Abstract
We found that the ability to develop genetic competence of a certain relaxed (relA) aspartate-auxotrophic strain of Bacillus subtilis is significantly lower than that of the isogenic stringent (relA+) strain. Transcriptional fusion analysis utilizing a lacZ reporter gene indicated that the amount of the ComK protein, known as the key protein for competence development, is greatly reduced in the relaxed strain than in the stringent strain. We also found that the addition of decoyinine, a GMP synthetase inhibitor, induces expression of a competence gene (comG) in the relaxed strain, accompanied by a pronounced decrease in the level of intracellular GTP as measured by high-performance liquid chromatography. The transformation efficiency of the relaxed strain increased 100-fold when decoyinine was added at t0 (the transition point between exponential to stationary growth phase). Conversely, supplementation of guanosine together with decoyinine completely abolished the observed effect of adding decoyinine on competence development. Furthermore, the impaired ability of the relaxed strain for competence development was completely restored by disrupting the codY gene, which is known to negatively control comK expression. Our results indicate that the RelA protein plays an essential role in the induction of competence development at least under certain physiological conditions by reducing the level of intracellular GTP and overcoming CodY-mediated regulation.
When Bacillus subtilis cells encounter adverse nutrient conditions, cells start to approach the stationary growth phase by initiating several development processes that contribute to its survival. Of these processes, genetic competence and sporulation have been studied in great detail. Genetic competence is a physiological state that enables cells to incorporate exogenous DNA, which is then incorporated into the endogenous DNA by crossing over. Competence development is tightly controlled by a complex regulatory network involving kinases, phosphatases, and transcriptional regulators (4). The most critical regulator is a transcriptional factor named ComK, which has been shown to be necessary for inducing the late competence genes such as comG (27, 28). It has been demonstrated that the cellular level of ComK is precisely controlled by several regulatory proteins at the transcriptional and posttranscriptional levels (2, 5, 6, 25, 26). During the exponential growth phase, ComK activity is inhibited by a complex of two proteins, MecA and ClpC (10, 26). The ComS protein, which is encoded by the srfA operon and is synthesized in response to quorum-sensing oligopeptide pheromones (13, 23), releases cells from ComK inhibition by dissociating the ternary complex with MecA and ClpC (25). In addition, Serror and Sonenshein (21) demonstrated that CodY, which is a global regulator for stationary-phase genes, directly binds to the srfA and comK promoter region. Thus, it is highly likely that CodY acts as a negative regulator for competence genes.
One of the most important adaptation systems for bacteria is the stringent response, which leads to the repression of stable RNA synthesis (reviewed by Cashel et al. [1]). The stringent response depends on the transient increase of hyperphosphorylated guanosine nucleotides [(p)ppGpp], which are synthesized from GDP or GTP by the relA gene product (called stringent factor [ppGpp synthetase]) in response to binding of uncharged tRNA to the ribosomal A site (reviewed by Cashel et al. [1]). Amino acid depletion in B. subtilis gives rise to the transient increase of (p)ppGpp, accompanied by the reduction of intracellular GTP, eventually leading to the induction of spore formation in relA+ (stringent) cells but not in relA (relaxed) mutant cells (11, 12, 16-18). In the course of studying the ribosomal function of B. subtilis, we have recently found that genetic competence in a certain aspartate-auxotrophic B. subtilis strain is affected significantly by the relA mutation. In this study, attempts were made to demonstrate that the RelA protein plays an essential role in induction of genetic competence by reducing the intracellular GTP level in certain genetic backgrounds. This paper is the first demonstration showing that GTP functions as a nutritional signal for competence development, as predicted by the regulatory role of CodY, a GTP binding protein, in competence, and that the reduction in GTP levels during the onset of stationary phase is partially dependent upon RelA in nutritionally rich culture conditions.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Bacterial strains used in this study are listed in Table 1. Strain 168 is the standard strain of B. subtilis used for studying sporulation of this organism. The aspartate-auxotrophic B. subtilis strains 61884 and 61883 are isogenic with respect to relA1 (17). This relA1 mutation is a leaky rel mutation (11, 17) and has an amino acid alteration at position 240 (Gly to Glu) (as determined by DNA sequencing in this study). B. subtilis relA null mutants are known to demand valine for growth in synthetic medium and to be partially auxotrophic for isoleucine, leucine, and methionine (29). Unlike relA null mutants, the relA1 strain does not demand any amino acids for growth on synthetic medium, possibly because of leaky rel character.
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype | Construction or source |
|---|---|---|
| 61884 | trpC2 aspB66 | Laboratory stock (17) |
| 61883a | trpC2 aspB66 relA1 | Laboratory stock (17) |
| 168 | trpC2 | Laboratory stock |
| YO-005 | hisH101 | Laboratory stock (8) |
| K2-007 | trpC2 relA1 | 61883→YO-005b |
| RIK720 | trpC2 lys-1 aprEΔ3 nprR2 nprE18 clpCΔS amyE::(comG-lacZ cat) | F. Kawamura (14) |
| RIK51 | trpC2 lys-1 aprEΔ3 nprR2 nprE18 amyE::(comK-lacZ cat) | F. Kawamura (5) |
| 61884G | trpC2 aspB66 amyE::(comG-lacZ cat) | RIK720→61884 |
| 61883G | trpC2 aspB66 relA1 amyE::(comG-lacZ cat) | RIK720→61883 |
| 61884K | trpC2 aspB66 amyE::(comK-lacZ cat) | RIK51→61884 |
| 61883K | trpC2 aspB66 relA1 amyE::(comK-lacZ cat) | RIK51→61883 |
| TI70 | trpC2 aspB66 codY::neo | pCR2.1-codY::neo→61884 |
| TI71 | trpC2 aspB66 relA1 codY::neo | pCR2.1-codY::neo→61883 |
The relA1 mutation was originally found by Swanton and Edlin (24) in 1972.
The strain to the right of the arrow was transformed with the chromosomal DNA or plasmid to the left of the arrow.
All of the strains used were grown aerobically at 37°C using L medium (10 g of tryptone, 5 g of yeast extract, and 5 g of NaCl [all amounts given per liter]) or Spizizen's salts medium [14 g of K2HPO4, 6 g of KH2PO4, 2 g of (NH4)2SO4, 1 g of sodium citrate, 0.2 g of MgSO4·7H2O, and 5 g of glucose (all amounts given per liter)]. For B. subtilis transformation, cells were grown in TF medium (Spizizen's salts medium supplemented with 0.05% yeast extract, 0.03% Casamino Acids, 2 μM MnCl2, and excess amounts of amino acids as required [50 μg of tryptophan per ml and 20 mM sodium aspartate]). For B. subtilis sporulation, TF medium was further supplemented with 1 mM Ca(NO3)2·4H2O, 0.1 mM MnCl2·4H2O, and 1 μM FeSO4·7H2O to promote sporulation. If necessary, neomycin (3 μg/ml) (for selection of codY mutants) or ampicillin (50 μg/ml) (for E. coli transformants) was added to the medium.
Disruption of the codY gene.
The codY gene was disrupted by insertion of the neo gene within the coding region as follows. The complete coding region of the codY gene (780 bp) was cloned into plasmid pCR2.1 after amplification by PCR with primers codY-F (5′-ATGGCTTTATTACAAAAAACAAGAAT-3′) and codY-R (5′-TTAATGAGATTTTAGATTTTCTAATTCAA-3′), and the resulting plasmid was designated pCR2.1-codY. A 1.3-kb SmaI fragment containing the neo gene derived from pBEST501 (9) was inserted at the SspI site within the codY gene in pCR2.1-codY, creating pCR2.1-codY::neo. Integration of pCR2.1-codY::neo into the chromosome of strain 61884 (relA+) or 61883 (relA) by double-crossover recombination gave rise to strain TI70 or TI71, respectively. The disruption of the codY gene was confirmed by PCR amplification using the codY-F and codY-R primers and subsequent sequencing of the flanking region.
Estimation of the transformation efficiency of B. subtilis.
B. subtilis strains 61883 and 61884 were grown at 37°C in TF medium containing 20 mM aspartate. Samples were taken at appropriate times as indicated in the figures and exposed to the chromosomal DNA (1 μg/ml) from strain 168 for 30 min at 37°C with gentle shaking. The cells were then spread on Spizizen's salts agar plate containing 50 μg of tryptophan per ml. After incubation for 2 days at 37°C, Asp+ transformants were scored.
β-Galactosidase assay.
β-Galactosidase assay was performed as described previously (14). The specific activity was calculated as follows: (A420 per minute per milliliter of culture per A650) × 1,000, where A420 is the absorbance of o-nitrophenol, the product of cleavage of the β-galactosidase substrate, o-nitrophenol-β-d-galactoside, and A650 is the absorbance of the culture at the time of sampling.
Measurement of the intracellular GTP and ppGpp pool sizes.
The intracellular GTP and ppGpp pool sizes were determined as described previously (17). The content of GTP (and also of ppGpp) was determined by high-performance liquid chromatography analysis using a Partisil-10 SAX column (GL Science). Concentrations of these nucleotides are expressed as picomoles per AM650 (picomoles per milliliter of culture per A650).
RESULTS
Effect of the relA mutation on competence development.
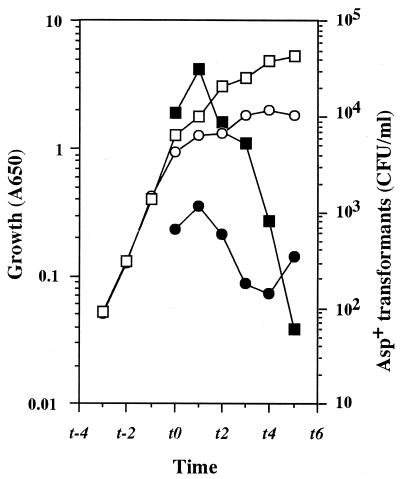
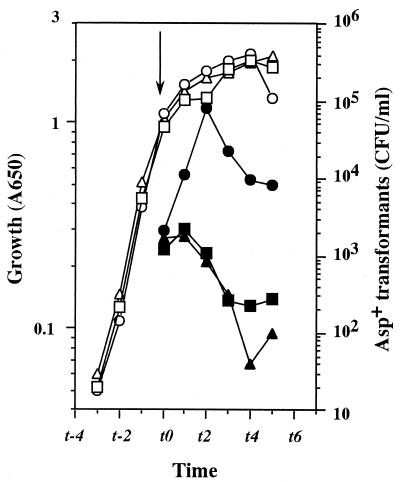
The aspartate-auxotrophic B. subtilis strain 61884 demands a high concentration (20 mM) of aspartate for full growth. Introduction of the relaxed relA mutation has a deleterious effect on growth (and sporulation) because more aspartate drains into the purine and pyrimidine nucleotide biosynthetic pathway (17). To determine the effect of the relA mutation on competence development in B. subtilis, we first compared the abilities to develop competence of the stringent (61884) and relaxed (61883) strains, since these isogenic strains offer a feasible system for studying the secondary metabolism of this organism (19). The transformation efficiency in the stringent strain peaked at t1 (1 h after the transition from exponential to stationary phase) (Fig. 1). In contrast, the transformation efficiency in the relA strain was shown to be significantly lower (by 40-fold) than that of the stringent strain (Fig. 1). Although the final cell mass of the relA strain was only one-third that of the stringent strain (Fig. 1), the difference in the transformation frequency is apparently much greater than the difference in the number of total viable cells. Although a B. subtilis relA null mutant requires valine, isoleucine, leucine, and methionine for growth in synthetic medium (29), the addition of these amino acids at a concentration of 50 μg/ml each did not restore the abilities to grow and to be transformed in strain 61883 (data not shown).
FIG. 1.
Transformation of relaxed (relA) and stringent (relA+) strains. B. subtilis strains 61884 (stringent) and 61883 (relaxed) were grown in TF medium containing 20 mM aspartate. Samples were taken at the indicated times to determine culture density (A650) and transformation efficiency (as discussed in Materials and Methods). The transformation efficiency is expressed as the number of asp+ transformants generated after exposure to chromosomal DNA from strain 168 (1 μg/ml) for 30 min. Growth (open symbols) and transformation efficiency (closed symbols) of strain 61883 (circles) and strain 61884 (squares) are shown.
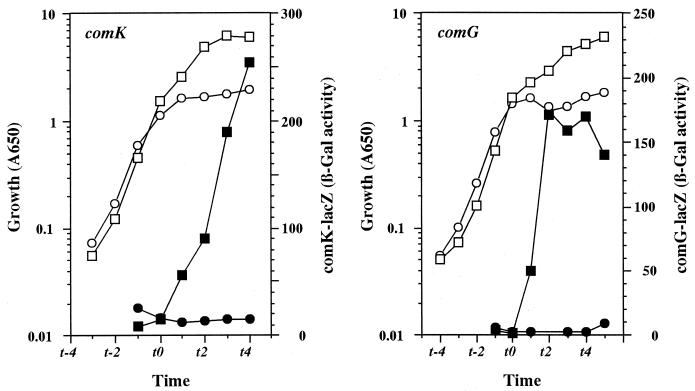
Extensive studies on the development of competence in B. subtilis have shown that the ComK protein can induce transcription of all the late competence genes that play a role in the uptake of exogenous DNA (2, 4, 7). To determine whether the ComK protein is produced in the relaxed strain, we monitored the expression of representative competence genes, comK and comG, using a transcriptional fusion of the target gene promoter to lacZ at the amyE locus. While both fusion genes were expressed at high levels in the stringent (rel+) strain, expression in the relA strain was 20-fold lower (comK) or was substantially undetectable (comG) compared to that in the stringent strain (Fig. 2). These results indicate that the level of ComK protein is dramatically reduced in the relA strain (61883).
FIG. 2.
Expression of competence genes comK and comG in the relaxed (relA) and stringent (relA+) strains. A stringent strain (strain 61884 [squares]) and relaxed strain (strain 61883 [circles]) carrying either the comK-lacZ or comG-lacZ transcriptional fusion gene at the amyE locus were grown in TF medium containing 20 mM aspartate. Culture samples were taken at the indicated times, and culture density (A650 [open symbols]) and β-galactosidase (β-Gal) activity (closed symbols) were measured.
Aspartate is responsible for competence efficiency.
B. subtilis strain 168 (asp+ relA+ strain) and its isogenic relA strain, K2-007 (asp+ relA), were transformed at similar efficiencies when grown in TF medium without aspartate, although the development of competence was delayed 1 h in K2-007 cells. The asp+ transformant of strain 61883 was also shown to be transformed as efficiently as strain 168 (data not shown). To explain these observations, we suggested two possibilities: (i) the combination of aspB and relA mutations is responsible for the decreased efficiency of competence, or (ii) the presence of an excess amount (20 mM) of aspartate in the culture medium for strain 61883 is responsible for decreased efficiency. The latter possibility was verified to be correct. Strain K2-007 (asp+ relA) revealed a 30- to 50-fold decrease in competence efficiency when cells were grown in TF medium supplemented with high concentrations (10 to 20 mM) of aspartate. Thus, the presence of aspartate, rather than the aspB mutation, is responsible for the decreased competence ability of the relA mutant 61883. Similar to the effect of adding aspartate, the transformation frequency of asp+ relA mutant K2-007 was also reduced by 30-fold by adding 20 mM glutamate to the TF medium (data not shown). Arginine had no such effect.
Effect of decoyinine on comG-lacZ expression.
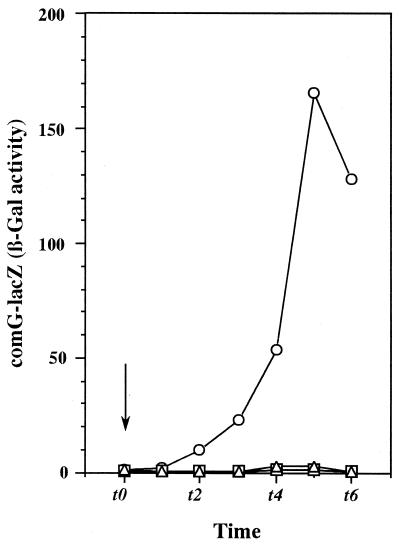
Previous studies have demonstrated that a reduction of GTP can induce spore formation in B. subtilis and that the RelA protein is involved in the reduction of GTP by synthesizing (p)ppGpp, which potently inhibits IMP dehydrogenase (11, 12, 17, 18). To determine whether a forced reduction in the GTP pool size could lead to an increase in the amount of active ComK in the relA strain, we added decoyinine (a GMP synthetase inhibitor) at t0 into the culture of strain 61883 grown in a high concentration (20 mM) of aspartate. Strikingly, decoyinine (2 mM) completely restored comG expression in the relA strain (61883) to the same level as that in the stringent strain (61884) (Fig. 3). When decoyinine was added at different growth phases (early, middle, or late exponential growth phase), the comG-lacZ expression was initiated at the same time (t2), irrespective of the addition time (data not shown). The addition of guanosine (2 mM) together with decoyinine abolished the decoyinine effect on comG expression (Fig. 3). The effect of guanosine on comG expression was no longer detected when guanosine was added 30 min after the addition of decoyinine (data not shown), indicating that a short time (30 min or less) is sufficient to commit the cells to produce ComG protein by decoyinine treatment.
FIG. 3.
Effect of decoyinine on comG-lacZ transcription. Strain 61883G carrying the comG-lacZ fusion gene was grown in TF medium containing 20 mM aspartate. Decoyinine (2 mM) (circles) or decoyinine plus guanosine (2 mM each) (triangles) were added to the culture at t0 as indicated by the arrow. Culture samples were taken at the indicated times, and β-galactosidase (β-Gal) activity was measured. Untreated culture (squares) is shown as a control.
Changes in the intracellular GTP level during growth.
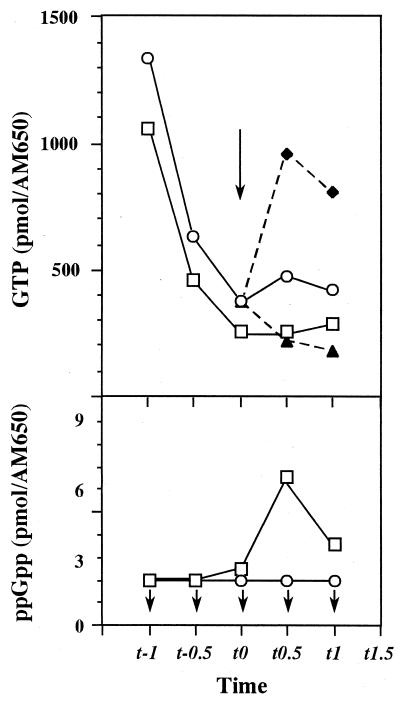
Next, we assumed that the impaired ability of the relA strain for competence development may result from insufficient reduction of intracellular GTP level. To assess this possibility, we monitored the level of intracellular GTP during growth of strains 61883 and 61884 in transformation medium (TF medium). The GTP level in the stringent strain (61884) decreased rapidly at t0 to approximately 200 pmol/AM650, accompanied by a transient increase in ppGpp. However, the GTP level in the relA strain (61883) remained at 400 pmol/AM650, and no increase in ppGpp was detected (Fig. 4). Similar results were reported previously by transferring cells from amino acid-sufficient to amino acid-limited conditions (17). When the relaxed mutant cells were treated with 2 mM decoyinine at t0, there was a significant reduction of GTP within 30 min, reaching 200 pmol/AM650 (Fig. 4). The addition of guanosine together with decoyinine resulted in a sharp increase of GTP to a level equivalent to that of exponentially growing cells (Fig. 4). Thus, it is reasonable to conclude that the inability to sufficiently reduce the level of GTP in the relA strain is a major cause for the inability of this strain to initiate genetic competence and sporulation (16-18). The observed intracellular concentrations of GTP during the transition phase (t0 to t1) were estimated to be 1.5 to 2.5 mM (for the stringent strain) and 3.5 to 4.5 mM (for the relaxed strain) by the method of Ochi (15).
FIG. 4.
Changes in the intracellular GTP and ppGpp concentrations during growth. The stringent (relA+) strain (61884) (squares) and the relaxed (relA) strain (61883) (circles) were grown in TF medium containing 20 mM aspartate. Decoyinine (2 mM) (closed triangles) or decoyinine plus guanosine (2 mM each) (closed diamonds) were added to the culture at t0, as indicated by the long black arrow in the top panel. Samples were taken at the indicated times to determine culture density (A650) and GTP and ppGpp concentrations. The short black arrows under the symbols in the bottom panel indicate that the values determined were below the detection limit by high-performance liquid chromatography (2 pmol/AM650 for ppGpp).
We further examined the effect of aspartate on the level of GTP using an asp+ strain. Strain K2-007 (asp+ relA) growing in TF medium without aspartate revealed GTP levels ranging from 450 to 220 pmol/AM650 during the transition phase (t0 to t1), while GTP levels ranged from 620 to 350 pmol/AM650 when cells were grown in TF medium supplemented with 20 mM aspartate, indicating that the intracellular GTP level is critically affected by the presence of aspartate even in the asp+ genetic background. These results, together with the results from strains 61883 and 61884 (see above), indicate a close relationship between the intracellular GTP level and acquisition of ability for competence.
Decoyinine fully induces competence in the relA strain.
We next studied the effect of decoyinine on transformation to assess the causal relationship between the level of GTP and the ability to be transformed. As expected from the results described above, competence of the relA strain (61883) was fully induced by decoyinine treatment. The transformation efficiency increased 100-fold with the addition of decoyinine at t0 (Fig. 5), reaching a level comparable to that of the stringent strain (Fig. 1). Apparently, the addition of decoyinine could fully induce a set of genes for competence. It was striking that the addition of guanosine together with decoyinine completely abolished the observed effect for decoyinine (Fig. 5).
FIG. 5.
Effect of decoyinine on transformation efficiency in the relaxed (relA) strain. The relaxed strain (61883) was grown in TF medium containing 20 mM aspartate. Decoyinine alone (2 mM) (circles) or with guanosine (2 mM each) (triangles) was added to the culture at t0 as indicated by the arrow. Samples were taken to determine culture density (A650 [open symbols]) and transformation efficiency (closed symbols). Transformation was performed as described in the legend to Fig. 1. Untreated culture (squares) is shown as a control.
Unlike the case for the relA strain, decoyinine addition was not effective in enhancing the frequency of transformation in wild-type (rel+) cells, apparently because rel+ cells can decrease GTP sufficiently without decoyinine (Fig. 4).
Effect of CodY on competence development.
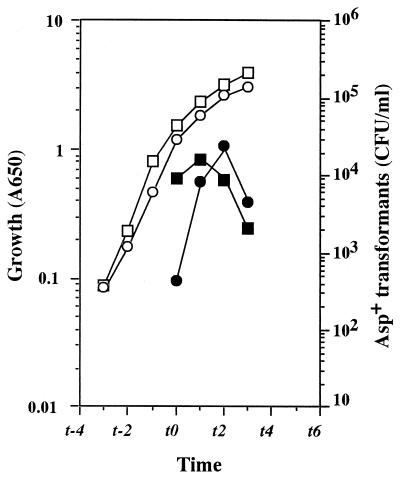
Serror and Sonenshein (21) demonstrated that CodY protein binds directly to the promoter region of the comK gene. More recently, Ratnayake-Lecamwasam et al. (20) working in the laboratory of A. L. Sonenshein have also demonstrated that the activity of the CodY protein is controlled by the level of intracellular GTP. These results raise the possibility that comK expression can be regulated by varying the level of intracellular GTP through CodY-mediated repression, eventually leading to full induction of genetic competence. To assess this possibility, we constructed codY-disruptant strains from the relaxed and stringent strains by inserting the neo gene as described in Materials and Methods. As expected, a disruption of the codY gene completely restored the competence ability in the relA strain (Fig. 6). In contrast, the disruption of codY did not give rise to any increase in competence ability when stringent strain 61884 was subjected to the gene disruption (compare Fig. 6 with Fig. 1), in agreement with the results from decoyinine addition (see above).
FIG. 6.
Effect of a codY disruption on the transformation of relaxed (relA) and stringent (relA+) strains. The stringent (TI70 [squares]) and relaxed (TI71 [circles]) strains were grown in TF medium containing 20 mM aspartate. Samples were taken to determine culture density (A650 [open symbols]) and transformation efficiency (closed symbols). Transformation was performed as described in the legend to Fig. 1.
Effect of CodY on sporulation.
As demonstrated by Ratnayake-Lecamwasam et al. (20) and Slack et al. (22) working in the laboratory of A. L. Sonenshein, CodY plays a crucial role in sporulation in B. subtilis. On the other hand, the relA mutation has a deleterious effect on sporulation (17). Likewise, the deleterious effect of the relA mutation was evident, as demonstrated by cultivating strains 61884 (rel+) and 61883 (relA) in TF medium supplemented with excess (20 mM) aspartate, displaying a 60-fold reduction in spore titers (Table 2). It was striking that the spore titer of a codY-disrupted relaxed strain TI71 was even greater than that of rel+ strain 61884 (Table 2). Moreover, strain TI71 formed heat-resistant spores earlier than strain 61884 did. The impaired sporulation ability of relA strain 61883 was also completely restored (2.0 × 108 spores produced per ml at 24 h) by adding 2 mM decoyinine at t0 (data not shown). Thus, under excess aspartate conditions, the stringent response plays a role in restoring not only competence development but also sporulation by reducing the intracellular GTP level sufficiently (Fig. 4), which eventually leads to inactivation of CodY as illustrated in Fig. 7.
TABLE 2.
Sporulation of B. subtilis strains grown in modified TF mediuma
| Strain | Time (h) | Total no. of viable cells (CFU/ml) | No. of heat- resistant spores (CFU/ml)b | Frequency of sporulation (%) |
|---|---|---|---|---|
| 61884 (rel+) | 12 | 6.0 × 108 | 2.5 × 104 | 0.004 |
| 18 | 1.7 × 108 | 2.8 × 104 | 0.016 | |
| 24 | 2.0 × 108 | 1.6 × 106 | 0.8 | |
| 36 | 6.8 × 108 | 4.2 × 107 | 6 | |
| 61883 (relA1) | 12 | 5.6 × 108 | 2.1 × 105 | 0.04 |
| 18 | 3.2 × 108 | 3.7 × 105 | 0.1 | |
| 24 | 7.3 × 108 | 7.2 × 105 | 0.1 | |
| 36 | 7.3 × 108 | 7.0 × 105 | 0.1 | |
| TI71 (relA1 ΔcodY) | 12 | 3.1 × 108 | 1.8 × 102 | <0.001 |
| 18 | 6.7 × 108 | 1.0 × 107 | 1 | |
| 24 | 7.8 × 108 | 6.4 × 107 | 8 | |
| 36 | 5.4 × 108 | 1.7 × 108 | 31 |
TF medium (see Materials and Methods) was supplemented with 1 mM Ca(NO3)2·4H2O, 0.1 mM MnCl2·4H2O, and 1 μM FeSO4·7H2O.
Heat-resistant spores were determined using L agar plate after heating the cultured broth at 80°C for 15 min.
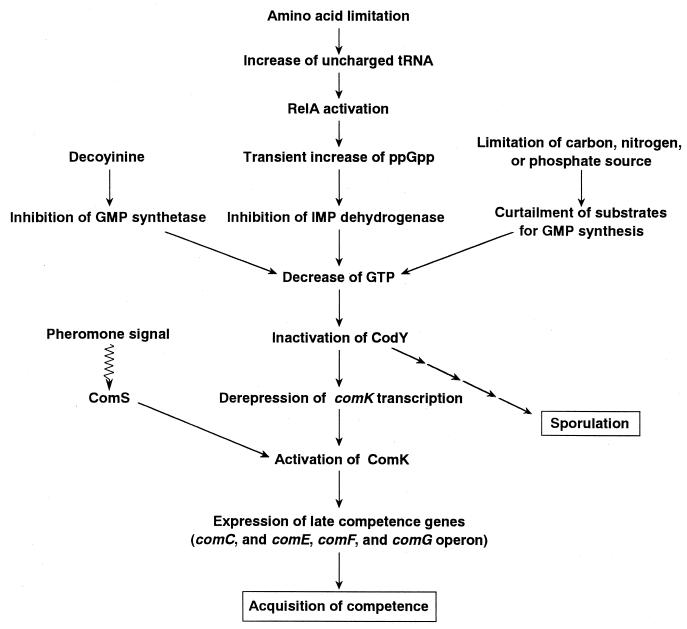
FIG. 7.
Schematic showing the signal transduction pathway starting from nutrient limitation to the acquisition of competence. This schematic was constructed on the basis of our present work and previously reported research in B. subtilis (2, 4, 7, 17, 18, 20, 21), including relevant work with Streptomyces griseus (15).
DISCUSSION
In this paper, we described the significance of the RelA protein in competence development, although the RelA protein is required for complete competence development only in the aspB relA mutant. As discussed by Dworkin and Losick (3), one of the most intriguing challenges in biology is to elucidate the mechanisms by which cells sense and respond to changes in extracellular nutritional conditions. However, the challenge of linking nutrient availability to alterations in gene expression has been met with success in only a few instances. Among prokaryotes, B. subtilis offers one of the best systems for studying such mechanisms, displaying a wide range of adaptations to nutrient limitations, including production and excretion of antibiotics and enzymes, entry into a state of genetic competence, elaboration of systems for motility and chemotaxis, and formation of endospores as an extreme response to nutrient limitation (3). In this light, recent work by Ratnayake-Lecamwasam et al. (20) and Slack et al.(22) in A. L. Sonenshein's laboratory should be highlighted, since they successfully linked nutritional status to sporulation, focusing mainly on CodY for sensing and responding to nutrient limitation. Our principal findings in this study were that (i) the RelA protein is essential for full induction of competence ability when cells were grown in the presence of high concentrations of aspartate (or glutamate), (ii) decoyinine treatment can elicit the competence ability, and (iii) aspartate significantly affects the nutritional status of B. subtilis. Thus, we successfully demonstrated that the development of competence and entry into the sporulation processes are tightly linked with nutrient limitations. This was verified by monitoring the changes in intracellular GTP and ppGpp levels and subsequent analysis of the expression of early (comK) and late (comG) competence genes as well as of the master protein CodY that controls both sporulation and competence. Thus, we can draw a scheme (Fig. 7) to describe the signal transduction system (starting from nutrient limitations to the eventual acquisition of competence) on the basis of our present work and previous research in other laboratories (2, 4, 7, 20, 21). Previous studies have shown that the stringent response initiates sporulation by reducing the level of intracellular GTP in B. subtilis (11, 12, 17, 18). When B. subtilis cells encounter a depletion of amino acids, cells immediately start to synthesize ppGpp via the RelA protein from ATP and GDP. The relA mutant lacks the ability to synthesize ppGpp (Fig. 4). It was also shown that ppGpp inhibits IMP dehydrogenase, the first enzyme in the GMP synthesis pathway, resulting in a rapid reduction in the level of GTP (18). Treatment with decoyinine, which inhibits GMP synthetase, the second enzyme in the GMP synthesis pathway, or limitation of the substrate for GMP synthesis also can cause a decrease of GTP, even in the relA mutant. Results of our present work support previous findings (Fig. 4). We also successfully demonstrated that a sufficient decrease in GTP caused by decoyinine treatment can fully induce genetic competence and sporulation in a relaxed strain. Therefore, we concluded that the RelA protein contributes significantly to the induction of genetic competence by reducing the level of GTP caused by the inhibitory effect of ppGpp (Fig. 7).
CodY mediates the inhibitory effects of glucose and amino acids on stationary-phase gene expression (22) and is considered to be a GTP-sensing transcriptional regulator having a predicted GTP binding pocket (20). Ratnayake-Lecamwasam et al. (20) working in A. L. Sonenshein's laboratory originally reported that the capacity of CodY to block transcription depends on the GTP concentration, but GTP does not have a strong effect on binding of CodY to DNA, although CodY actually binds GTP. However, in more-recent experiments, it was found that the difference in affinity between CodY and DNA varies as much as 10-fold in environments with low (0.2 mM) and high (2 mM) concentrations of GTP (K. Tachikawa and A. L. Sonenshein, personal communication). It is important to point out that blocking of transcription by CodY requires physiological amounts (2.2 mM) of GTP, as examined for dpp transcription (20), and that our calculated GTP concentrations in transition-phase cells were 1.5 to 2.5 mM for the stringent strain and 3.5 to 4.5 mM for the relaxed strain. Therefore, at these calculated concentrations, the CodY protein can no longer repress transcription in the stringent strain but can repress transcription in the relaxed strain. A sufficient decrease in GTP can lead to the derepression of CodY-mediated genes including comK (21), which correlates well with the results from our present work (Fig. 2). It is of interest that the impaired growth of the relA strain (Fig. 1) was restored by the codY disruption (Fig. 6). These observations suggest that the deleterious effects caused by the relaxed mutation resulted from the CodY-mediated repression, implying a wide variety of functions for CodY in late growth phase as originally predicted by Slack et al. (22).
Our results were further corroborated with research using the aspartate-auxotrophic strains. Since the asp+ relaxed strain could be transformed efficiently unless excess aspartate was present, the RelA protein is not thought to be crucial to the development of genetic competence. Nevertheless, it should be stressed that the effect of the RelA protein can be pronounced under specified physiological conditions, i.e., growth with abundant sources for carbon, nitrogen, and phosphate. Under such nutritional conditions, cells would not need to meet the severe requirements for curtailing the amount of substrates used in GMP synthesis. Therefore, modulation of the GTP level depends largely upon the stringent response (Fig. 7). The relaxed mutant cannot initiate the ppGpp system and therefore is dependent on the secondary pathway, i.e., curtailing the substrates used in GMP synthesis to reduce the level of GTP. Aspartate serves as a substrate for both purine (including GMP) and pyrimidine nucleotide biosynthesis as well as various other amino acids. Apparently, excess amounts of aspartate can block the consumption of substrates for GMP synthesis, resulting in an insufficient decrease in the GTP level in the relaxed mutants (see Results).
It is important to point out that although decoyinine addition was effective for competence development, the acquisition of competence was detected at the specified time (t2), irrespective of the time of decoyinine addition (see Results). Moreover, expression of comK and comG was not enhanced (but suppressed to some degree) when the rel+ strain 61884 was subjected to tryptophan deprivation (accompanied by the stringent response) at mid-exponential growth phase (A650 = 0.5) (unpublished results). These observations suggest that factors besides the ppGpp-GTP-CodY signal transduction system (Fig. 7) could promote the development of competence. In the framework of this notion, the pheromone signal transduction system (13, 23) should be highlighted. Since quorum-sensing oligopeptide pheromones have been demonstrated to govern a ComS signal transduction system, the development of competence is thought to be regulated by these mutually independent systems, i.e., the GTP signal system and pheromone signal system, as illustrated in Fig. 7.
Acknowledgments
This work was supported in part by a grant from the Organized Research Combination System (ORCS) of the Science and Technology Agency of Japan.
We thank Chie Kobayashi for technical assistance in some experiments and F. Kawamura for providing strains RIK720 and RIK51.
REFERENCES
- 1.Cashel, M., D. R. Gentry, V. J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1458-1496. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. American Society for Microbiology, Washington, D.C.
- 2.Dubnau, D. 1993. Genetic exchange and homologous recombination, p. 555-584. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 3.Dworkin, J., and R. Losick. 2001. Linking nutritional status to gene activation and development. Genes Dev. 15:1051-1054. [DOI] [PubMed] [Google Scholar]
- 4.Grossman, A. D. 1995. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu. Rev. Genet. 29:477-508. [DOI] [PubMed] [Google Scholar]
- 5.Hahn, J., L. Kong, and D. Dubnau. 1994. The regulation of competence transcription factor synthesis constitutes a critical control point in the regulation of competence in Bacillus subtilis. J. Bacteriol. 176:5753-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn, J., A. Luttinger, and D. Dubnau. 1996. Regulatory inputs for the synthesis of ComK, the competence transcription factor of Bacillus subtilis. Mol. Microbiol. 21:763-775. [DOI] [PubMed] [Google Scholar]
- 7.Haijema, B. J., D. van Sinderen, K. Winterling, J. Kooistra, G. Venema, and L. W. Hamoen. 1996. Regulated expression of the dinR and recA genes during competence development and SOS induction in Bacillus subtilis. Mol. Microbiol. 22:75-85. [DOI] [PubMed] [Google Scholar]
- 8.Inaoka, T., K. Kasai, and K. Ochi. 2001. Construction of an in vivo nonsense readthrough assay system and functional analysis of ribosomal proteins S12, S4, and S5 in Bacillus subtilis. J. Bacteriol. 183:4958-4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itaya, M., K. Kondo, and T. Tanaka. 1989. A neomycin resistance gene cassette selectable in a single copy state in the Bacillus subtilis chromosome. Nucleic Acids Res. 17:4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong, L., and D. Dubnau. 1994. Regulation of competence-specific gene expression by Mec-mediated protein-protein interaction in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 91:5793-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez, J., A. Dromerick, and E. Freese. 1981. Response of guanosine 5′-triphosphate concentration to nutritional changes and its significance for Bacillus subtilis sporulation. J. Bacteriol. 146:605-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez, J., C. L. Marks, and E. Freese. 1979. The decrease of guanine nucleotides initiates sporulation of Bacillus subtilis. Biochim. Biophys. Acta 587:238-252. [DOI] [PubMed] [Google Scholar]
- 13.Magnuson, R., J. Solomon, and A. D. Grossman. 1994. Biochemical and genetic characterization of a competence pheromone. Cell 77:207-216. [DOI] [PubMed] [Google Scholar]
- 14.Nanamiya, H., Y. Ohashi, K. Asai, S. Moriya, N. Ogasawara, M. Fujita, Y. Sadaie, and F. Kawamura. 1998. ClpC regulates the fate of a sporulation initiation sigma factor, sigmaH protein, in Bacillus subtilis at elevated temperatures. Mol. Microbiol. 29:505-513. [DOI] [PubMed] [Google Scholar]
- 15.Ochi, K. 1987. Changes in nucleotide pools during sporulation of Streptomyces griseus in submerged culture. J. Gen. Microbiol. 133:2787-2795. [Google Scholar]
- 16.Ochi, K., and E. Freese. 1983. Effect of antibiotics on sporulation caused by the stringent response in Bacillus subtilis. J. Gen. Microbiol. 129:3709-3720. [Google Scholar]
- 17.Ochi, K., J. C. Kandala, and E. Freese. 1981. Initiation of Bacillus subtilis sporulation by the stringent response to partial amino acid deprivation. J. Biol. Chem. 256:6866-6875. [PubMed] [Google Scholar]
- 18.Ochi, K., J. C. Kandala, and E. Freese. 1982. Evidence that Bacillus subtilis sporulation induced by the stringent response is caused by the decrease in GTP or GDP. J. Bacteriol. 151:1062-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochi, K., and S. Ohsawa. 1984. Initiation of antibiotic production by the stringent response of Bacillus subtilis marburg. J. Gen. Microbiol. 130:2473-2482. [DOI] [PubMed] [Google Scholar]
- 20.Ratnayake-Lecamwasam, M., P. Serror, K.-W. Wong, and A. L. Sonenshein. 2001. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev. 15:1093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serror, P., and A. L. Sonenshein. 1996. CodY is required for nutritional repression of Bacillus subtilis genetic competence. J. Bacteriol. 178:5910-5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slack, F. J., P. Serror, E. Joyce, and A. L. Sonenshein. 1995. A gene required for nutritional repression of the Bacillus subtilis dipeptide permease operon. Mol. Microbiol. 15:689-702. [DOI] [PubMed] [Google Scholar]
- 23.Solomon, J. M., B. A. Lazazzera, and A. D. Grossman. 1996. Purification and characterization of an extracellular peptide factor that affects two developmental pathways in Bacillus subtilis. Genes Dev. 10:2014-2024. [DOI] [PubMed] [Google Scholar]
- 24.Swanton, M., and G. Edlin. 1972. Isolation and characterization of an RNA relaxed mutant of B. subtilis. Biochem. Biophys. Res. Commun. 46:583-588. [DOI] [PubMed] [Google Scholar]
- 25.Turgay, K., J. Hahn, J. Burghoorn, and D. Dubnau. 1998. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 17:6730-6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turgay, K., L. W. Hamoen, G. Venema, and D. Dubnau. 1997. Biochemical characterization of a molecular switch involving the heat shock protein ClpC, that controls the activity of ComK, the competence transcription factor of Bacillus subtilis. Genes Dev. 11:119-128. [DOI] [PubMed] [Google Scholar]
- 27.van Sinderen, D., A. Luttinger, L. Kong, D. Dubnau, G. Venema, and L. Hamoen. 1995. comK encodes the competence transcription factor, the key regulatory protein for competence development in Bacillus subtilis. Mol. Microbiol. 15:455-462. [DOI] [PubMed] [Google Scholar]
- 28.van Sinderen, D., A. ten Berge, B. J. Hayema, L. Hamoen, and G. Venema. 1994. Molecular cloning and sequence of comK, a gene required for genetic competence in Bacillus subtilis. Mol. Microbiol. 11:695-703. [DOI] [PubMed] [Google Scholar]
- 29.Wendrich, T. M., and M. A. Marahiel. 1997. Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol. Microbiol. 26:65-79. [DOI] [PubMed] [Google Scholar]