Abstract
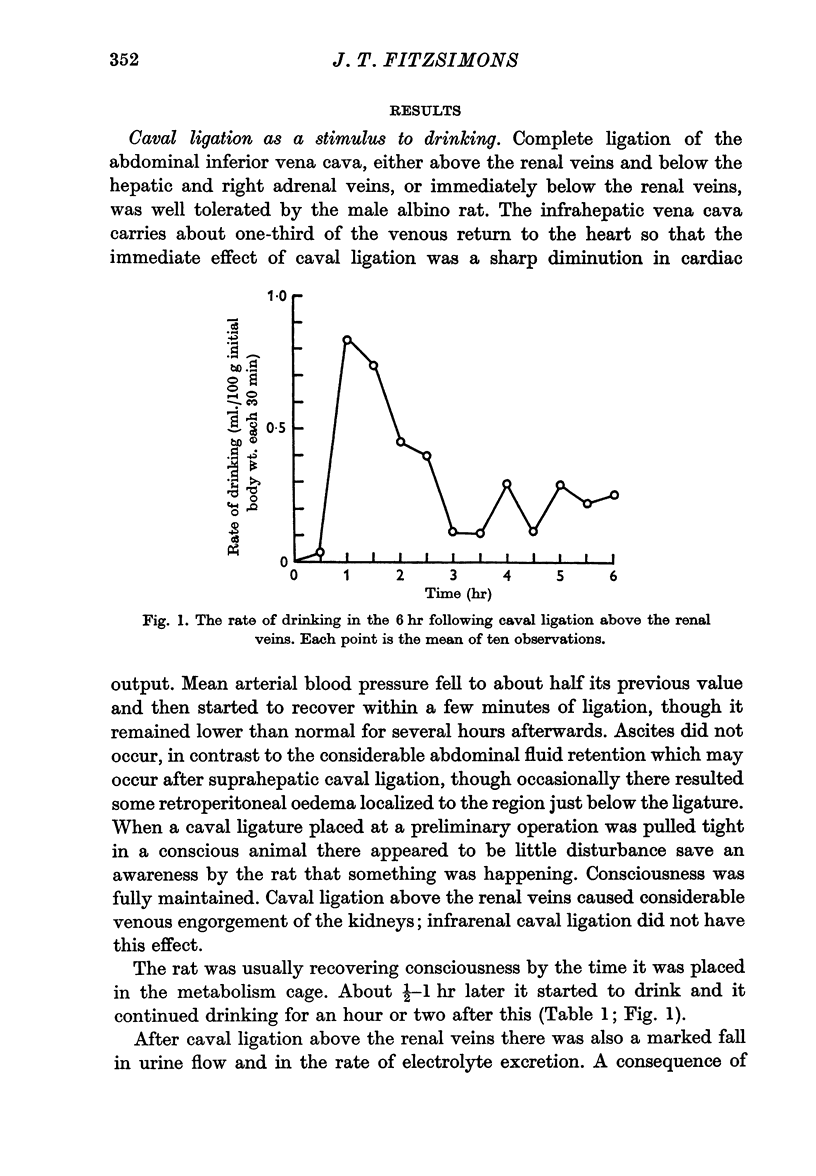
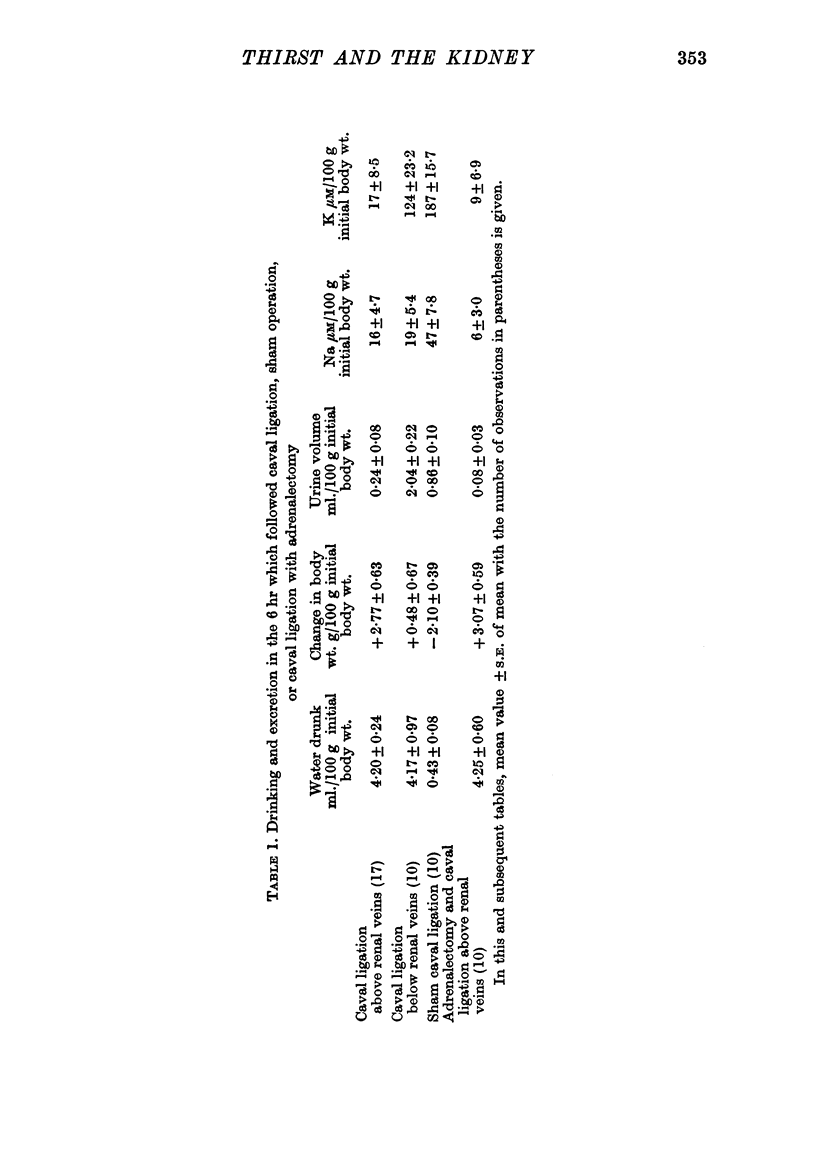
1. Rats in normal fluid balance drank water 1-2 hr after complete ligation of the inferior vena cava either above or below the renal veins. At the same time there was a fall in urine flow and excretion of electrolyte, especially after caval ligation above the renal veins, so that the animals ended the initial 6 hr period in positive fluid balance.
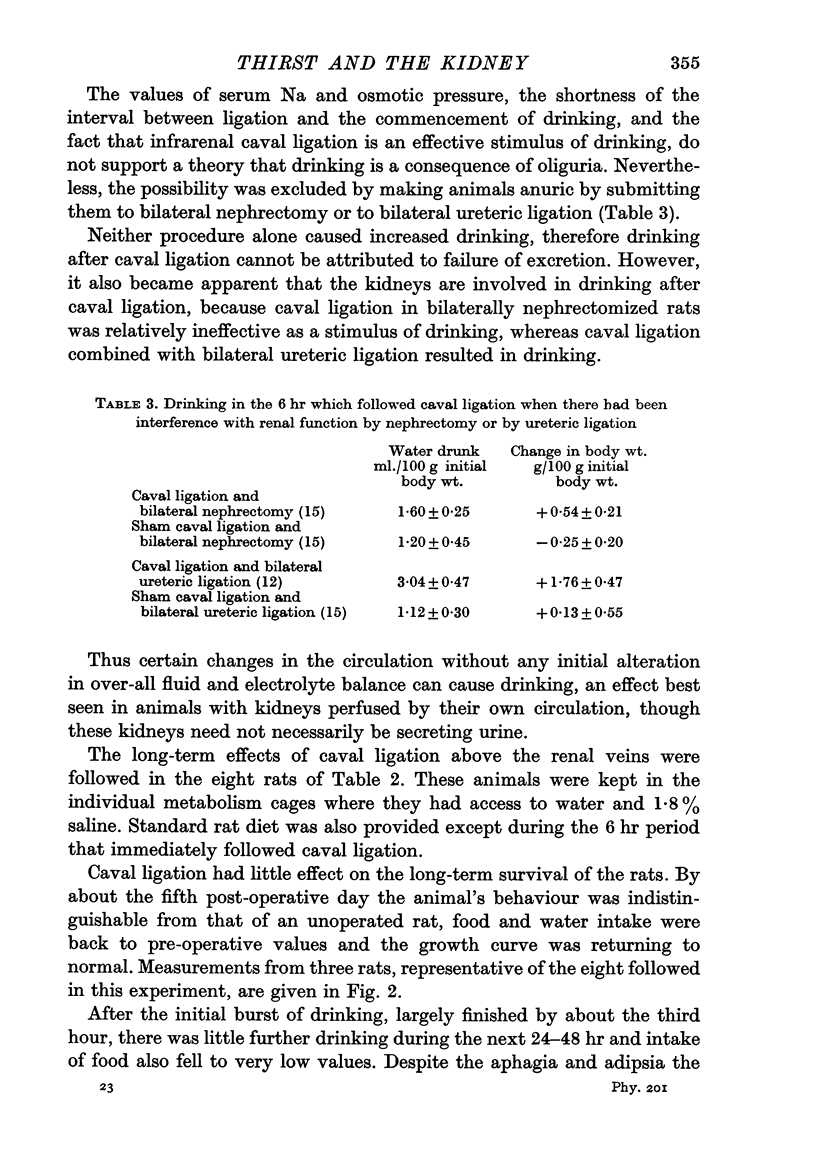
2. Caval ligation was relatively ineffective as a stimulus to drinking after bilateral nephrectomy, but was effective in rats made anuric by ureteric ligation.
3. Rats subjected to caval ligation and offered a choice between water and 1·8% saline (w/v) drank water, despite the increasing hypotonicity of the body fluids thereby resulting.
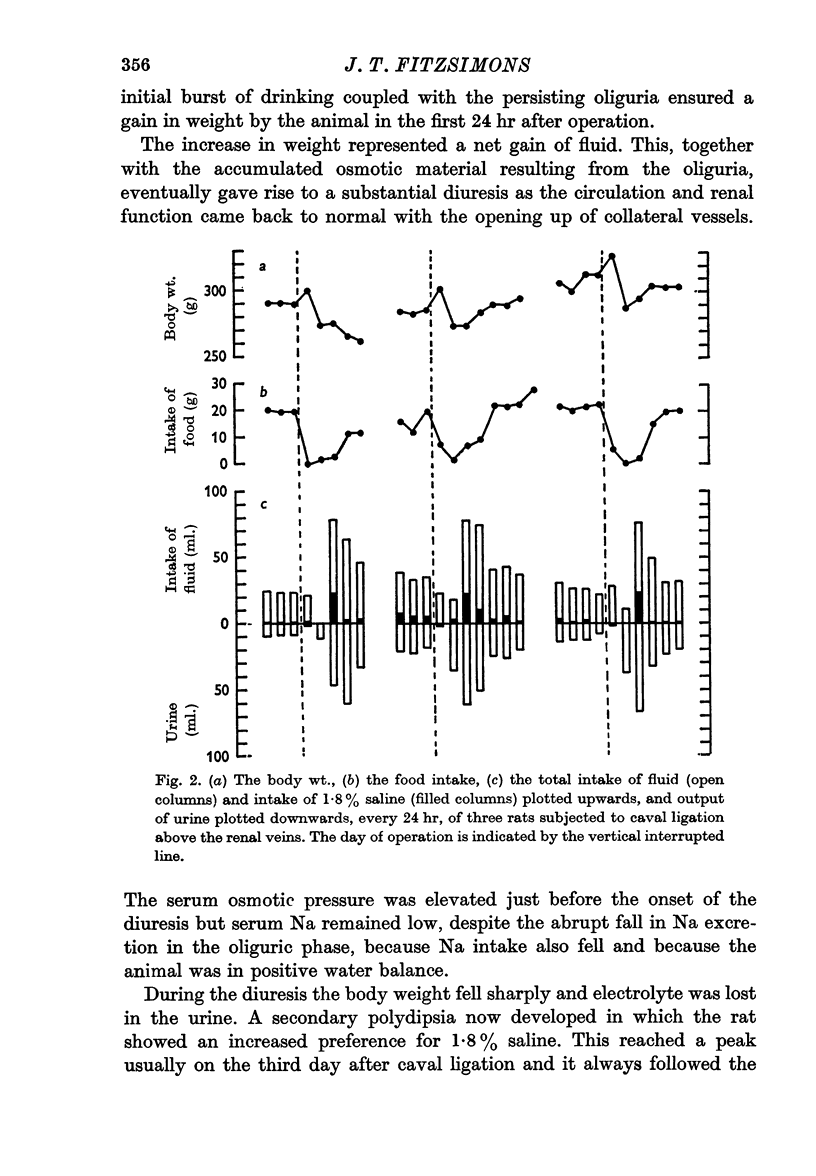
4. During the secondary polydipsia, which generally occurred on about the third day after caval ligation as renal function was recovering, there was an increased preference for 1·8% saline.
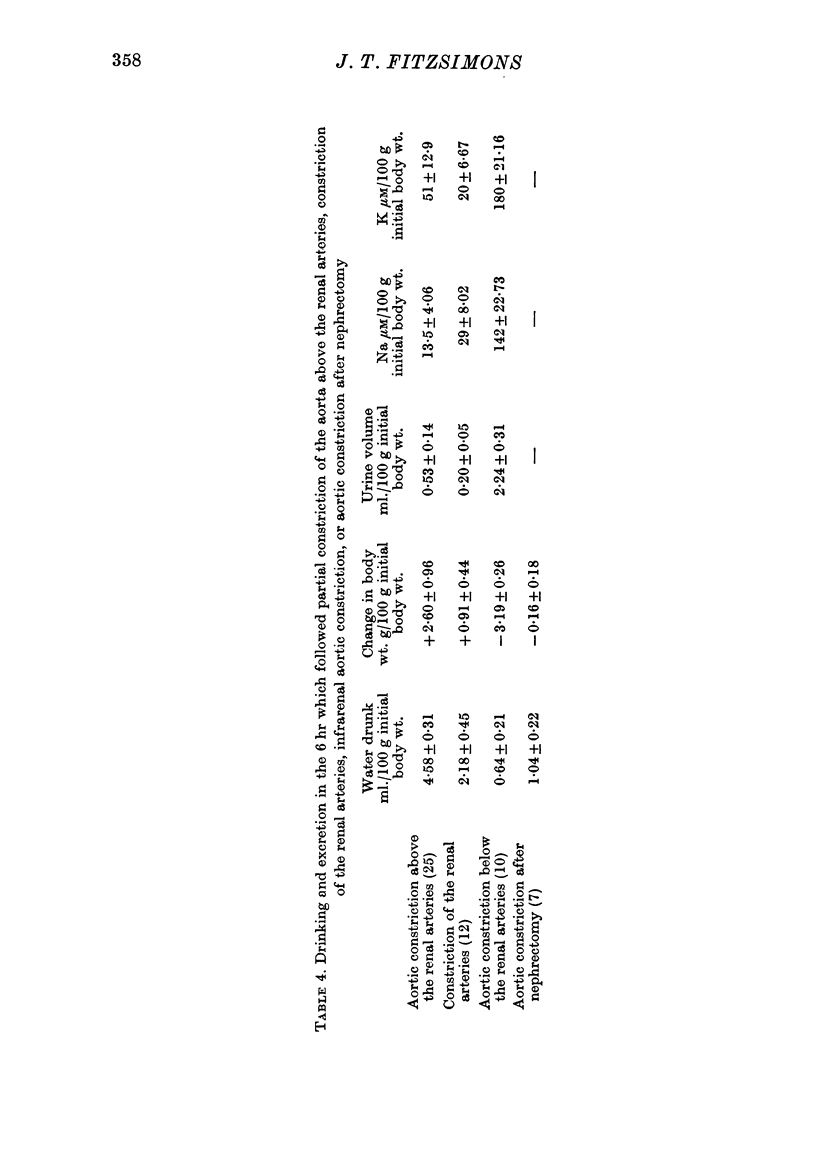
5. Constriction of the aorta above the renal arteries, or constriction of both renal arteries, also caused drinking, oliguria and the development of positive fluid balance.
6. Constriction of the aorta below the renal arteries, or after nephrectomy, was ineffective as a stimulus to drinking.
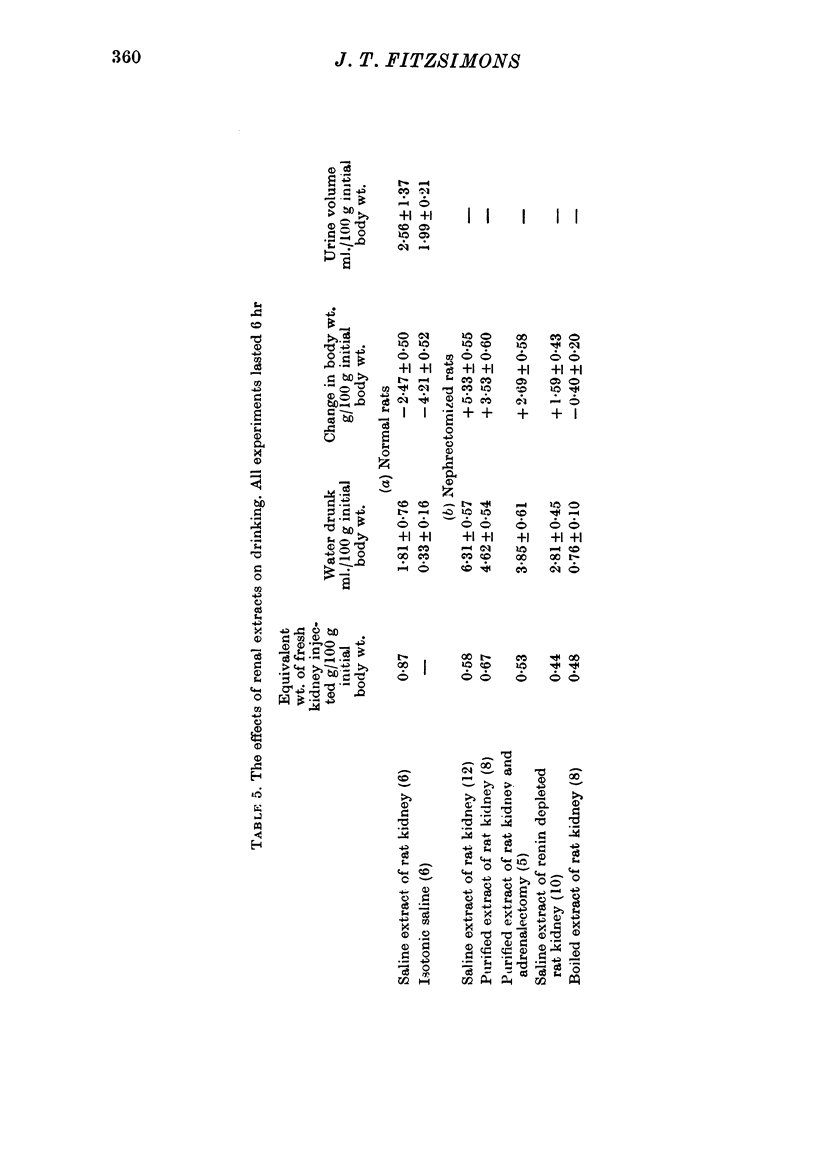
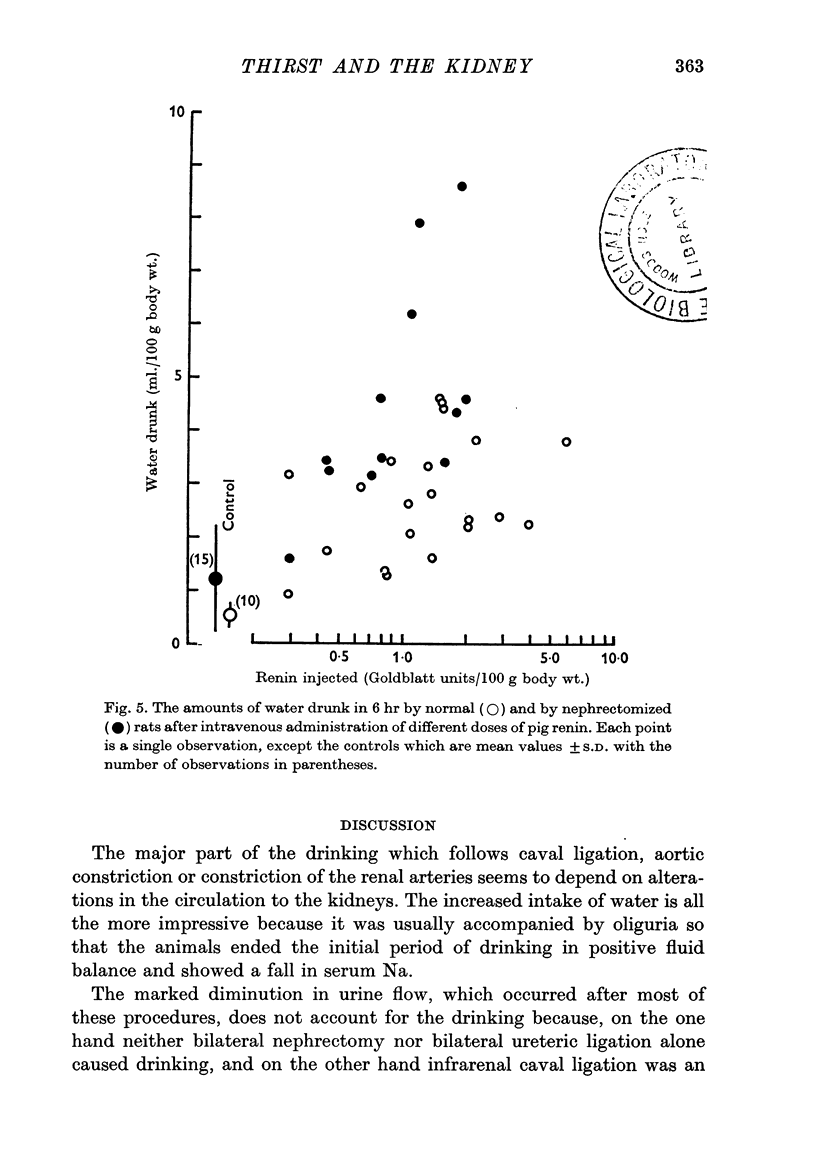
7. Saline extracts of renal cortex caused rats in normal water balance to drink. Activity was destroyed by boiling the extract for 10 min. Renal medullary and hepatic extracts were without effect on drinking.
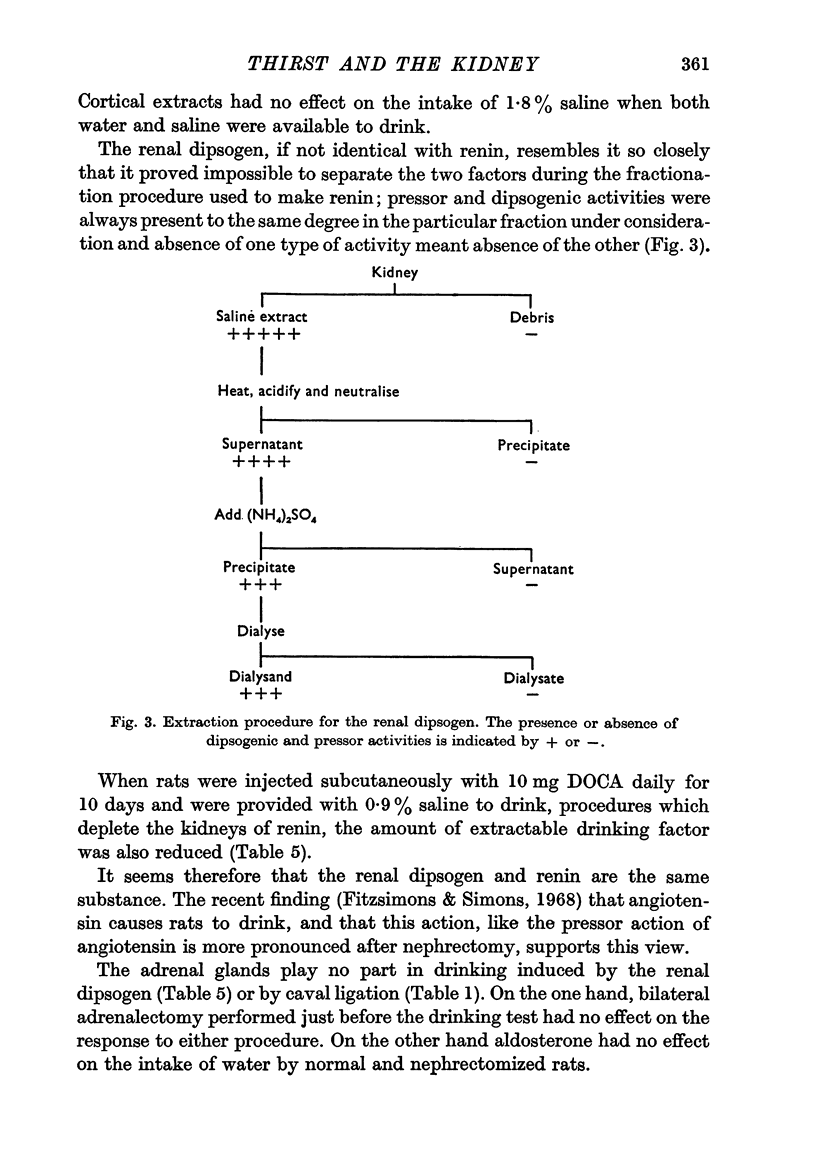
8. It proved impossible to separate dipsogenic and pressor activities of renal extracts during the different stages of fractionation which lead to the production of renin; disappearance of one activity was invariably accompanied by disappearance of the other.
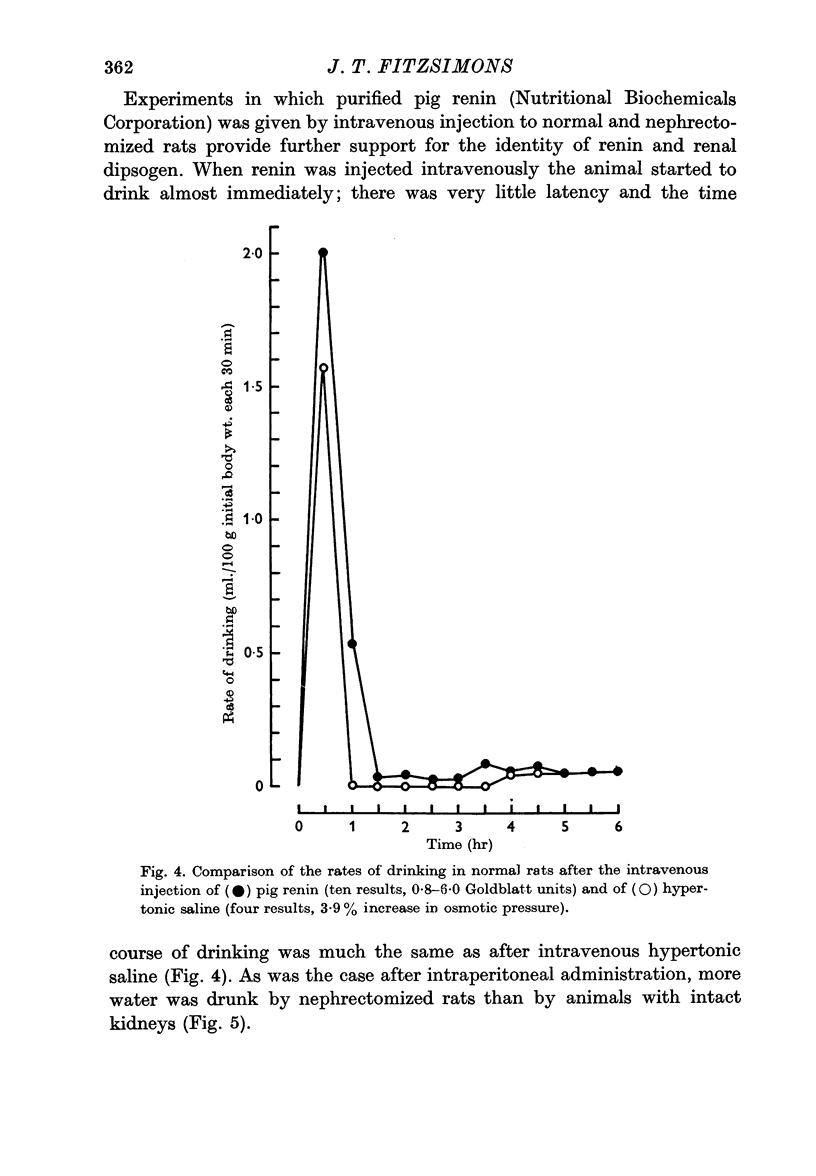
9. Dipsogenic and pressor actions were greater in nephrectomized rats than in normal rats.
10. Both extractable dipsogenic factor and extractable pressor activity were reduced by treating the rat with DOCA and saline for several weeks beforehand.
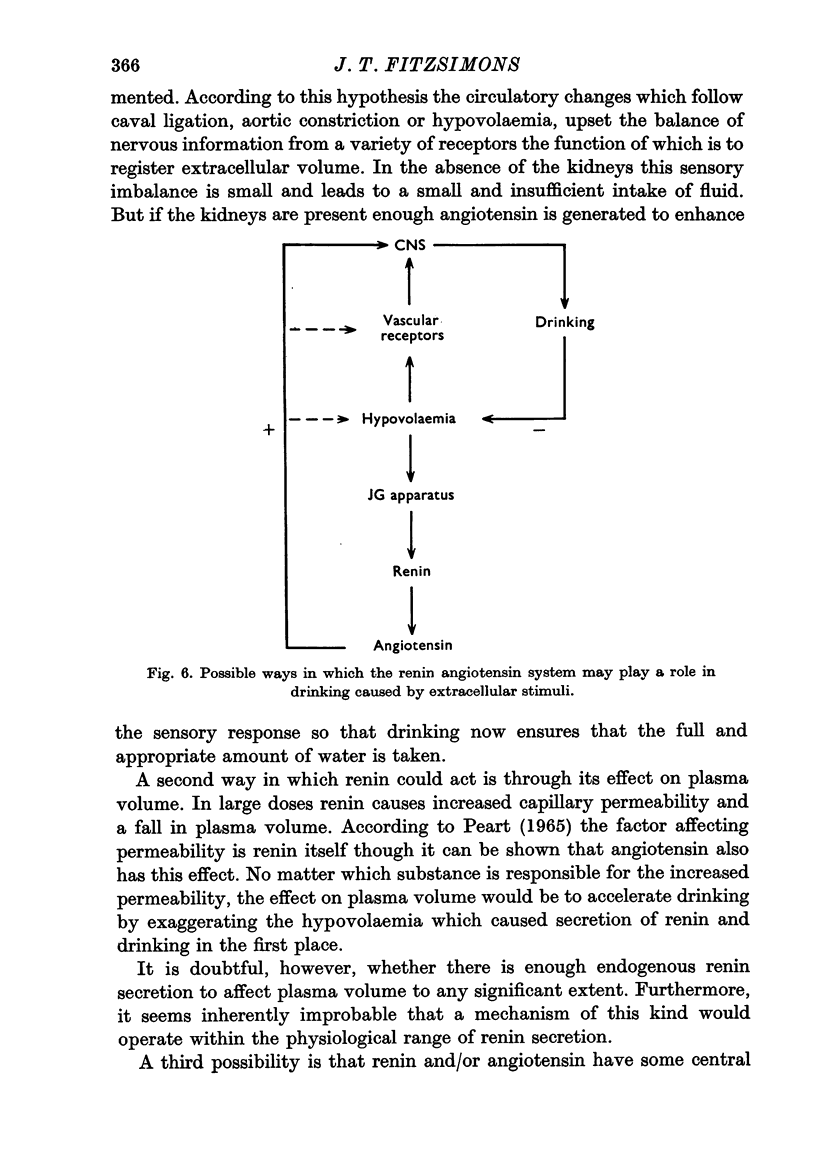
11. The renal dipsogen therefore has similar properties to renin. It may prove to be identical with renin, particularly in view of the fact that angiotensin also stimulates drinking.
12. Adrenalectomy did not affect drinking induced by renin or by caval ligation.
13. It is concluded that the renin angiotensin system may play a role in the genesis of the thirst which follows certain extracellular stimuli.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASSCHER A. W., ANSON S. G. A vascular permeability factor of renal origin. Nature. 1963 Jun 15;198:1097–1098. doi: 10.1038/1981097a0. [DOI] [PubMed] [Google Scholar]
- Booth D. A. Mechanism of action of norepinephrine in eliciting an eating response on injection into the rat hypothalamus. J Pharmacol Exp Ther. 1968 Apr;160(2):336–348. [PubMed] [Google Scholar]
- Carretero O., Gross F. Renin substrate in plasma under various experimental conditions in the rat. Am J Physiol. 1967 Sep;213(3):695–700. doi: 10.1152/ajplegacy.1967.213.3.695. [DOI] [PubMed] [Google Scholar]
- Cuthbert M. F., Asscher A. W., Jones J. H. Characterization of a vascular permeability factor of renal origin. Clin Sci. 1966 Dec;31(3):325–336. [PubMed] [Google Scholar]
- Epstein A. N., Fitzsimons J. T., Simons B. J. Drinking caused by the intracranial injection of angiotensin into the rat. J Physiol. 1969 Feb;200(2):98P–100P. [PubMed] [Google Scholar]
- FITZSIMONS J. T. DRINKING CAUSED BY CONSTRICTION OF THE INFERIOR VENA CAVA IN THE RAT. Nature. 1964 Oct 31;204:479–480. doi: 10.1038/204479a0. [DOI] [PubMed] [Google Scholar]
- FITZSIMONS J. T. Drinking by rats depleted of body fluid without increase in osmotic pressure. J Physiol. 1961 Dec;159:297–309. doi: 10.1113/jphysiol.1961.sp006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons J. T. The kidney as a thirst receptor. J Physiol. 1967 Jul;191(2):128P–129P. [PubMed] [Google Scholar]
- HAAS E., LAMFROM H., GOLDBLATT H. A simple method for the extraction and partial purification of renin. Arch Biochem Biophys. 1954 Feb;48(2):256–260. doi: 10.1016/0003-9861(54)90339-2. [DOI] [PubMed] [Google Scholar]
- LINAZASORO J. M., JIMENEZ DIAZ C., CASTRO MENDOZA H. The kidney and thirst regulation. Bull Inst Med Res Kuala Lumpur. 1954 Apr-Jun;7(2):53–61. [PubMed] [Google Scholar]
- NAIRN R. C., MASSON G. M., CORCORAN A. C. The production of serous effusions in nephrectomised animals by the administration of renal extracts and renin. J Pathol Bacteriol. 1956 Jan;71(1):155–163. doi: 10.1002/path.1700710121. [DOI] [PubMed] [Google Scholar]
- OATLEY K. CHANGES OF BLOOD VOLUME AND OSMOTIC PRESSURE IN THE PRODUCTION OF THIRST. Nature. 1964 Jun 27;202:1341–1342. doi: 10.1038/2021341a0. [DOI] [PubMed] [Google Scholar]
- PEART W. S. THE RENIN-ANGIOTENSIN SYSTEM. Pharmacol Rev. 1965 Jun;17:143–182. [PubMed] [Google Scholar]
- SCHAECHTELIN G., REGOLI D., GROSS F. QUANTITATIVE ASSAY AND DISAPPEARANCE RATE OF CIRCULATING RENIN. Am J Physiol. 1964 Jun;206:1361–1364. doi: 10.1152/ajplegacy.1964.206.6.1361. [DOI] [PubMed] [Google Scholar]
- SEMPLE R. E. Compensatory changes in the rat following removal of electrolytes by intraperitoneal dialysis. Am J Physiol. 1952 Jan;168(1):55–65. doi: 10.1152/ajplegacy.1951.168.1.55. [DOI] [PubMed] [Google Scholar]
- Stricker E. M. Extracellular fluid volume and thirst. Am J Physiol. 1966 Jul;211(1):232–238. doi: 10.1152/ajplegacy.1966.211.1.232. [DOI] [PubMed] [Google Scholar]


