Abstract
1. One caudate nucleus of the anaesthetized cat was superfused by perfusing the anterior horn of one lateral cerebral ventricle. The perfusates were examined for their content in acetylcholine (ACh), dopamine, homovanillic acid (HVA) and 5-hydroxytryptamine (5-HT), at rest and after a variety of stimuli.
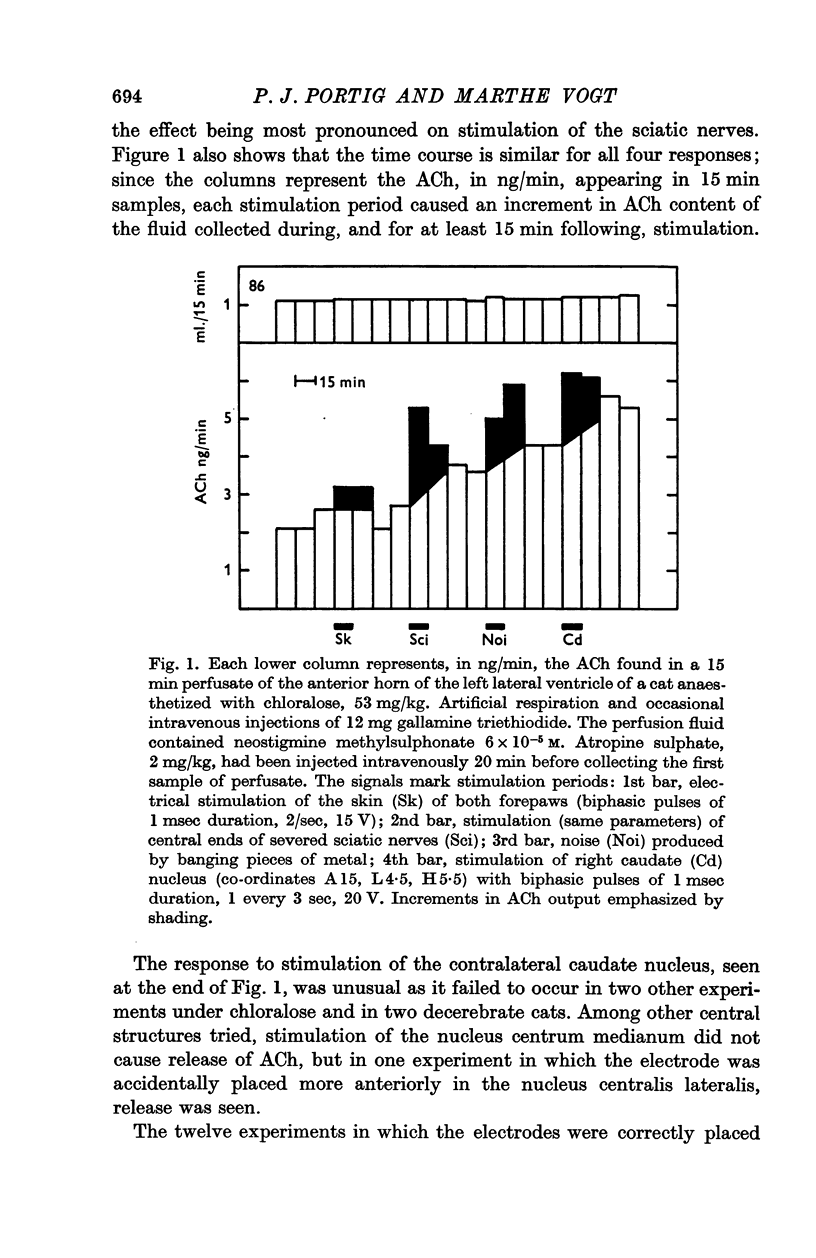
2. When prostigmine was added to the perfusion fluid, ACh appeared in the effluent; its concentration tended to rise in the course of an experiment. Various afferent stimuli, all of which caused evoked responses recorded from the contra-lateral caudate nucleus, increased the ACh content of the effluent. Effective stimuli were noise and electrical stimulation of afferent nerves or of certain regions of the brain including the ipsi-lateral substantia nigra.
3. The dopamine content of the effluent was extremely low (of the order of 50 pg/min) at rest, but, on occasion, rose sharply when the substantia nigra was stimulated electrically with trains of pulses repeated once every 3 sec. The results were inconsistent.
4. Since dopamine in tissue is rapidly transformed enzymically into HVA, the appearance of this acid in the perfusate was examined.
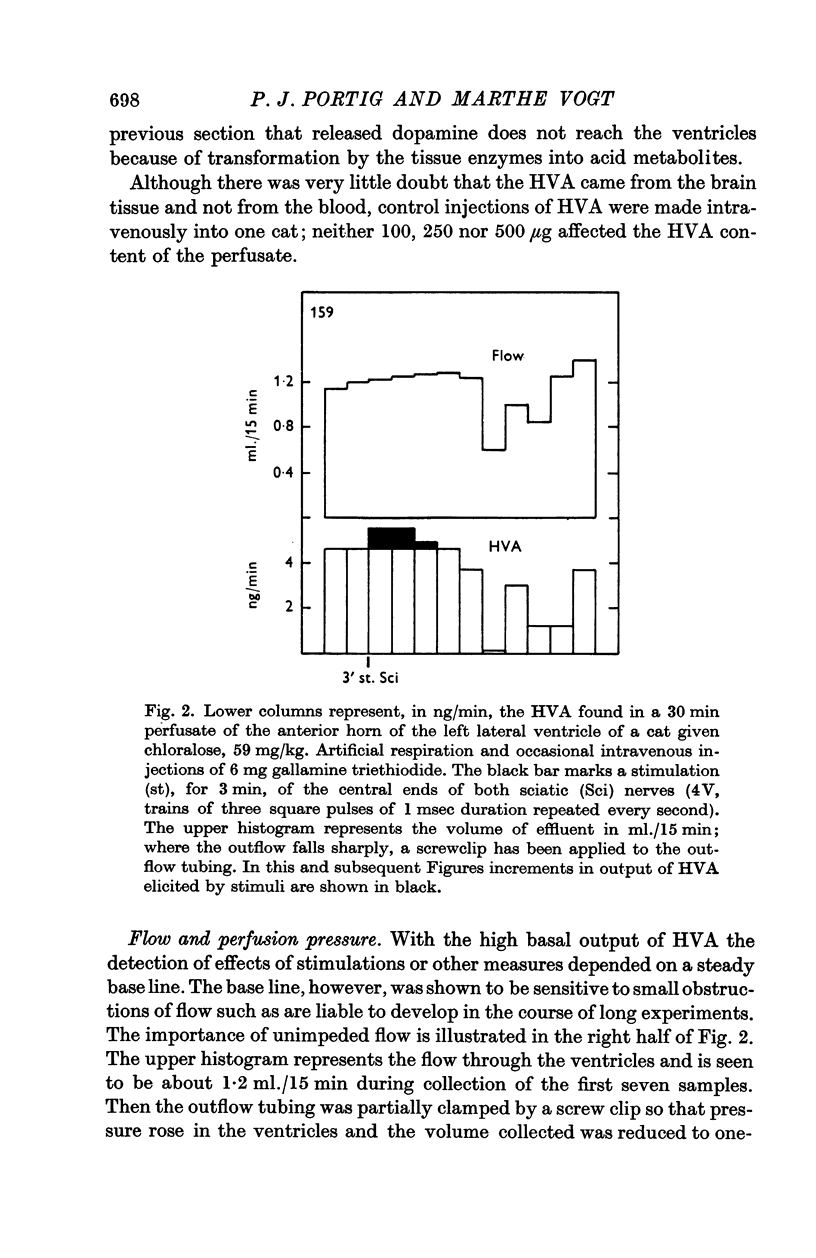
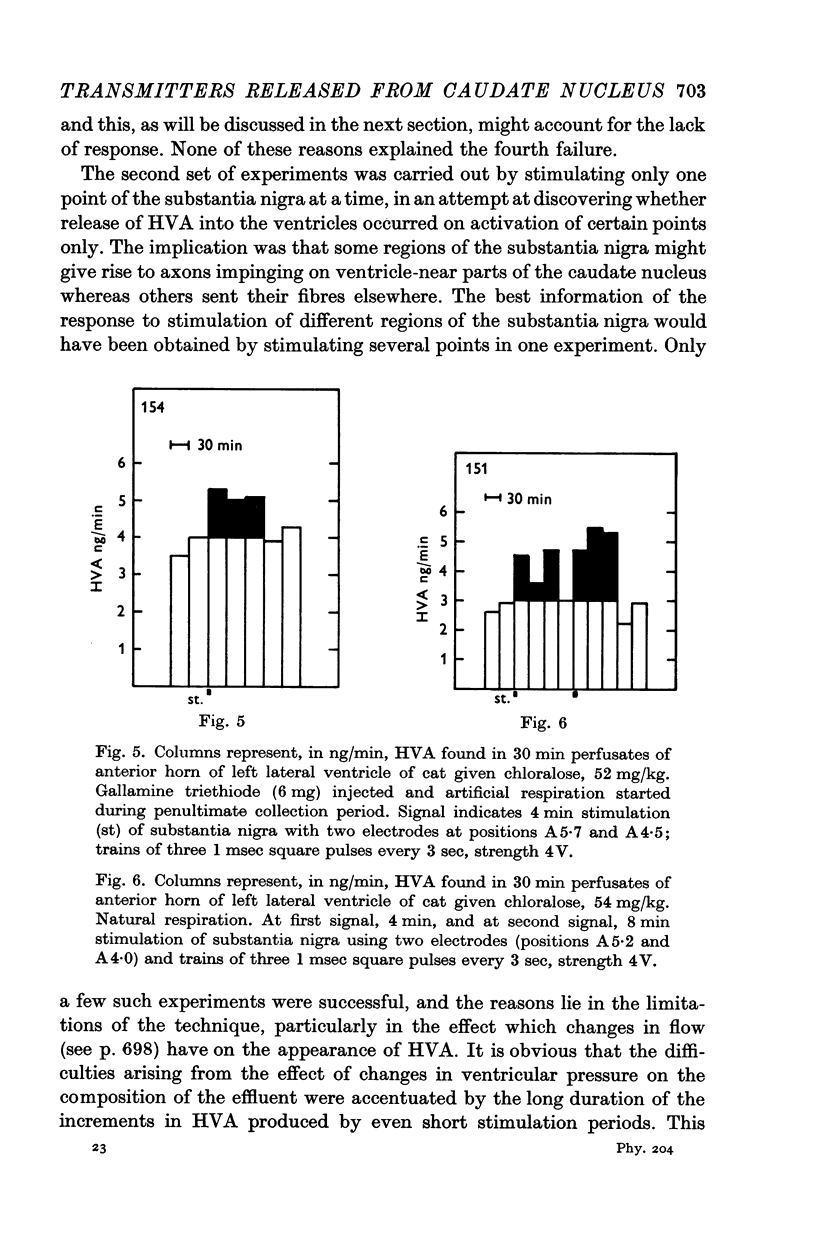
5. At rest, HVA was found to appear in the effluent at a rate of 2-8 ng/min. Its concentration was rapidly depressed by increasing the depth of anaesthesia.
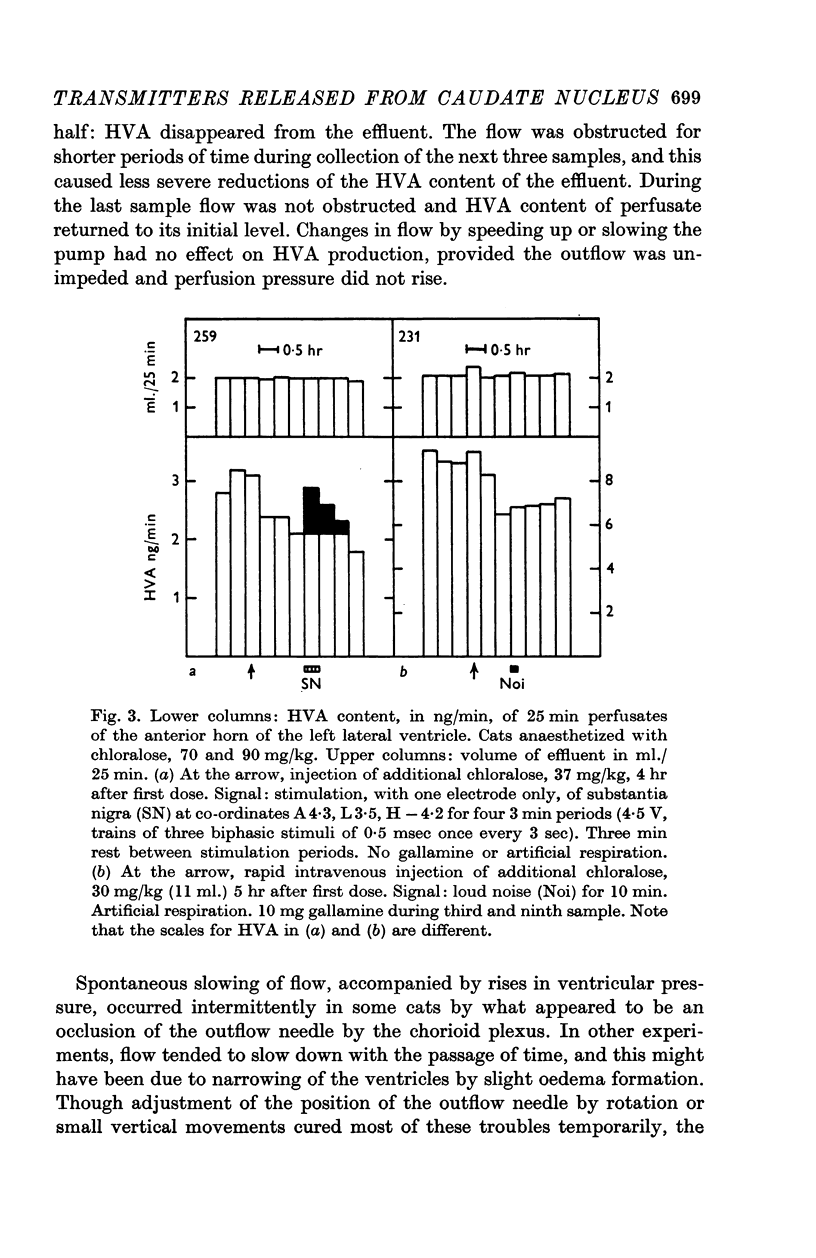
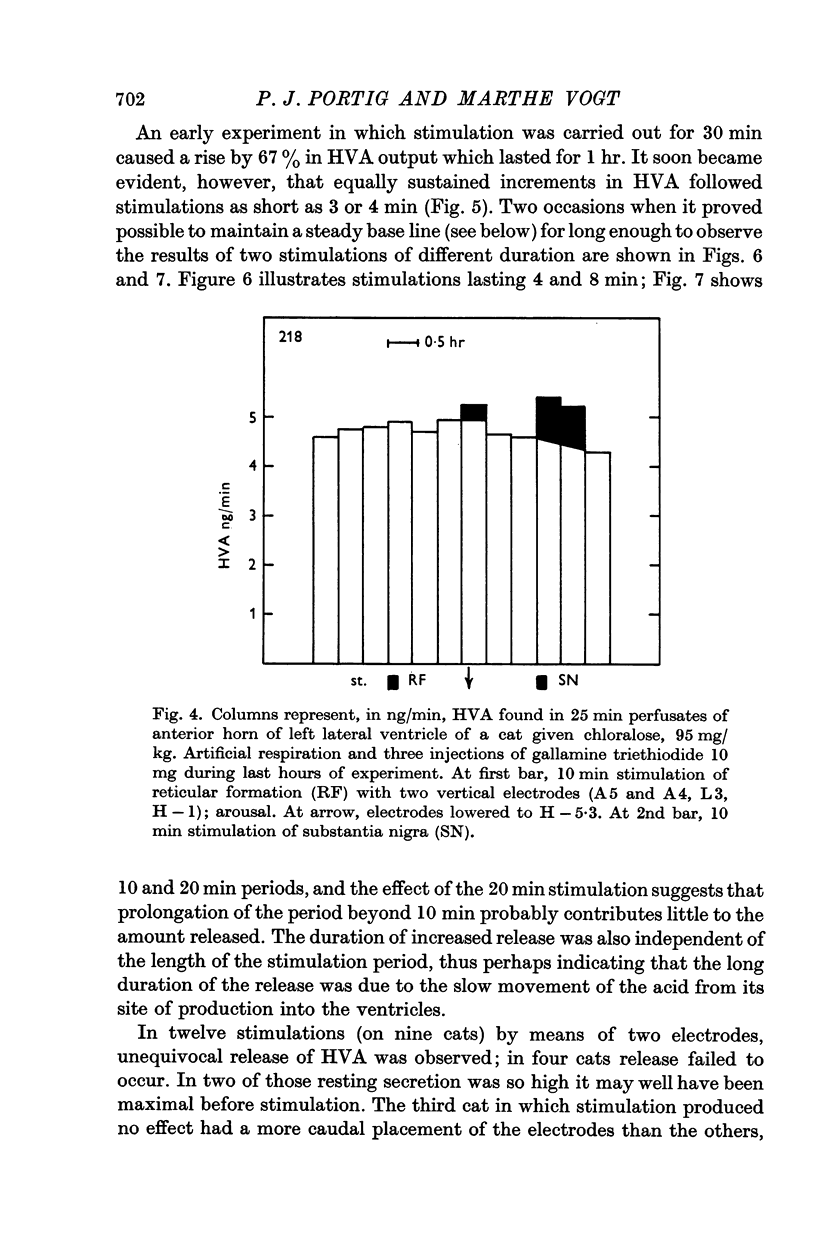
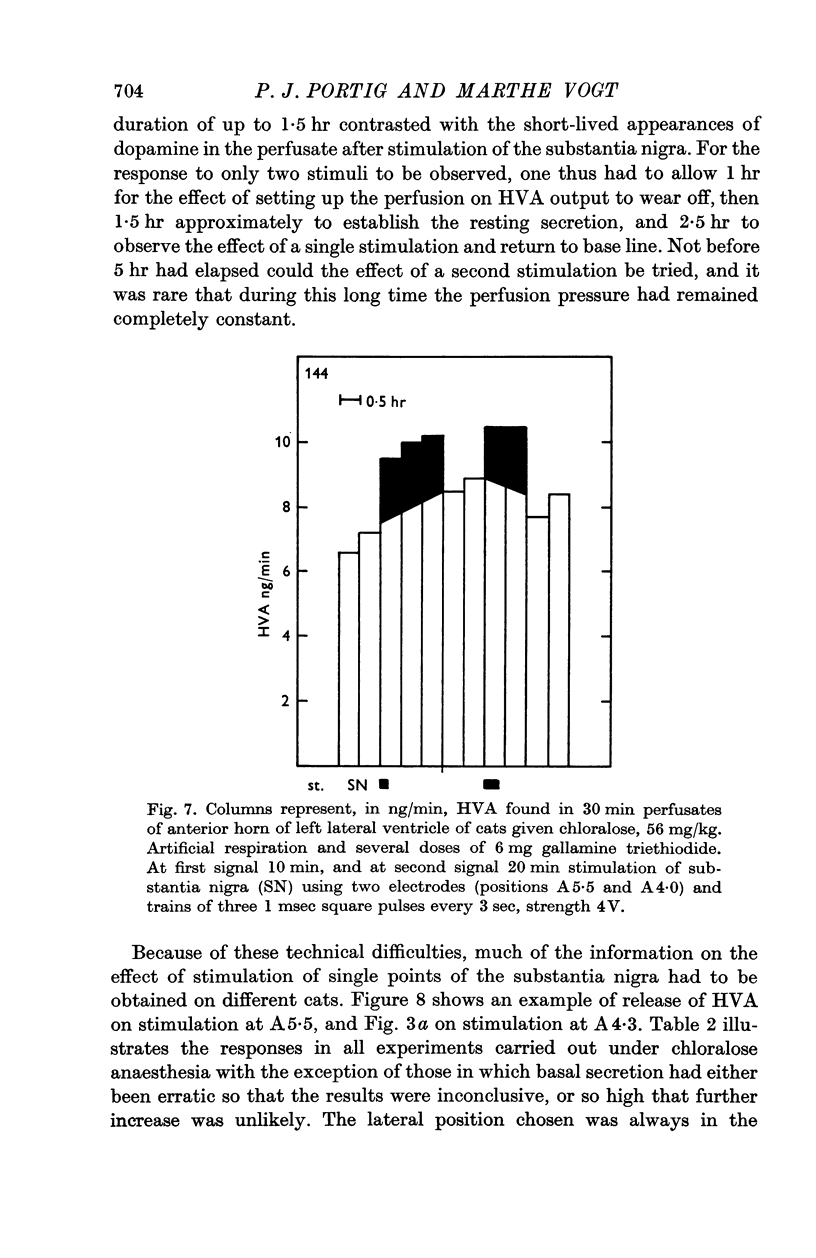
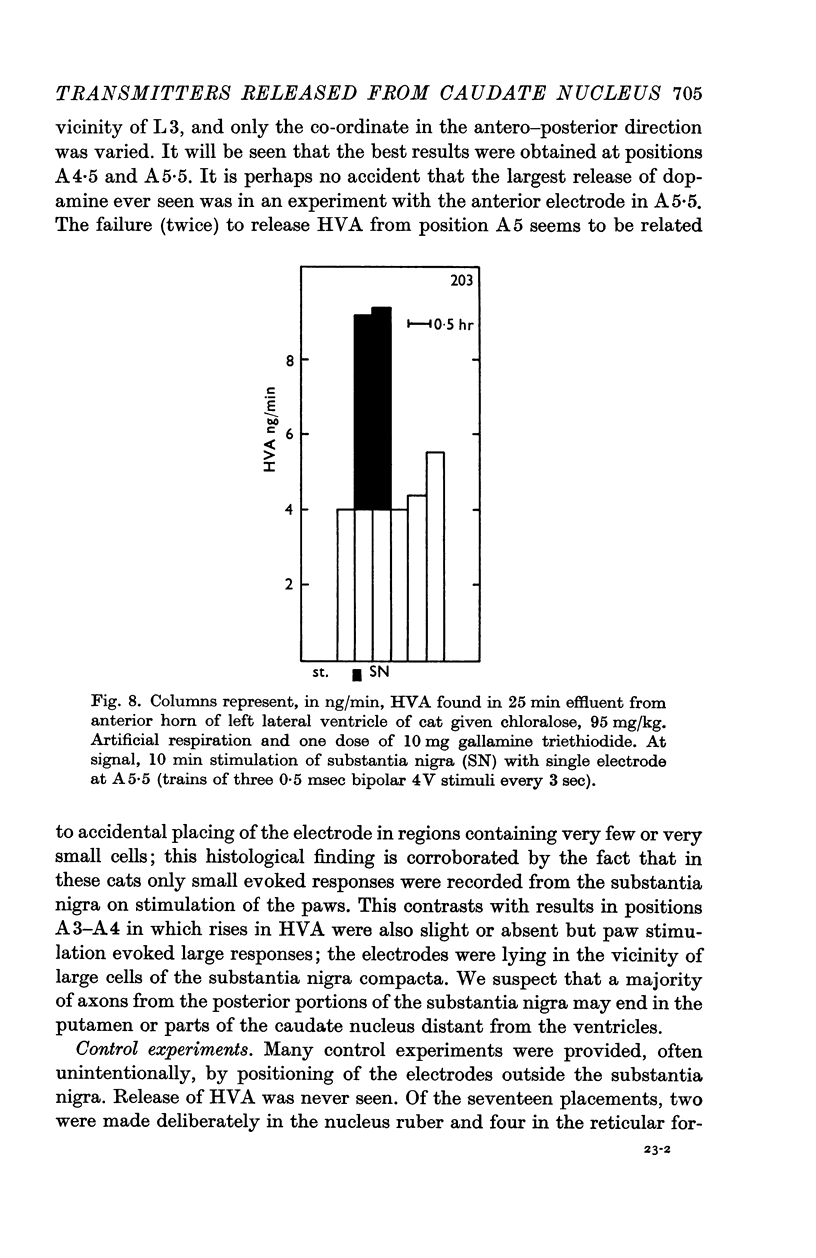
6. Stimulation of the substantia nigra for periods of 3 or 4 min caused an increment in the HVA content of the effluent lasting 1 hr or more. It was frequently seen when two points of the substantia nigra were stimulated simultaneously, less regularly with only one stimulating electrode, and rarely if this was placed in the most caudal part of the substantia nigra.
7. These results strongly support the view that there is a dopaminergic nigro-striatal pathway. The following assumption would explain the erratic appearance of dopamine and the long duration of increments in HVA: many of the axons originating in the substantia nigra end either in the putamen or in parts of the caudate nucleus which are far away from the ventricular surface; any dopamine released from these axons will not reach the ventricular surface at all, and HVA will, at best, reach it very slowly.
8. Small amounts of 5-HT appeared in the ventricular perfusate, and the quantity rose after the administration of monoamine oxidase inhibitors. It was not increased by the type of stimuli used in this work to elicit the release of ACh or HVA.
Full text
PDF





















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDEN N. E., CARLSSON A., DAHLSTROEM A., FUXE K., HILLARP N. A., LARSSON K. DEMONSTRATION AND MAPPING OUT OF NIGRO-NEOSTRIATAL DOPAMINE NEURONS. Life Sci. 1964 Jun;3:523–530. doi: 10.1016/0024-3205(64)90161-4. [DOI] [PubMed] [Google Scholar]
- Albe-Fessard D., Raieva S., Santiago W. Sur les relations entre substance noire et noyau caudé. J Physiol (Paris) 1967;59(4 Suppl):324–325. [PubMed] [Google Scholar]
- Andén N. E., Hfuxe K., Hamberger B., Hökfelt T. A quantitative study on the nigro-neostriatal dopamine neuron system in the rat. Acta Physiol Scand. 1966 Jul-Aug;67(3):306–312. doi: 10.1111/j.1748-1716.1966.tb03317.x. [DOI] [PubMed] [Google Scholar]
- BERTLER A., FALCK B., ROSENGREN E. THE DIRECT DEMONSTRATION OF A BARRIER MECHANISM IN THE BRAIN CAPILLARIES. Acta Pharmacol Toxicol (Copenh) 1963;20:317–321. doi: 10.1111/j.1600-0773.1964.tb01752.x. [DOI] [PubMed] [Google Scholar]
- BHATTACHARYA B. K., FELDBERG W. Perfusion of cerebral ventricles: assay of pharmacologically active substances in the effluent from the cisterna and the aqueduct. Br J Pharmacol Chemother. 1958 Jun;13(2):163–174. doi: 10.1111/j.1476-5381.1958.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONVALLET M., DELL P., HUGELIN A. Projections olfactives, gustatives, viscérales, vagales, visuelles et auditives au niveau des formation grises du cerveau antérieur du chat. J Physiol (Paris) 1952;44(2):222–224. [PubMed] [Google Scholar]
- Bloom F. E., Costa E., Salmoiraghi G. C. Anesthesia and the responsiveness of individual neurons of the caudate nucleus of the cat to acetylcholine, norepinephrine and dopamine administered by microelectrophoresis. J Pharmacol Exp Ther. 1965 Nov;150(2):244–252. [PubMed] [Google Scholar]
- CARLSSON A. The occurrence, distribution and physiological role of catecholamines in the nervous system. Pharmacol Rev. 1959 Jun;11(2 Pt 2):490–493. [PubMed] [Google Scholar]
- CARMICHAEL E. A., FELDBERG W., FLEISCHHAUER K. METHODS FOR PERFUSING DIFFERENT PARTS OF THE CAT'S CEREBRAL VENTRICLES WITH DRUGS. J Physiol. 1964 Oct;173:354–367. doi: 10.1113/jphysiol.1964.sp007461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARPENTER M. B., MCMASTERS R. E. LESIONS OF THE SUBSTANTIA NIGRA IN THE RHESUS MONKEY. EFFERENT FIBER DEGENERATION AND BEHAVIORAL OBSERVATIONS. Am J Anat. 1964 Mar;114:293–319. doi: 10.1002/aja.1001140209. [DOI] [PubMed] [Google Scholar]
- Carlsson A., Fuxe K., Hamberger B., Lindqvist M. Biochemical and histochemical studies on the effects of imipramine-like drugs and (+)-amphetamine on central and peripheral catecholamine neurons. Acta Physiol Scand. 1966 Jul-Aug;67(3):481–497. doi: 10.1111/j.1748-1716.1966.tb03334.x. [DOI] [PubMed] [Google Scholar]
- Chase T. N., Katz R. I., Kopin I. J. Release of [3H]serotonin from brain slices. J Neurochem. 1969 Apr;16(4):607–615. doi: 10.1111/j.1471-4159.1969.tb06860.x. [DOI] [PubMed] [Google Scholar]
- Chase T. N., Kopin I. J. Stimulus-induced release of substances from olfactory bulb using the push-pull cannula. Nature. 1968 Feb 3;217(5127):466–467. doi: 10.1038/217466a0. [DOI] [PubMed] [Google Scholar]
- Connor J. D. Caudate unit responses to nigral stimuli: evidence for a possible nigro-neostriatal pathway. Science. 1968 May 24;160(3830):899–900. doi: 10.1126/science.160.3830.899. [DOI] [PubMed] [Google Scholar]
- DAVEY M. J., FARMER J. B., REINERT H. The effects of nialamide on adrenergic functions. Br J Pharmacol Chemother. 1963 Feb;20:121–134. doi: 10.1111/j.1476-5381.1963.tb01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlström A., Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964 Jul 15;20(7):398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- EHRINGER H., HORNYKIEWICZ O. [Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system]. Klin Wochenschr. 1960 Dec 15;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- Feldberg W., Myers R. D. Appearance of 5-hydroxytryptamine and an unidentified pharmacologically active lipid acid in effluent from perfused cerebral ventricles. J Physiol. 1966 Jun;184(4):837–855. doi: 10.1113/jphysiol.1966.sp007951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigyesi T. L., Purpura D. P. Electrophysiological analysis of reciprocal caudato-nigral relations. Brain Res. 1967 Nov;6(3):440–456. doi: 10.1016/0006-8993(67)90057-1. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Hamberger B., Malmfores T. Inhibition of amine uptake in tubero-infundibular dopamine neurones and in catecholamine cell bodies of the area postrema. J Pharm Pharmacol. 1966 Aug;18(8):543–544. doi: 10.1111/j.2042-7158.1966.tb07924.x. [DOI] [PubMed] [Google Scholar]
- Glowinski J., Axelrod J., Iversen L. L. Regional studies of catecholamines in the rat brain. IV. Effects of drugs on the disposition and metabolism of H3-norepinephrine and H3-dopamine. J Pharmacol Exp Ther. 1966 Jul;153(1):30–41. [PubMed] [Google Scholar]
- Hunt R. The Fall of Blood-pressure resulting from the Stimulation of Afferent Nerves. J Physiol. 1895 Nov 16;18(5-6):381–410. doi: 10.1113/jphysiol.1895.sp000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. S., Roberts M. H., Sobieszek A., Straughan D. W. Excitation of cortical neurones by noradrenaline. Br J Pharmacol. 1968 Sep;34(1):221P–222P. [PMC free article] [PubMed] [Google Scholar]
- Levinger I. M., Edery H. Casts of the cat cerebro-ventricular system. Brain Res. 1968 Nov;11(2):294–304. doi: 10.1016/0006-8993(68)90025-5. [DOI] [PubMed] [Google Scholar]
- Lisch H. J., Aigner A., Hornykiewicz O. Dopamin-Stoffwechsel im Nucleus caudatus des Kaninchens nach partieller Hemmung der Gehirn-Monoaminoxydase mittels Nialamid. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;261(4):289–298. [PubMed] [Google Scholar]
- MARLEY E., PATON W. D. The output of sympathetic amines from the cat's adrenal gland in response to splanchnic nerve activity. J Physiol. 1961 Jan;155:1–27. doi: 10.1113/jphysiol.1961.sp006610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLENNAN H. THE RELEASE OF ACETYLCHOLINE AND OF 3-HYDROXYTYRAMINE FROM THE CAUDATE NUCLEUS. J Physiol. 1964 Oct;174:152–156. doi: 10.1113/jphysiol.1964.sp007478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie G. M., Szerb J. C. The effect of dihydroxyphenylalanine, pheniprazine and dextroamphetamine on the in vivo release of dopamine from the caudate nucleus. J Pharmacol Exp Ther. 1968 Aug;162(2):302–308. [PubMed] [Google Scholar]
- McLennan H. The release of dopamine from the putamen. Experientia. 1965 Dec 15;21(12):725–726. doi: 10.1007/BF02138500. [DOI] [PubMed] [Google Scholar]
- Mitchell J. F. The spontaneous and evoked release of acetylcholine from the cerebral cortex. J Physiol. 1963 Jan;165(1):98–116. doi: 10.1113/jphysiol.1963.sp007045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. F., Robinson D., Sharman D. F. The effect of tropolone on the formation of 3,4-dihydroxyphenylacetic acid and 4-hydroxy-3-methoxyphenylacetic acid in the brain of the mouse. Br J Pharmacol. 1969 May;36(1):107–115. doi: 10.1111/j.1476-5381.1969.tb08308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POIRIER L. J., SOURKES T. L. INFLUENCE OF THE SUBSTANTIA NIGRA ON THE CATECHOLAMINE CONTENT OF THE STRIATUM. Brain. 1965 Mar;88:181–192. doi: 10.1093/brain/88.1.181. [DOI] [PubMed] [Google Scholar]
- Polak R. L. Effect of hyoscine on the output of acetylcholine into perfused cerebral ventricles of cats. J Physiol. 1965 Nov;181(2):317–323. doi: 10.1113/jphysiol.1965.sp007763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portig P. J., Sharman D. F., Vogt M. Release by tubocurarine of dopamine and homovanillic acid from the superfused caudate nucleus. J Physiol. 1968 Feb;194(2):565–572. doi: 10.1113/jphysiol.1968.sp008425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECH R. H., DOMINO E. F. Observations on injections of drugs into the brain substance. Arch Int Pharmacodyn Ther. 1959 Sep 1;121:429–442. [PubMed] [Google Scholar]
- Richardson K. C. Electron microscopic identification of autonomic nerve endings. Nature. 1966 May 14;210(5037):756–756. doi: 10.1038/210756a0. [DOI] [PubMed] [Google Scholar]
- SEGUNDO J. P., MACHNE X. Unitary responses to afferent volleys in lenticular nucleus and claustrum. J Neurophysiol. 1956 Jul;19(4):325–339. doi: 10.1152/jn.1956.19.4.325. [DOI] [PubMed] [Google Scholar]
- SPECTOR S. Monoamine oxidase in control of brain serotonin and norepinephrine content. Ann N Y Acad Sci. 1963 Jul 8;107:856–864. doi: 10.1111/j.1749-6632.1963.tb13329.x. [DOI] [PubMed] [Google Scholar]
- Shute C. C., Lewis P. R. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967 Sep;90(3):497–520. doi: 10.1093/brain/90.3.497. [DOI] [PubMed] [Google Scholar]
- Spector S., Gordon R., Sjoerdsma A., Udenfriend S. End-product inhibition of tyrosine hydroxylase as a possible mechanism for regulation of norepinephrine synthesis. Mol Pharmacol. 1967 Nov;3(6):549–555. [PubMed] [Google Scholar]
- Thoenen H., Haefely W., Staehelin H. Potentiation by tetraethylammonium of the response of the cat spleen to postganglionic sympathetic nerve stimulation. J Pharmacol Exp Ther. 1967 Sep;157(3):532–540. [PubMed] [Google Scholar]
- VANE J. R. A sensitive method for the assay of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1957 Sep;12(3):344–349. doi: 10.1111/j.1476-5381.1957.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGT M. Catecholamines in brain. Pharmacol Rev. 1959 Jun;11(2 Pt 2):483–489. [PubMed] [Google Scholar]
- VOGT M. The concentration of sympathin in different parts of the central nervous system under normal conditions and after the administration of drugs. J Physiol. 1954 Mar 29;123(3):451–481. doi: 10.1113/jphysiol.1954.sp005064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M. Transmitter released in the cat uterus by stimulation of the hypogastric nerves. J Physiol. 1965 Jul;179(1):163–171. doi: 10.1113/jphysiol.1965.sp007655. [DOI] [PMC free article] [PubMed] [Google Scholar]


