have been used to measure plasma levels of neurohypophysial hormones in man, following either a single injection or a continuous infusion.
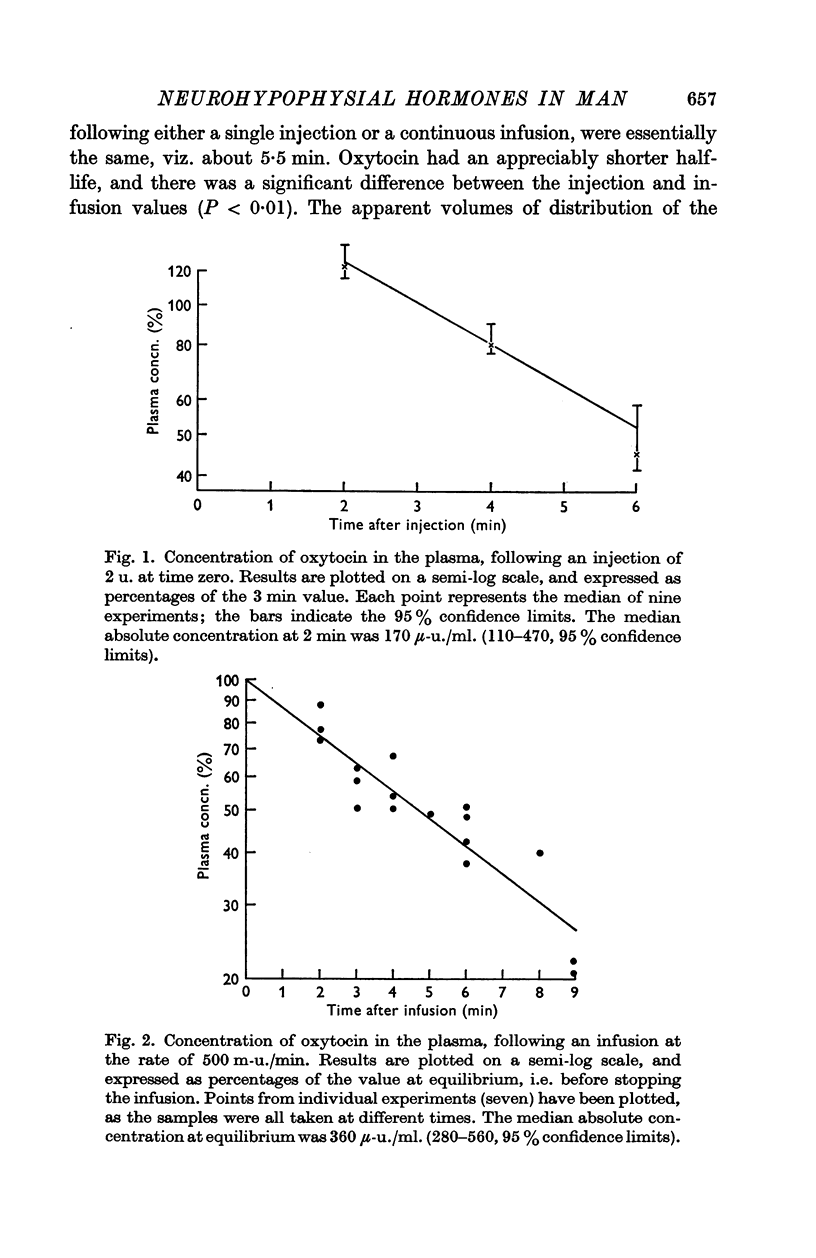
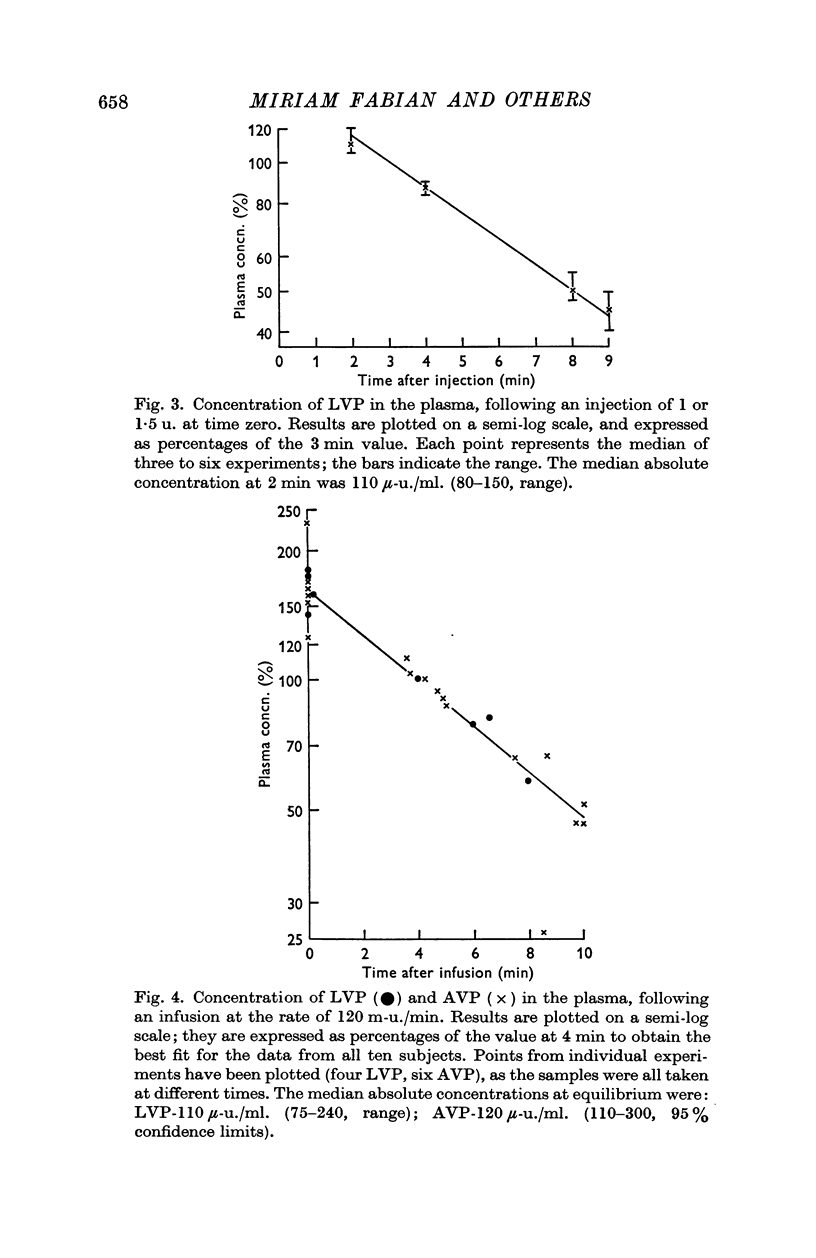
2. The median half-life of oxytocin after a single injection of 2 u. was 3·2 min (2·0-5·7, 95% confidence limits); this increased significantly (P < 0·01) to 4·8 min (4·4-6·1) when the hormone was infused at a rate of 500 m-u./min. The vasopressins had appreciably longer half-lives. After a single injection of 1 or 1·5 u. 8-lysine vasopressin (LVP), the half-life was 5·7 min (3·6-6·0). Continuous infusions of the hormones at a rate of 120 m-u./min yielded half-lives of 5·5 min (5·0-7·1) for LVP, and 5·6 min (3·9-9·5) for 8-arginine vasopressin (AVP).
3. The apparent volumes of distribution of the hormones were all of the order of two thirds the extracellular volume.
4. In accordance with its shorter half-life, the clearance of oxytocin was greater than that of the vasopressins (1·5 l./min, compared with 1·0 l./min).
5. The antidiuretic potencies of the hormones were studied in over-hydrated subjects, by measuring the rate of urine excretion following an I.V. injection. Duration of antidiuretic action increased in the order: oxytocin, LVP, AVP. A 5:1 mixture of oxytocin and AVP was not as long-lasting as AVP alone. 8·5% (4-22) of an administered dose of AVP was excreted in the urine, and this amount was significantly correlated with urine volume (r = +0·67, P < 0·05).
6. Ultrafiltration of human plasma containing exogenous hormones showed that 30% (13-50) of AVP was bound, the degree of binding being independent of concentration over the range used (50-400 μ-u./ml.) In contrast, oxytocin was completely unbound.
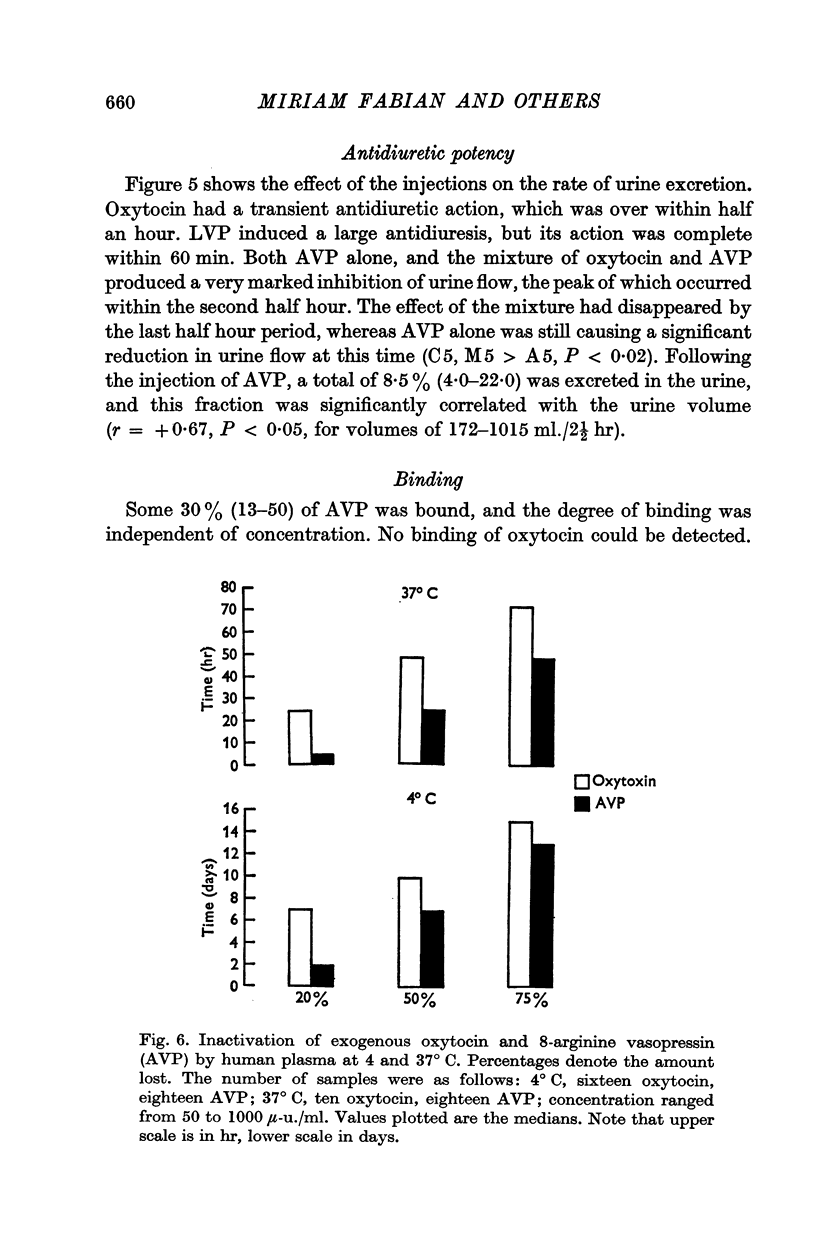
7. Exogenous oxytocin was more stable than exogenous AVP in human plasma. At 4° C there was no significant loss of oxytocin until 7 days, whereas 20% of AVP was inactivated in 2 days. At 37° C a 20% loss of AVP occurred within 4 hr, and a 50% loss within 24 hr; corresponding times for oxytocin were 24 and 48 hr.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. B., George B. C., Gonzalez-Auvert C., Dingman J. F. Increased plasma arginine vasopressin in clinical adrenocortical insufficeincy and its inhibition by glucosteroids. J Clin Invest. 1967 Jan;46(1):111–123. doi: 10.1172/JCI105504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURN G. P., GREWAL R. S. The antidiuretic response to and excretion of pituitary (posterior lobe) extract in man, with reference to the action of nicotine. Br J Pharmacol Chemother. 1951 Sep;6(3):471–482. doi: 10.1111/j.1476-5381.1951.tb00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J., Haldar J., Harris M. C., Lewis G. P., Rocha e Silva R., Jr The assay of milk-ejecting activity in the lactating rat. Br J Pharmacol Chemother. 1967 Nov;31(3):537–549. doi: 10.1111/j.1476-5381.1967.tb00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook A. H., Share L. On the question of protein binding and the diffusibility of circulating antidiuretic hormone in the dog. Endocrinology. 1966 Apr;78(4):779–785. doi: 10.1210/endo-78-4-779. [DOI] [PubMed] [Google Scholar]
- CZACZKES J. W., KLEEMAN C. R., KOENIG M. PHYSIOLOGIC STUDIES OF ANTIDIURETIC HORMONE BY ITS DIRECT MEASUREMENT IN HUMAN PLASMA. J Clin Invest. 1964 Aug;43:1625–1640. doi: 10.1172/JCI105038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKER S. E. A method for the assay of very small amounts of antidiuretic activity with a note on the antidiuretic titre of rat's blood. J Physiol. 1953 Oct;122(1):149–157. doi: 10.1113/jphysiol.1953.sp004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIECKMANN W. J., EGENOLF G. F., MORLEY B., POTTINGER R. E. The inactivation of the antidiuretic hormone of the posterior pituitary gland by blood from pregnant patients. Am J Obstet Gynecol. 1950 Nov;60(5):1043–1050. doi: 10.1016/0002-9378(50)90509-6. [DOI] [PubMed] [Google Scholar]
- FREY J., KERP L., REICHARDT W. Zum Vasopressin-Oxytocin-Antagonismus beim Menschen. Klin Wochenschr. 1959 Apr 1;37(7):325–329. doi: 10.1007/BF01483481. [DOI] [PubMed] [Google Scholar]
- Fabian M., Forsling M. L., Jones J. J., Lee J. The release, clearance and plasma protein binding of oxytocin in the anaesthetized rat. J Endocrinol. 1969 Feb;43(2):175–189. doi: 10.1677/joe.0.0430175. [DOI] [PubMed] [Google Scholar]
- Forsling M. L., Jones J. J., Lee J. Factors influencing the sensitivity of the rat to vasopressin. J Physiol. 1968 May;196(2):495–505. doi: 10.1113/jphysiol.1968.sp008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUHL U. [Antidiuretic and pressor effects of arginine-8-vasopressin, lysine-8-vasopressin, phenylalanine-2-lysine-8-vasopressin in man]. Schweiz Med Wochenschr. 1961 Jul 8;91:798–804. [PubMed] [Google Scholar]
- HOLLIDAY M. A., BURSTIN C., HARRAH J. EVIDENCE THAT THE ANTIDIURETIC SUBSTANCE IN THE PLASMA OF CHILDREN WITH NEPHROGENIC DIABETES INSIPIDUS IS ANTIDIURETIC HORMONE. Pediatrics. 1963 Sep;32:384–388. [PubMed] [Google Scholar]
- Jones J. J., Lee J. The value of rats with hereditary hypothalamic diabetes insipidus for the bioassay of vasopressin. J Endocrinol. 1967 Mar;37(3):335–344. doi: 10.1677/joe.0.0370335. [DOI] [PubMed] [Google Scholar]
- Lauson H. D. Metabolism of antidiuretic hormones. Am J Med. 1967 May;42(5):713–744. doi: 10.1016/0002-9343(67)90091-5. [DOI] [PubMed] [Google Scholar]
- Miller L., Fisch L., Kleeman C. R. Relative potency of arginine-8-vasopressin and lysine-8-vasopressin in humans. J Lab Clin Med. 1967 Feb;69(2):270–291. [PubMed] [Google Scholar]
- PETTMAN J. G. Water intoxication due to oxytocin. Report of a case. N Engl J Med. 1963 Feb 28;268:481–482. doi: 10.1056/NEJM196302282680908. [DOI] [PubMed] [Google Scholar]
- RYDEN G., SJOHOLM I. Assay of oxytocin by rat mammary gland in vitro. Br J Pharmacol Chemother. 1962 Aug;19:136–141. doi: 10.1111/j.1476-5381.1962.tb01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHROEDER R., ROTT D. [On the determination and behavior of ADH in human plasma]. Klin Wochenschr. 1959 Nov 15;37:1175–1181. doi: 10.1007/BF01491358. [DOI] [PubMed] [Google Scholar]
- SILVER L., SCHWARTZ I. L., FONG C. T., DEBONS A. F., DAHL L. K. Disappearance of plasma radioactivity after injection of H3- or I-131-labeled arginine vasopressin. J Appl Physiol. 1961 Nov;16:1097–1099. doi: 10.1152/jappl.1961.16.6.1097. [DOI] [PubMed] [Google Scholar]
- Silva P., Allan M. S. Water intoxication due to high doses of synthetic oxytocin. Report of a case. Obstet Gynecol. 1966 Apr;27(4):517–520. doi: 10.1097/00006250-196604000-00013. [DOI] [PubMed] [Google Scholar]
- Vane J. R. The release and fate of vaso-active hormones in the circulation. Br J Pharmacol. 1969 Feb;35(2):209–242. doi: 10.1111/j.1476-5381.1969.tb07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorherr H., Kleeman C. R., Hoghoughi M. Factors influencing the sensitivity of rats under ethanol anesthesia to antidiuretic hormone (ADH). Endocrinology. 1968 Feb;82(2):218–224. doi: 10.1210/endo-82-2-218. [DOI] [PubMed] [Google Scholar]


